Abstract
OBJECTIVE:
Persons with opioid use disorder who take benzodiazepines are at high risk for overdose. Limited data exist on the association of benzodiazepine and Z-drug use with drug-related poisonings in patients receiving buprenorphine maintenance treatment.
METHODS:
This case-crossover study focused on prescription claims in persons (ages 12–64) with opioid use disorder who were prescribed buprenorphine in the IBM® MarketScan® databases (2006–2016), encompassing 14,213,075 person-days of observation time among 23,036 individuals who experienced drug-related poisoning. The exposures were buprenorphine prescriptions and benzodiazepine prescriptions, standardized as daily diazepam-equivalent milligram doses and separated by pharmacologic properties (short-acting, long-acting, Z-drug). The outcome of interest was non-fatal drug-related poisonings. Conditional logistic regression was used to evaluate variation in benzodiazepine and buprenorphine use between poisoning and non-poisoning days.
RESULTS:
Buprenorphine treatment days were associated with a nearly 40% reduction in risk of poisoning events (OR=0.63, [95% CI: 0.60–0.66]) relative to non-treatment days, whereas benzodiazepine treatment days were associated with an 88% increase in the risk of such events (1.78–1.98). In stratified analyses by dose, we observed a 78% (1.67–1.88) and 122% (2.03–2.43) increase in poisonings associated with low-dose and high-dose benzodiazepine treatment days respectively. High-dose, but not low-dose, benzodiazepine treatment was associated with increased poisonings in combination with buprenorphine co-treatment (OR=1.64 [1.39–1.93]), but this was lower than the odds risk associated with benzodiazepine treatment in the absence of buprenorphine (OR=1.69 [1.60–1.79] for low-dose, OR=2.23 [2.04–2.45] for high-dose).
CONCLUSIONS:
Increased risk of non-fatal drug-related poisoning are associated with benzodiazepine treatment in patients with opioid use disorder, but these risks are partially mitigated by buprenorphine treatment. Dose reduction of benzodiazepines while maintaining buprenorphine treatment may have the advantage of lowering drug-related poisoning risk.
Introduction
Buprenorphine is an effective treatment for opioid use disorder (OUD), contributing to significant reductions in all-cause and opioid-associated mortality amid the current United States (U.S.) opioid epidemic.(1) Among OUD patients taking buprenorphine, benzodiazepine use is highly prevalent; some studies have estimated as many as 30% of OUD patients on opioid maintenance treatment received benzodiazepine prescriptions, (2) with over one-third of these patients endorsing past-month problematic use of benzodiazepines.(3) Although benzodiazepines are frequently prescribed for treatment of comorbid mood and anxiety disorders that are common in the OUD population, recent research has shed light on respiratory depression, overdose risk, and addictive potential associated with benzodiazepine use in patients taking chronic opioids such as buprenorphine.(4–7) It is unclear if risks associated with benzodiazepine use outweigh treatment benefits of buprenorphine.
The relationship between benzodiazepine use and buprenorphine treatment outcomes is poorly characterized. Existing observational studies investigating mortality risk associated with benzodiazepine prescriptions in OUD patients have produced contradictory results.(8–10) While some findings suggest that benzodiazepines may enhance retention in buprenorphine maintenance treatment (4, 11), benzodiazepines have also been associated with increased drug-related poisoning, (11–14) all-cause mortality,(11) non-overdose deaths(8), decreased retention in treatment,(12, 15–17) and accidental-injury related emergency room visits.(5) Notably, no studies have specifically studied potential additive or interactive effects between buprenorphine and benzodiazepines.
Another limitation of the current research base on benzodiazepine-related morbidity and mortality is that the different pharmacologic properties of sedative/hypnotics –such as half-life and potency— have seldom been explored.(18) This is an important clinical and scientific gap given that benzodiazepines with shorter onset such as alprazolam are thought to have higher addictive potential than longer-acting medications such as clonazepam. (6, 19) In fact, previous work in non-OUD populations have shown significant intra-benzodiazepine variation in health outcomes; for instance, certain benzodiazepines appear to be associated with greater injury risk in the elderly than others.(18) Furthermore, very little research has investigated drug-related poisoning associated with selective benzodiazepine receptor modulators (Z drugs: zolpidem, zaleplon, eszopiclone); these medications have increasingly been found to have a spectrum of adverse effects similar to benzodiazepines, with similar dose response effects on all-cause mortality in the general population.(20, 21) Since studies to date have relied on relatively small numbers of benzodiazepine users, they have precluded examination of how drug subclasses (e.g., short- vs. long-acting; Z drugs) or dosage regimens impact OUD outcomes.
In light of the research gap on OUD outcomes among patients taking both buprenorphine and benzodiazepines, our study used the IBM® MarketScan® databases (2006–2016) to: (1) quantify odds of non-fatal drug-related poisoning (including overdoses) associated with benzodiazepine use in OUD patients; (2) determine if BZD use improves, nullifies, or reverses the protective effect of buprenorphine in OUD patients; (3) evaluate if different sedative/hypnotic subtypes (e.g., long-acting vs short-acting agents, Z drugs) correspond to different poisoning risk.
Methods
Dataset and Participants
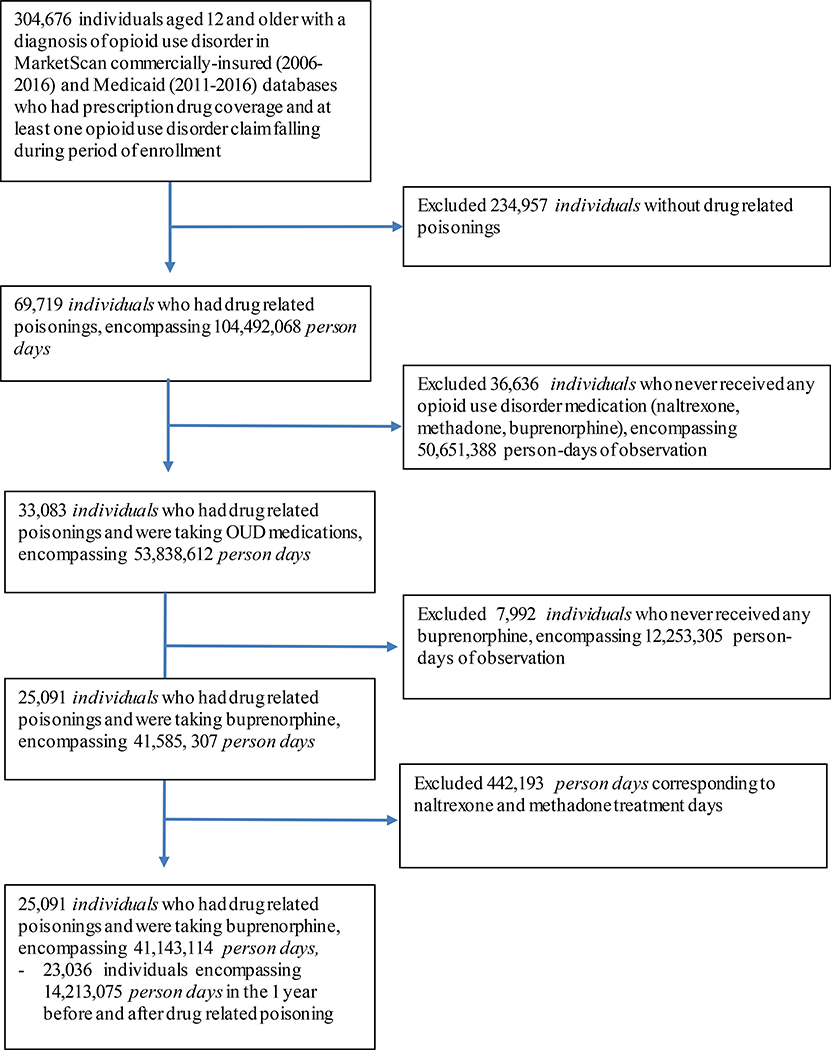
Our analysis used pharmaceutical claims data of 304,676 individuals ages 12–64 in the IBM® MarketScan® Commercial and Multi-State Medicaid Databases who received buprenorphine treatment for OUD (Figure 1). Our cohort, spanning from January 1, 2006 to December 31, 2016, represents claims for insured active employees, early (non-Medicare) retirees, and dependents insured by employer-sponsored plans as well as Medicaid insurance holders, spanning all 50 states.(22) Details on data extraction are in the eMethods 1. As all data were de-identified, our analysis was exempt from human subjects review by the Washington University Institutional Review Board.
Figure 1.
shows derivation of the study’s final analytic sample during follow-up. There were 304,676 individuals ages 12 and older with a diagnosis of OUD, prescription drug coverage, and at least one OUD claim during period of enrollment. 234,957 individuals without drug related poisonings were excluded from the sample, resulting in 69,719 individuals (23%) with poisoning events that occurred between 2006 and 2016 in the MarketScan databases. We then excluded individuals who never received any opioid use disorder medication (naltrexone, methadone, buprenorphine), resulting in 33,083 individuals with both drug related poisonings and were taking OUD medications. We excluded individuals who never received buprenorphine, resulting in 25,091 individuals with poisoning events and insurance claims for buprenorphine coverage. Finally, we excluded person days of naltrexone and methadone treatment and narrowed our window of observation to up to 1 year before and after index poisoning.
We included individuals ages 12–64 with insurance claims indicating an OUD diagnosis, at least one buprenorphine prescription, and at least one non-fatal drug-related poisoning. Buprenorphine prescriptions were defined by associated diagnostic codes selected using established methods(22) and are listed in eTable 1. We included persons who had time periods of buprenorphine-free treatment as long as they received the medication at another point in time. Buprenorphine use was characterized in terms of strength, quantity, and days’ supply in order to calculate a daily milligram dose. This was further stratified into daily buprenorphine doses > 12 mg and ≤12 mg, given previous analyses suggesting differences in treatment retention associated with this dose.(23) Since each individual serves as their own control in case-crossover designs, all individuals in the sample must experience the outcome (drug-related poisoning) at least once. Thus we excluded all individuals who did not experience a drug-related poisoning.
Design
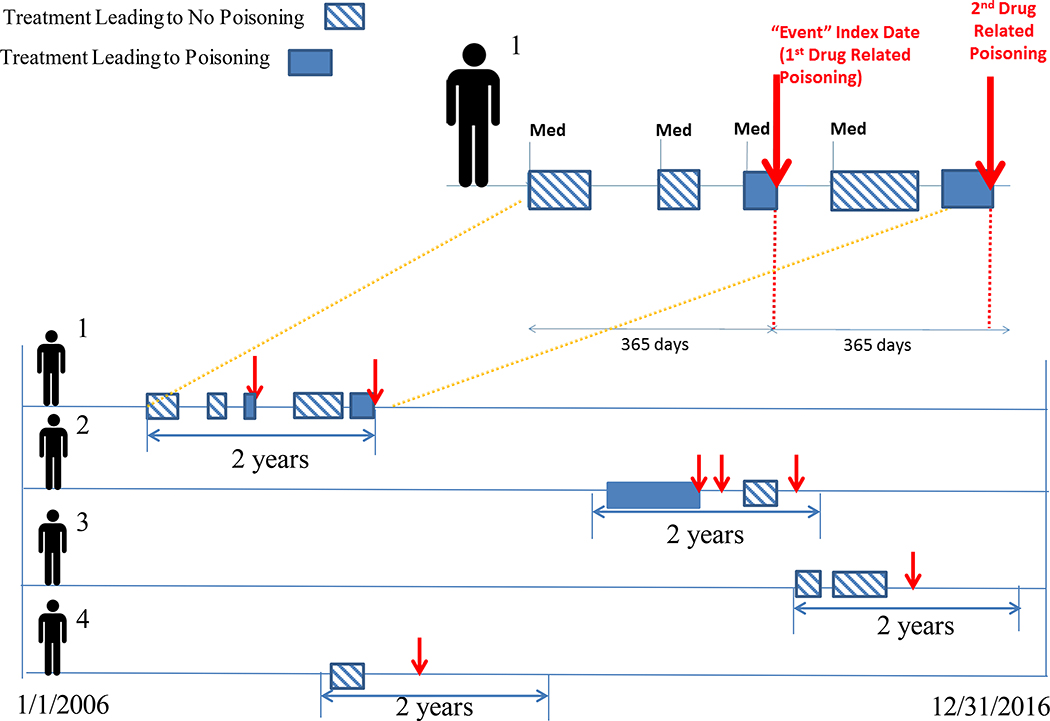
We used a case-crossover study design that exploited within-subject variation in exposure and outcome; this addresses unobserved, time-invariant confounding by allowing each individual to serve as their own control. Units of observation were person-days, denoting days during which participants were enrolled in insurance. Case periods were days when a participant experienced non-fatal drug-related poisonings. Control periods were nearby days without poisoning events.(24, 25) We characterized each person-day of observation by: (1) presence or absence of benzodiazepine treatment; (2) presence or absence of buprenorphine treatment. We permitted each participant to have multiple poisoning events as long as these fell within the interval of at most 365 days before and after index poisoning (Figure 2).Individuals with fewer observation days on either side of the index event were included with missing days treated as censored. We evaluated participants during the year prior to and after a patient’s first drug-related poisoning (index date), thus limiting participants to two-year periods of observation to reduce heterogeneity in observation time per person. We included periods both before and after the index poisoning so as to define the poisoning as a repeatable event, allowing for the inclusion of time as a covariate.(25, 26) Thus, the unit of analysis was days, stratified within persons, and the comparison of interest was drug exposure status concurrent with drug-related poisoning (case period) versus exposure status during referent periods when poisoning events did not occur (control period).
Figure 2.
provides an overview of the study’s case-crossover design. In this figure, treatment days during which an individual received a medication, such as, benzodiazepines or buprenorphine, that culminated in drug-related poisoning (hatched boxes) are compared to days that do not coincide with poisoning event for the same medication (shaded boxes). We used an observation period spanning up to at most one year before and after index poisoning. Consequently, each individual can potentially contribute multiple drug-related poisoning events to the final analysis, as long as such events fall within a maximum of one year before and after index poisoning event.
For individual #1, Figure 2 depicts five treatment periods, two of which culminate in a drug-related poisoning. This contrasts with individual #2, who has two treatment periods, only one of which culminates in a drug-related poisoning; individual #2 has three drug-related poisonings, two of which are not preceded by medication treatment. Individuals #3 and #4 notably do not have any treatment periods culminating in drug-related poisoning.
Given their high prevalence in clinical practice and overlapping indications as benzodiazepines, selective serotonin reuptake inhibitors (SSRIs: sertraline, fluoxetine, escitalopram, and citalopram) were included in our conditional logistic models as an active comparator analysis. This is a validated approach employed by previous papers in this topic area(27) to control for unobserved confounding. In other words, the SSRI term assesses whether underlying conditions for which patients were receiving benzodiazepines were contributing to drug-related poisonings.
Ascertainment of Outcomes and Exposures:
As shown in Figure 2, our primary outcome was non-fatal drug-related poisonings (including overdoses). Controls were adjacent person-days when poisoning event did not occur. Poisonings were defined using International Classification of Disease codes and Healthcare Common Procedure Coding System codes for naloxone reversal. Per guidelines compiled by Center for Disease Control consensus recommendations for poisoning surveillance,(28) our codes encompassed not only opioids but also alcohol, benzodiazepines, psychotropic medication, and overdose with other substances (eTable 1). This was intended to capture conditions commonly associated with chronic drug misuse, as well as avoid misclassification bias and underestimation of overdose risks. We searched all emergency room visits, outpatient ambulatory visits, and hospitalizations for relevant diagnostic codes.(28)
Our primary exposure was benzodiazepine prescriptions, ascertained through pharmacy files. A person is assumed to be exposed if they were covered by a prescription for a drug on a given day. We characterized initial benzodiazepine, Z drug, and buprenorphine prescription in terms of strength, quantity dispensed, and days’ supply. To standardize daily dosage, we calculated each benzodiazepine or Z drug’s strength in terms of total diazepam equivalent milligrams(29) using known pharmacologic conversion factors (eTable 2).(30–32) We calculated a daily diazepam equivalent dose by multiplying number supplied by strength (in diazepam equivalent milligrams) and dividing by days’ supply. Benzodiazepine dosage was stratified into high dose (diazepam equivalent mg dose > 30 mg) and low dose (≤ 30 mg) based on established thresholds.(33) Benzodiazepine exposure was categorized based on duration of action, namely short-acting (half-life ≤24 hours) or long-acting (half-life >24 hours), guided by established classifications.(6) Specific classifications of benzodiazepine agents and Z drugs, based on previously published definitions, are shown in eTable 2.(34, 35) Furthermore, we collected data on age, sex, relationship of patient to the primary beneficiary, and insurance status. Because our cases are self-matched in case-crossover design, our models did not warrant adjustment for time-invariant measured confounders (e.g., sex or race).
Statistical Analysis:
Analyses were conducted on a day-level dataset in which each medication coverage date was identified. All analyses were conducted via SAS® 9.4. Prior to evaluating variation in medication exposure between case and control days, we calculated descriptive statistics for the primary analytical sample (i.e., participants who had non-fatal drug-related poisonings). To compare OUD treatment characteristics between those with and without poisoning events, we obtained a random sample of persons who never experienced poisonings, for which we compared descriptive statistics of those who had poisoning events (eMethods 2, eTables 3–4).
To evaluate the effect of medication exposure, we estimated conditional logistic regression models stratified by subject and modeled the risk of poisoning as a function of drug exposure by day. We first built a crude model containing benzodiazepine prescription status as the only predictor variable. We then built additional models that included predictor variables for both benzodiazepine and buprenorphine prescription status. Simultaneous inclusion of benzodiazepine and buprenorphine permitted us to model additive and/or interactive effects of benzodiazepines and buprenorphine in association with drug-related poisonings. SSRIs were included in our models as an active comparator analysis. Subgroup analyses were conducted to assess the effect of buprenorphine treatment days, in comparison to days without treatment, on drug-related poisoning among those who received benzodiazepine prescriptions and those who did not. Controls for both calendar time and time from index poisoning were included using cubic spline methods described further in the eMethods 3. Several sensitivity analyses were conducted to evaluate the possibility of persistent user bias and assess the robustness of our findings to alternative time samplings (eFigure 1, eMethods 3–4, eTable 5).
Results
Sample and Treatment Characteristics
As seen in Figure 1, out of 304,676 individuals ages 12–64 with an OUD diagnosis, prescription drug coverage, and at least one OUD claim during period of enrollment, we excluded individuals without drug-related poisonings, individuals who never received OUD medication, days of naltrexone and methadone treatment, and days of observation outside 1 year before and after index poisoning. Our final analytic sample included 23,036 participants (mean age 30 years, 51% male, mean observation time 299 days), spanning 14,213,075 person-days of insurance coverage. 2,210,927 person-days (15.6%) entailed claims for buprenorphine (mean daily dose 15.4 mg). 1,968,944 person-days (13.9%) entailed claims for benzodiazepines or Z drugs, of which 474,181 person-days entailed concurrent buprenorphine treatment. We calculated the mean daily dose of any benzodiazepine or Z drug to be 23.4 diazepam milligram equivalents, with short-acting benzodiazepines, long-acting benzodiazepines, and Z drugs respectively 25.3, 31.3, and 4.9 diazepam milligram equivalents (Table 2).
Table 2.
Opioid Use Disorder Treatment Characteristics at the Person-Days Levela
| 14,213,075 Treatment Days | ||
|---|---|---|
| Person-Days | (%) | |
| Treatment Days Marked by Drug-Related Poisoning | 26,243 | 0.18 |
| Days Treated with Buprenorphine | 2,210,927 | 15.56 |
| Mean Dose (mg daily) (sd) | 15.44 | 7.31 |
| Low-dose, ≤12 mg daily | 758,261 | 5.33 |
| High-dose, > 12 mg daily | 1,367,893 | 9.62 |
| Days Treated with SSRIs | 1,715,489 | 12.07 |
| Days Treated with Benzodiazepines or Z drugs | 2,493,800 | 17.55 |
| Mean Dose (diazepam equivalent mg daily) (sd) | 23.39 | 25.88 |
| Days Treated with Benzodiazepines excluding Z drugs | 1,968,944 | 13.85 |
| Mean Dose (diazepam equivalent mg daily) (sd) | 27.58 | 26.98 |
| Low-dose, ≤ 30 diazepam equivalent mg daily) | 1,453,110 | 10.22 |
| High-dose, > 30 diazepam equivalent mg daily) | 515,834 | 3.63 |
| Days Treated with Short Acting Benzodiazepines | 1,584,424 | 11.15 |
| Mean Dose (diazepam equivalent mg daily) (sd) | 25.33 | 20.53 |
| Days Treated with Long Acting Benzodiazepines | 452,820 | 3.19 |
| Mean Dose (diazepam equivalent mg daily) (sd) | 31.28 | 38.10 |
| Days Treated with Z Drugs | 825,610 | 5.81 |
| Mean Dose (diazepam equivalent mg daily) (sd) | 4.88 | 1.24 |
| Concurrent Usage of Buprenorphine and/or Benzodiazepines or Z drugs | ||
| Days Without Buprenorphine or Benzodiazepine or Z drug Treatment | 9,982,529 | 70.23 |
| Days Treated with Benzodiazepines or Z drugs only | 2,019,619 | 14.21 |
| Days Treated with Buprenorphine only | 1,736,746 | 12.22 |
| Days Treated with Concurrent Buprenorphine and Benzodiazepines or Z drugs | 474,181 | 3.34 |
This table depicts opioid use disorder treatment characteristics at the person-days level. Among all individuals with history of drug-related poisoning during the study’s observation window, we calculated the number of person-days for which insurance claims were filed for medication treatment. Because the data in this table is not displayed at the individual level, it is possible for an individual participant to contribute multiple person-days.
Benzodiazepines and Drug-Related Poisonings
Table 3 shows that buprenorphine treatment days were associated with 37% lower odds of drug-related poisoning (95% confidence interval: 0.60–0.66) relative to non-treatment days, whereas odds of poisoning increased 81% on days when individuals were treated with benzodiazepines or Z drugs (1.73–1.91, Model 1). When we evaluated benzodiazepines and Z drugs separately, Z drug treatment days were associated with increased odds of poisoning events (OR=1.29 [1.19–1.39]), but this was notably lower than the odds associated with benzodiazepine treatment days (OR=1.88 [1.78–1.98], Model 2). We found no association between SSRI treatment days and drug-related poisonings (OR=0.95 [0.90–1.00], Model 3). We observed no difference in magnitude of protective effect against poisoning conferred by buprenorphine treatment days when conducting stratified analyses among benzodiazepine or Z drug users (OR=0.64 [0.60–0.69], Model 4) and those who never used benzodiazepine or Z drugs during the study’s observation period (OR=0.64 [0.59–0.69], Model 5).
Table 3.
Odds of Drug-Related Poisoning Associated with Benzodiazepine Use
| Odds Ratio Estimates | ||||
|---|---|---|---|---|
| Effect | Point Estimate | 95% Confidence Intervals | ||
| Model 1 | Buprenorphine | 0.63 | 0.60 | 0.66 |
| Any Benzodiazepine or Z Drug | 1.81 | 1.73 | 1.91 | |
| Model 2 | Buprenorphine | 0.63 | 0.60 | 0.67 |
| Benzodiazepines, excluding Z Drugs | 1.88 | 1.78 | 1.98 | |
| Z Drugs | 1.29 | 1.19 | 1.39 | |
| Model 3 | Buprenorphine | 0.63 | 0.60 | 0.67 |
| Benzodiazepines, excluding Z Drugs | 1.88 | 1.79 | 1.99 | |
| Z Drugs | 1.29 | 1.19 | 1.40 | |
| SSRIs | 0.95 | 0.90 | 1.00 | |
| Model 4 | Buprenorphine (among benzodiazepine or Z drug users) | 0.64 | 0.60 | 0.69 |
| Model 5 | Buprenorphine (among benzodiazepine or Z drug non-users) | 0.64 | 0.59 | 0.69 |
| Model 6 | Buprenorphine | 0.63 | 0.60 | 0.66 |
| Short-acting Benzodiazepines | 1.86 | 1.75 | 1.97 | |
| Long-acting Benzodiazepines | 1.68 | 1.54 | 1.83 | |
| Z Drugs | 1.29 | 1.19 | 1.39 | |
| Model 7 | Buprenorphine | 0.63 | 0.60 | 0.66 |
| Low-dose Benzodiazepines a | 1.78 | 1.67 | 1.88 | |
| High-dose Benzodiazepines b | 2.22 | 2.03 | 2.43 | |
| Z Drugs | 1.29 | 1.19 | 1.39 | |
| Model 8 | Buprenorphine | 0.64 | 0.62 | 0.67 |
| Any Benzodiazepine or Z Drug, low dose c | 1.86 | 1.77 | 1.95 | |
| Any Benzodiazepine or Z Drug, high dose d | 2.53 | 2.35 | 2.73 | |
| Model 9 | Low-dose Buprenorphine e | 0.62 | 0.57 | 0.67 |
| High-dose Buprenorphine f | 0.63 | 0.59 | 0.67 | |
| Low-dose Benzodiazepines | 1.78 | 1.68 | 1.88 | |
| High-dose Benzodiazepines | 2.22 | 2.03 | 2.43 | |
| Z Drugs | 1.29 | 1.19 | 1.39 | |
| Model 10 | Buprenorphine only | 0.61 | 0.58 | 0.65 |
| Benzodiazepine or Z Drug, low dose (+ Buprenorphine) | 1.11 | 1.00 | 1.23 | |
| Benzodiazepine or Z Drug, high dose (+ Buprenorphine) | 1.64 | 1.39 | 1.93 | |
| Benzodiazepine or Z Drug, low dose (no buprenorphine) | 1.69 | 1.60 | 1.79 | |
| Benzodiazepine or Z Drug, high dose (no buprenorphine) | 2.23 | 2.04 | 2.45 | |
Low-dose Benzodiazepines: ≤ 30 diazepam equivalent milligrams daily
High-dose Benzodiazepines: > 30 mg diazepam equivalent milligrams daily
Low-dose Benzodiazepines or Z drugs: ≤ 30 diazepam equivalent milligrams daily
High-dose benzodiazepines or Z drugs: > 30 diazepam equivalent milligrams daily
Low-dose buprenorphine: ≤12 milligrams daily
High-dose buprenorphine: > 12 milligrams daily
Subgroup and Sensitivity Analyses
We stratified benzodiazepines by mechanism and observed similarly elevated odds of drug-related poisoning for short-acting (OR=1.86 [1.75–1.97], Model 6) and long-acting benzodiazepine treatment days (OR=1.68 [1.54–1.83], Model 6). When we stratified benzodiazepines by low and high doses, we found a 78% (1.67–1.88, Model 6) and 122% (2.03–2.43, Model 7) increase in odds of a poisoning event respectively. To assess if this dose-response relationship remained significant across all sedative/hypnotic drugs, we grouped benzodiazepines and Z drugs together and stratified them into low-dose and high-dose strata, observing a similar pattern of increased poisoning odds associated with dose (OR=1.86 [1.77–1.79] for low-dose; OR=2.53 [2.35–2.73] for high-dose, Model 8). In contrast to benzodiazepines, we found similar protective effects of buprenorphine irrespective of daily dose threshold (OR=0.62 for low dose, OR=0.63 for high dose buprenorphine, Model 9).
Interaction between Benzodiazepines and Buprenorphine
Table 3 (Model 10) depicts odds of drug-related poisoning associated with benzodiazepine use, stratified by benzodiazepine dose and by buprenorphine co-use. Stratification on these variables allowed for observation of possible interaction effects between benzodiazepine dose and buprenorphine use. For high-dose benzodiazepines, poisoning odds associated with buprenorphine co-use were higher than would have been expected based on the assumption of additive risks (Wald Chi-Square= 4.02, p=0.045). Odds ratios associated with low- and high-dose benzodiazepine co-use with buprenorphine were respectively 1.11 (1.00–1.23) and 1.64 (1.39–1.93). Notably, these effects were lower than those for low- and high-dose benzodiazepines in absence of buprenorphine, which were respectively OR=1.69 (1.60–1.79) and OR=2.23 (2.04–2.45). In other words, individuals with OUD taking both buprenorphine and benzodiazepines had lower net-risk of poisoning than those taking benzodiazepines without buprenorphine.
Discussion
In this study, we used the IBM MarketScan® databases to analyze non-fatal drug-related poisoning (including overdose events) associated with specific benzodiazepines and dose regimens in OUD patients. Even though buprenorphine treatment days conferred a nearly 40% reduction in poisonings, benzodiazepine treatment days corresponded to a near-doubling in poisoning risk. While individuals taking both buprenorphine and benzodiazepines are at elevated risk of poisoning, they still had a lower net-risk than those taking benzodiazepines without buprenorphine. We found no association between SSRIs and drug-related poisoning, making it less likely that underlying conditions for which patients were receiving benzodiazepines were contributing to overdoses. Additionally, we found no dose-dependence for the association between buprenorphine and poisoning risk.
Our findings may have significant implications for clinical practice. First, our results show a clear, dose-dependent pattern of worsened overdose-related outcomes associated with benzodiazepine use, indicating that, among OUD patients for whom benzodiazepine cessation is risky, lower doses and shorter treatment duration for sedative/hypnotics may reduce risk. In addition, we found slightly lower-risk for long-acting benzodiazepines relative to short-acting, and substantially lower risk associated with Z-drugs as compared to either, which may be related to lower mean standardized dosages observed among Z-drugs. Overall, these results suggest that switching benzodiazepine users from short-acting medications to long-acting agents or Z drugs may hold promise in lowering overdose risk.
Importantly, buprenorphine’s beneficial effect was observed among both benzodiazepine users and nonusers. This is an important finding because the safety of benzodiazepine use in buprenorphine users has been a topic of contentious debate in the past, leading to restrictions on buprenorphine treatment among benzodiazepine users that were later reversed by the U.S. Food and Drug Administration.(4, 36) Our results demonstrate that even though benzodiazepines may increase drug-related poisonings, buprenorphine’s protective effect is not eliminated by benzodiazepine treatment. Overall, our findings suggest that for patients taking both benzodiazepines and buprenorphine, dose reduction of benzodiazepines—while maintaining buprenorphine treatment-- may have the advantage of decreasing overdoses associated with both benzodiazepines and the benzodiazepine/buprenorphine interaction. As previous studies have found benzodiazepines to be associated with improved treatment retention in patients receiving buprenorphine,(4, 11) dose reduction of benzodiazepines may be more preferable to abrupt cessation.
There are several limitations of this study. Firstly, despite use of an active comparator and case-crossover design, we cannot completely exclude the possibility of residual confounding by indication. For instance, benzodiazepines may constitute a proxy for underlying anxiety symptoms that lead to sedative/hypnotic prescriptions, substance use, and overdose, as opposed to directly contributing to poisoning. Unmeasured exposures, such as illicit substances and non-prescribed benzodiazepines, have commonly been found in the opioid user population (37) and warrant further investigation. Secondly, secular time trends in exposure and outcome may introduce confounding into case-crossover designs;(24, 25) we cannot rule out the possibility of unmeasured time-varying factors associated with drug-related poisoning and benzodiazepine exposure. Mitigating this, we made efforts to control for temporal variation and reduce heterogeneity in observation time per person, using calendar-time and time-from event as a covariate and restricting participants to two-year periods of observation bracketing the index event. In addition to an active comparator analysis, we used bidirectional sampling (before and after index poisoning), which has been found to reduce overlap bias resulting from control period selection as a function of event times.(25)
In addition, our study is limited by its focus on non-fatal drug-related poisonings as opposed to poisoning deaths. While our reliance on non-fatal poisoning allows for the creation of a more robust clinical risk profile that can be used to prevent fatal cases, future research evaluating the effect of benzodiazepine use on overdose deaths is warranted in light of our findings. Furthermore, although the MarketScan data boast a large sample size, nationally representative sample, and strong longitudinal follow-up at the patient level, it is limited by measurement error such that medication coverage does not always reflect the actual dose consumed. The MarketScan data’s generalizability is also limited to insured patients with observed drug-related poisonings; our findings may not be generalizable to lower-risk patients who did not experience poisoning events within the study observation period.
A key strength of our study was its use of a case-crossover approach to harness within-person variation (i.e., individuals serving as their own controls) and estimate the degree to which poisonings were reduced on days when participants were taking benzodiazepines, as opposed to non-treatment days. Because each participant acted as his or her own control, we reduced selection and sampling bias resulting from recruitment of different cases and controls in conventional observational study design. This also allows us to study the relationship between common exposures and less common acute outcomes such as overdoses.(18, 38, 39)This strategy also enables us to examine subtle changes in exposure (i.e., variation by benzodiazepine dose, subtype) that may result in poisoning.(18) We furthermore used SSRIs as an active comparator to control for unobserved confounders and serve as a proxy for underlying conditions leading to benzodiazepine prescriptions, given their overlapping indications with benzodiazepines and no known association with drug-related poisoning or mortality in adults. The lack of association between SSRI prescriptions and poisoning is notable, suggesting that increased odds of poisoning following receipt of benzodiazepine is more attributable to benzodiazepines as opposed to underlying conditions leading to a benzodiazepine or SSRI prescription. Finally, our findings were pharmacologically-informed, using the high statistical power of the MarketScan data to identify subgroups of patients receiving buprenorphine who may be at elevated risk of treatment failure, such as those taking short-acting benzodiazepines and higher-potency agents.
Conclusion
Opioid use disorder patients prescribed buprenorphine have double the odds of a drug-related poisoning when co-prescribed benzodiazepines, with short-acting and high-dose benzodiazepines conferring additional risk. Buprenorphine remains protective against overdose in this high-risk population. Our findings contribute to emerging evidence that OUD patients receiving buprenorphine may benefit from cessation or dose-reduction of benzodiazepine use while remaining on buprenorphine.
Supplementary Material
Figure 1 - Flowchart for development of analytic sample
Figure 2- Overview of the study’s case cross-over design
eMethods 1: Data Extraction from the MarketScan databases
eMethods 2: Characteristics of Individuals With and Without Drug-related Poisonings
eMethods 3: Assessment of Time-Varying Factors and Procedure for Time-Varying, Multiple Window Sensitivity Analysis
eTable 1: Overview of Diagnosis Codes for Opioid Use Disorder Diagnosis, Drug-Related Poisonings, Treatment Services, and Medications
eTable 2: Conversion Factors for the Standardization of Benzodiazepine Strength
eTable 3: Individual Level Comparison of Opioid Use Disorder Treatment Characteristics, in the 1 year Before and After Index Drug-Related Poisoning, between Participants with and without History of Drug-Related Poisoning
eTable 4: Person-Days Level Comparison of Opioid Use Disorder Treatment Characteristics Between Participants With and Without History of Drug-Related Poisoning
eTable 5: Proportion of Days Covered for Benzodiazepine or Z Drugs and Buprenorphine in Comparison to Other Selected Long-Term Medications
eFigure 1: Time varying, multiple-window sensitivity analysis for interaction between benzodiazepine and buprenorphine on drug-related poisonings (Model 10, Table 3)
Table 1.
Opioid Use Disorder Treatment Characteristics at the Individual Participant Level a
| Individual level N=23,036 Participants | ||
|---|---|---|
| Individuals | (%) | |
| Buprenorphine Use | 16,451 | 71.41 |
| Low Dose, ≤12 mg daily | 9,469 | 41.11 |
| High Dose, > 12 mg daily | 11,690 | 50.75 |
| Use of Benzodiazepines or Z drugs | 12,890 | 55.96 |
| Use of Benzodiazepines excluding Z drugs | 11,839 | 51.39 |
| Low Dose (≤ 30 diazepam equivalent mg daily) | 10,356 | 44.96 |
| High Dose (> 30 diazepam equivalent mg daily) | 5,227 | 22.69 |
| Use of Short Acting Benzodiazepine | 9,292 | 40.34 |
| Alprazolam | 6,210 | 26.96 |
| Lorazepam | 4,433 | 19.24 |
| Oxazepam | 130 | 0.56 |
| Triazolam | 248 | 1.08 |
| Estazolam | 19 | 0.08 |
| Temazepam | 1,127 | 4.89 |
| Midazolam | 47 | 0.2 |
| Use of Long Acting Benzodiazepine | 6,660 | 28.91 |
| Clonazepam | 3,885 | 16.86 |
| Diazepam | 3,612 | 15.68 |
| Chlordiazepoxide | 206 | 0.89 |
| Clobazam | 1 | 0 |
| Flurazepam | 33 | 0.14 |
| Quazepam | 2 | 0.01 |
| Use of Z Drugs | 5,068 | 22 |
| Zolpidem | 4,640 | 20.14 |
| Eszopiclone | 1,025 | 4.45 |
| Zaleplon | 216 | 0.94 |
| Methadone Use | 420 | 1.82 |
| Naltrexone XR Use | 746 | 3.24 |
| Naltrexone Use | 1,449 | 6.29 |
| Selective Serotonin Reuptake Inhibitor (SSRI) Use | 10,286 | 44.65 |
| Mean Age (sd) | 30.05 | 12.15 |
| Mean Year of Birth | 1980 | |
| Sex (Male) | 11,713 | 50.85 |
| Mean Days of Observation (sd) | 298.73 | 107.88 |
| Relationship of Patient to Primary Beneficiary | ||
| Employee | 4,345 | 28.30 |
| Spouse | 3,746 | 24.40 |
| Child/other | 7,263 | 47.30 |
| Medicaid | 7,682 | 33.35 |
This table illustrates opioid use disorder treatment characteristics at the individual participant level among those with a history of drug-related poisoning during the study’s observation window (1 year before and after index overdose). While all participants had prescriptions for buprenorphine in the MarketScan data (a requirement for inclusion in our final analytic sample), only 71% of participants had buprenorphine claims during the 1 year before and after index overdose. The prevalence of using specific medications of interest within the study’s observation period is depicted, with stratification by dose and individual benzodiazepine types. The bottom of the table depicts demographic characteristics for participants.
Acknowledgments:
This work was supported by National Institutes of Health (NIH R25 MH112473-01, KYX; R21 AA024888-01 and UL1 TR002345, SMH; R21 DA044744, RAG and SMH; U10 AA008401, R01 DA036583 LJB; K12 DA041449 CMM; R21 AA02568901 and F32 AA027941, JTB). These funding sources had no role in the study design, implementation, or interpretation of results. We acknowledge the support of Dr. Nuri Farber and the Psychiatry Residency Research Education Program (PRREP) of Washington University.
In addition, we acknowledge John Sahrmann and the Center for Administrative Data Research (CADR) at Washington University for assistance with data acquisition, management, and storage. CADR is supported in part by the Washington University Institute of Clinical and Translational Sciences via grants UL1 TR002345 (from the National Center for Advancing Translational Sciences of the National Institutes of Health) and R24 HS19455 (from the Agency for Healthcare Research and Quality).
Abbreviations:
- OUD
Opioid use disorder
- OR
odds ratio
- CI
confidence interval
- mg
milligram
- SSRI
selective serotonin reuptake inhibitors
Footnotes
Disclosures: LJB is also listed as an inventor on US Patent8080371,’Markers for Addiction’, covering use of SNPs in determining the diagnosis, prognosis and treatment of addiction. JTB serves on board of directors and is treasurer of the non-profit MySafeRx Inc. but does not receive any financial compensation for this work. All other authors (NP, CMM, SMH, RAG) declare no financial interests. All authors do not have financial relationships with organizations that may have an interest in our submitted work.
Footnotes
Ethical approval: Not required.
Data sharing Statement: No additional data available. We intend to provide relevant code on written reasonable request
Dissemination Declaration: Dissemination to study participants and or patient organizations is not possible/applicable due to the de-identified nature of our data.
Transparency declaration: The manuscript’s guarantors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported. We affirm that no important aspects of the study have been omitted. We affirm that any discrepancies from the study as planned and registered have been explained.
Patient and Public Involvement (PPI) Statement: This research was done without direct patient involvement.
Previous Presentation: Not applicable
Additional supporting information may be found online in the following supplements.
References
- 1.Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med. 2018;169(3):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramness JG, Kornor H. Benzodiazepine prescription for patients in opioid maintenance treatment in Norway. Drug Alcohol Depend. 2007;90(2–3):203–9. [DOI] [PubMed] [Google Scholar]
- 3.Lavie E, Fatseas M, Denis C, Auriacombe M. Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug Alcohol Depend. 2009;99(1–3):338–44. [DOI] [PubMed] [Google Scholar]
- 4.Park TW, Larochelle MR, Saitz R, Wang N, Bernson D, Walley AY. Associations between prescribed benzodiazepines, overdose death and buprenorphine discontinuation among people receiving buprenorphine. Addiction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuman-Olivier Z, Hoeppner BB, Weiss RD, Borodovsky J, Shaffer HJ, Albanese MJ. Benzodiazepine use during buprenorphine treatment for opioid dependence: clinical and safety outcomes. Drug Alcohol Depend. 2013;132(3):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soyka M Treatment of Benzodiazepine Dependence. N Engl J Med. 2017;376(12):1147–57. [DOI] [PubMed] [Google Scholar]
- 7.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94(7):961–72. [PubMed] [Google Scholar]
- 8.Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58–64. [DOI] [PubMed] [Google Scholar]
- 9.Franklyn AM, Eibl JK, Gauthier G, Pellegrini D, Lightfoot NE, Marsh DC. The impact of benzodiazepine use in patients enrolled in opioid agonist therapy in Northern and rural Ontario. Harm Reduct J. 2017;14(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macleod J, Steer C, Tilling K, Cornish R, Marsden J, Millar T, et al. Prescription of benzodiazepines, z-drugs, and gabapentinoids and mortality risk in people receiving opioid agonist treatment: Observational study based on the UK Clinical Practice Research Datalink and Office for National Statistics death records. PLoS Med. 2019;16(11):e1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein ZM, Cheng DM, Quinn E, Hui D, Kim H, Gryczynski G, et al. Psychoactive medications and disengagement from office based opioid treatment (obot) with buprenorphine. Drug Alcohol Depend. 2017;170:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansone RA, Sansone LA. Buprenorphine treatment for narcotic addiction: not without risks. Innov Clin Neurosci. 2015;12(3–4):32–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Hakkinen M, Launiainen T, Vuori E, Ojanpera I. Benzodiazepines and alcohol are associated with cases of fatal buprenorphine poisoning. Eur J Clin Pharmacol. 2012;68(3):301–9. [DOI] [PubMed] [Google Scholar]
- 15.Fareed A, Eilender P, Ketchen B, Buchanan-Cummings AM, Scheinberg K, Crampton K, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345–50. [DOI] [PubMed] [Google Scholar]
- 16.Ferri M, Finlayson AJ, Wang L, Martin PR. Predictive factors for relapse in patients on buprenorphine maintenance. Am J Addict. 2014;23(1):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118–23. [DOI] [PubMed] [Google Scholar]
- 18.Tamblyn R, Abrahamowicz M, du Berger R, McLeod P, Bartlett G. A 5-year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc. 2005;53(2):233–41. [DOI] [PubMed] [Google Scholar]
- 19.Ait-Daoud N, Hamby AS, Sharma S, Blevins D. A Review of Alprazolam Use, Misuse, and Withdrawal. J Addict Med. 2018;12(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunja N The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintz CM PNJ, Sahrman JM, Borodovsky JT, Glaser PEA, Bierut LJ, Grucza RA Age disparities in six-month treatment retention for opioid use disorder. Drug and Alcohol Dependence. 2020;213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett PG, Rodgers JH, Bloch DA. A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction. 2001;96(5):683–90. [DOI] [PubMed] [Google Scholar]
- 24.Allison P, editor Fixed Effects Regression Methods in SAS. SUGI 31 Proceedings; 2006; San Francisco, CA. [Google Scholar]
- 25.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43(5):1645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison P, Christakis NA Fixed-effects methods for the analysis of nonrepeated events. Sociological Methodology. 2006;36:155–72. [Google Scholar]
- 27.Patorno E, Glynn RJ, Levin R, Lee MP, Huybrechts KF. Benzodiazepines and risk of all cause mortality in adults: cohort study. BMJ. 2017;358:j2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guide to ICD9 and ICD10 codes related to poisoning and pain. 2013. [Available from: https://www.cdc.gov/drugoverdose/pdf/pdo_guide_to_icd-9-cm_and_icd-10_codes-a.pdf.
- 29.Sheehy O, Zhao JP, Berard A. Association Between Incident Exposure to Benzodiazepines in Early Pregnancy and Risk of Spontaneous Abortion. JAMA Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashton H Ashton Manual: Benzodiazepine Equivalence Table 2007. Available from: www.benzo.org/uk.
- 31.Howard P TR, Shuster J, Mihalyo M, Wilcock A. Therapeutic Reviews: Benzodiazepines. Journal of Pain and Symptom Management. 2014;47(5):955–64. [DOI] [PubMed] [Google Scholar]
- 32.Sadock B SV, Kaplan Ruiz P. and Sadock’s Comprehensive Textbook of Psychiatry.2009. [Google Scholar]
- 33.Liebrenz M, Schneider M, Buadze A, Gehring MT, Dube A, Caflisch C. High-Dose Benzodiazepine Dependence: A Qualitative Study of Patients’ Perceptions on Initiation, Reasons for Use, and Obtainment. PLoS One. 2015;10(11):e0142057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorevski E, Bian B, Kelton CM, Martin Boone JE, Guo JJ. Utilization, spending, and price trends for benzodiazepines in the US Medicaid program: 1991–2009. Ann Pharmacother. 2012;46(4):503–12. [DOI] [PubMed] [Google Scholar]
- 35.Bushnell GA, Sturmer T, Gaynes BN, Pate V, Miller M. Simultaneous Antidepressant and Benzodiazepine New Use and Subsequent Long-term Benzodiazepine Use in Adults With Depression, United States, 2001–2014. JAMA Psychiatry. 2017;74(7):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Communication FDS. FDA urges caution about withholding opioid addiction medications from patients taking benzodiazepines or CNS depressants: careful medication management can reduce risks. In: FDA, editor. 2017. [Google Scholar]
- 37.Mateu-Gelabert P, Jessell L, Goodbody E, Kim D, Gile K, Teubl J, et al. High enhancer, downer, withdrawal helper: Multifunctional nonmedical benzodiazepine use among young adult opioid users in New York City. Int J Drug Policy. 2017;46:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu LT, Anthony JC. The use of the case-crossover design in studying illicit drug use. Subst Use Misuse. 2000;35(6–8):1035–50. [DOI] [PubMed] [Google Scholar]
- 39.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 - Flowchart for development of analytic sample
Figure 2- Overview of the study’s case cross-over design
eMethods 1: Data Extraction from the MarketScan databases
eMethods 2: Characteristics of Individuals With and Without Drug-related Poisonings
eMethods 3: Assessment of Time-Varying Factors and Procedure for Time-Varying, Multiple Window Sensitivity Analysis
eTable 1: Overview of Diagnosis Codes for Opioid Use Disorder Diagnosis, Drug-Related Poisonings, Treatment Services, and Medications
eTable 2: Conversion Factors for the Standardization of Benzodiazepine Strength
eTable 3: Individual Level Comparison of Opioid Use Disorder Treatment Characteristics, in the 1 year Before and After Index Drug-Related Poisoning, between Participants with and without History of Drug-Related Poisoning
eTable 4: Person-Days Level Comparison of Opioid Use Disorder Treatment Characteristics Between Participants With and Without History of Drug-Related Poisoning
eTable 5: Proportion of Days Covered for Benzodiazepine or Z Drugs and Buprenorphine in Comparison to Other Selected Long-Term Medications
eFigure 1: Time varying, multiple-window sensitivity analysis for interaction between benzodiazepine and buprenorphine on drug-related poisonings (Model 10, Table 3)