Abstract
Outcomes for triple negative breast cancer (TNBC) are poor and may be improved by increasing CD8+ tumor infiltrating lymphocytes (TIL) to augment anti-tumor immunity. Radiation (RT) can promote immunogenic cell death with increased anti-tumor T-cell activity but also stimulates suppressive regulatory T-cells (Tregs). Since metabolic alterations affect immune homeostasis and prior studies show caloric restriction (CR) combined with RT improves preclinical TNBC outcomes, we hypothesized that CR augments RT, in part, by altering intratumoral immunity. Using an in vivo model of TNBC, mice were treated with ad libitum (AL) diet, radiation, a CR diet, or CR+RT, and demonstrated an immune suppressive environment with a significant increase in CD4+CD25+Foxp3+ Tregs after RT but not CR-fed mice. CD8:Treg ratio in CR+RT TIL increased 4 fold compared with AL+RT mice. In vivo CD8 depletion was performed to assess the role of effector T-cells in mitigating the effects of CR and it was found that in mice undergoing CR, depletion of CD8 T-cells resulted in increased tumor progression and decreased median survival compared with isotype control treated mice. In addition, PD-1 expression on CD3+CD8+ T-cells within the tumor microenvironment was significantly increased in CR+RT versus AL+RT treated mice as per immunofluorescence. Serum from breast cancer patients undergoing RT alone or CR and RT was collected pre- and post-intervention and a cytokine array demonstrated that patients treated with CR+RT had notable decreases in immunosuppressive cytokines such as IL-2Rγ, IL-10Rβ, and TGF-β2 and 3, compared with patients receiving RT alone. In conclusion, combining CR with RT decreases intratumoral Tregs, increases CD8:Treg and increases PD-1 expression via a process dependent on CD8 T-cells in a TNBC model. Breast cancer patients undergoing CR concurrently with RT experience also had significant reduction in immunosuppressive cytokine levels compared with those receiving RT alone.
SUMMARY
Combining caloric restriction with RT alters intratumoral immunity by decreasing Tregs and increasing effector T cells/Treg ratio in a murine triple negative breast cancer model. The immune mediating effects of caloric restriction are also seen in breast cancer patients undergoing radiation and caloric restriction. These results increase our understanding of how immunity can be altered in the setting of radiation and suggest a potential use of caloric restriction in the clinical setting.
INTRODUCTION
Triple-negative breast cancer (TNBC) accounts for approximately 20% of all newly diagnosed cases of breast cancer and is more common in young patients, those of African-American ethnicity, and in individuals with BRCA1 germline mutations1,2. TNBC typically presents at a more advanced stage, is of higher histologic grade, and has a higher risk of relapse, metastasis, and disease progression compared with hormone receptor positive variants3,4. Despite standard adjuvant radiation there is an increased incidence of locoregional recurrence in these patients compared with those that have luminal subtypes.4 Furthermore, there are fewer treatment options for TNBC due to the lack estrogen receptor (ER) and progesterone receptor (PR) expression and human epidermal growth factor receptor 2 (HER2) amplification, which makes targeted therapies ineffective.
Recent attention has turned towards treatment strategies involving immune modulation since TNBC tumors have been shown to have significant lymphocyte infiltrate compared to other breast cancer subtypes. Inhibitory immune checkpoints, such as the program cell death protein-1 (PD-1) receptor expressed by CD8+ effector T-cells, are safeguards built into the immune response to prevent autoimmunity but is often exploited by cancer to evade immune mediated destruction5–7. The ligand for this receptor, PD-L1, is expressed in multiple solid tumors including lung, bladder, colorectal, melanoma, and squamous cell carcinomas of the head and neck, and has been shown to be a predictor of response to blockade of the PD-1 pathway8. In locally advanced non-small cell lung cancer for instance, PD-L1 blockade after definitive treatment with concurrent chemo-radiation therapy resulted in a significant improvement in progression free survival and overall survival9. In breast cancer, PD-L1 expression is observed in just under 2% of tumors but may be present in up to 20–30% of TNBCs.10 Indeed the phase III clinical trial, Keynote-522, which randomized patients with Stage II or III untreated TNBC to either neoadjuvant pembrolizumab, a PD-1 inhibitor, plus chemotherapy or to placebo plus chemotherapy, demonstrated a significant improvement in pathologic complete response with the addition of immunotherapy compared with chemotherapy alone11.
Despite this, improvement in overall survival and overall response rates with PD-1 checkpoint blockade remains low12. For instance, Impassion130 was a phase 3 clinical trial that randomized patients with untreated metastatic TNBC to receive either atezolizumab, a PD-L1 inhibitor, plus nab-paclitaxel, or placebo plus nab-paclitaxel. Although progression free survival was significantly improved in patients receiving atezolizumab compared with those receiving placebo, median overall survival was not significantly improved. Furthermore the objective response rate was only 56% in the atezolizumab arm compared with 45.9% in the control arm, while the complete response rate was only 7.1% in the experimental arm13. Together, these studies highlight the need for the development of complementary therapies.
In a pre-clinical animal model caloric restriction (CR), defined as 10–40% reduction in daily caloric intake, delays tumor growth, initiation of metastatic disease, and the growth of metastatic lesions when compared with animals fed an ad libitum (AL) diet14. Recently studies showed that mice challenged with the syngeneic 4T1 triple negative breast cancer cell line and treated with radiation experienced a significant improvement in overall survival (OS) and decreased metastatic disease burden when kept on CR rather than an AL diet15,16. Similar observations were noted on combining chemotherapy consisting of either cisplatin or docetaxel with CR compared with AL-fed animals17. Lee et al., support these findings in the setting of short-term starvation and went on to demonstrate that this is in part mediated by increased oxidative damage, apoptosis, and DNA damage. These findings suggest a role for CR in the adjuvant setting in combination with RT and/or chemotherapy.
Investigations into the effects of metabolism on the immune response have revealed that the metabolic environment can dramatically impact immune phenotype. For instance, fatty acid oxidation is utilized primarily by immune suppressive regulatory T-cells (Tregs) while fatty acid synthesis and glycolysis is the primary means of energy production for effector T-cells18. In a pre-clinical lung cancer model, short term starvation indeed resulted in an increase in the CD8/Treg ratio within the tumor microenvironment resulting in delays in tumor growth19. We therefore sought to evaluate the effects of CR in combination with radiation therapy on the immune response in a pre-clinical murine TNBC model.
METHODS
Mice, tumors, diet, and radiation
Female 12-week old BALB/c mice were obtained from Charles River Laboratories under an Institutional Animal Care and Use Committee approved protocol 01859. At week 13, 40 mice underwent orthotopic injection with 50,000 4T1 triple negative murine breast cancer cells (ATCC CRL-2539) into the #4 mammary fat pad. Once tumors reached a palpable size, approximately 5 x 5 mm, the animals were randomized into 1 of 4 groups (Figure 1A): ad libitum (AL) fed diet, caloric restriction (CR), AL plus radiation therapy (RT), or CR+RT. AL mice had unrestricted access to normal chow (LabDiet 5010). CR mice were singly housed one week prior to tumor implantation and food consumption was monitored daily. Mean food consumption per mouse was calculated and used to reduce available food mass by 10% beginning on the day 7 after tumor implantation. Food mass was further reduced by 20% on day 10 post tumor implantation, and by 30% on day 13 post tumor implantation. CR mice were maintained at 30% caloric intake for the duration of the experiment. Mice receiving RT were treated to a total dose of 12 Gy delivered in 3 daily fractions of 4 Gy delivered to the primary tumor with shielding of the remainder of the body (Pantak H-320 (320 kV) Precision X-Ray, N. Bradford, CT) using a custom jig.
Figure 1. After RT, CR reduces intratumoral Tregs and increases effector T cells.
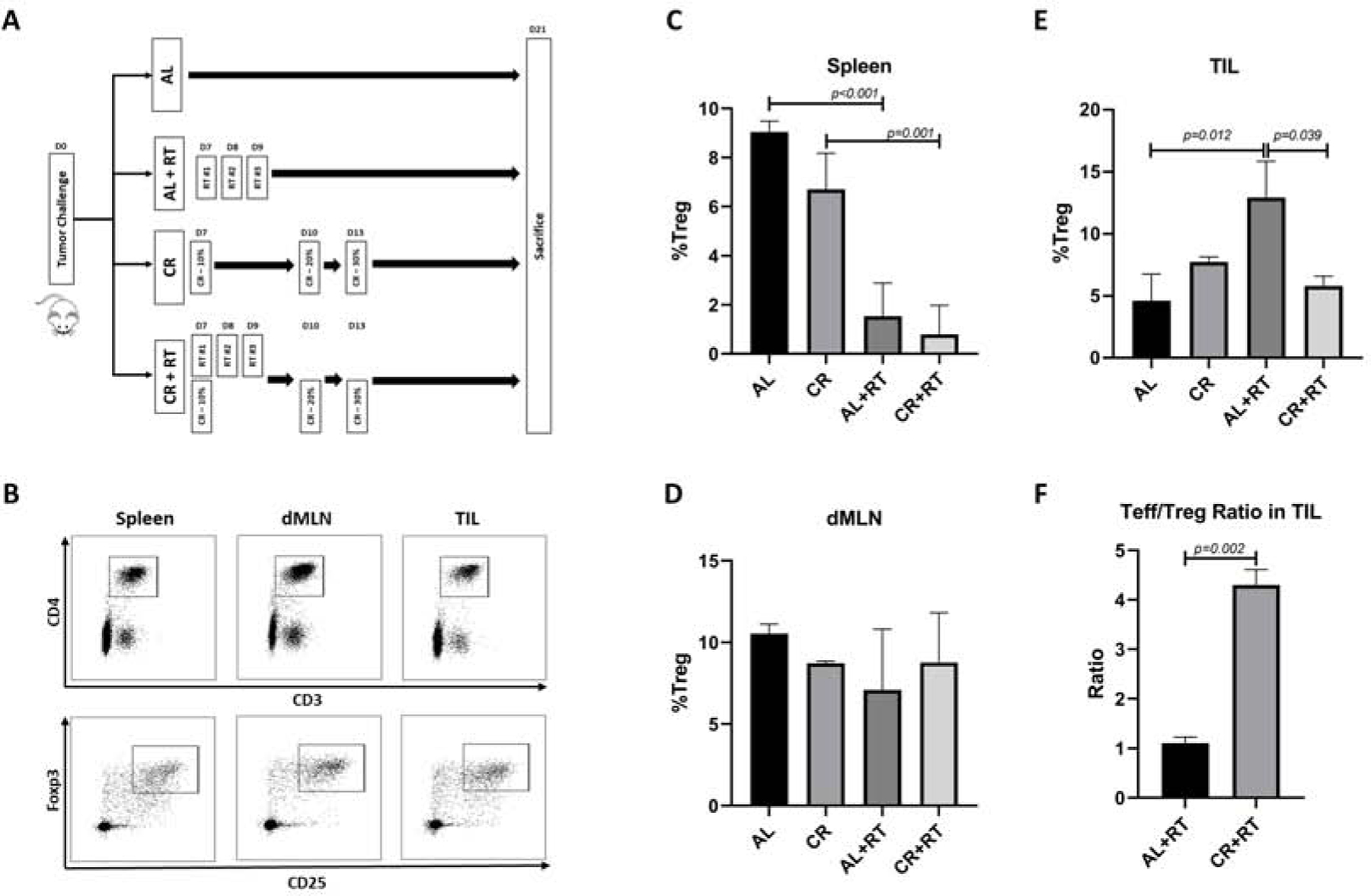
A) Gating strategy for identifying regulatory T cells defined as CD3+CD4+CD25+Foxp3+. B,C,D. Changes in the Treg population in spleen, dMLN, and TIL is animals treated with either AL, CR, AL+RT, or CR+RT. E) Change in the CD8:Treg ratio within TIL in animals treated with either AL+RT or CR+RT.
Animal monitoring and tissue extraction
After tumor challenge, mice were monitored with weights (Supplemental Figure 1) and tumor measurements three times per week. Tumor length (L) and width (W) was measured using calipers, and tumor volume was calculated using the formula L x W x W x π/6. Depending on the experimental endpoint, mice were euthanized at either day 21 or once tumors had reached a calculated volume of 2500 mm3. Animals were euthanized using CO2 asphyxiation as per protocol. Whole blood was obtained using cardiac puncture immediately after death. Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Paque PLUS density gradient medium (GE Healthcare Life Sciences) according to the manufacturer’s protocol. Tumors and spleens were surgically excised. Half of the tumor was stored in optimal cutting temperature (OCT) compound for immunofluorescence (IF) microscopy. Spleens and remaining tumor were ground against 70 µm filters in order to generate a single cell suspension. Splenocytes were treated with Ammonium-Chloride-Potassium Lysing Buffer (Thermofisher) to lyse red blood cells according to the manufacturer’s protocol. Draining mesenteric lymph nodes (dMLN) were isolated as previously described20. PBMCs, dMLN, splenocytes, and tumor cells were all filtered through 70 µm filters prior to use in additional downstream assays.
CD8+ T cell depletion
Forty mice were injected with 20 µg of either anti-CD8 (Clone 53–6.7, eBioscience) or isotype control antibodies (Clone eBR2a, eBioscience) via intraperitoneal injection on days 6, 5, and 4 prior to tumor challenge. Ten mice treated with anti-CD8 and ten with isotype control were placed on a caloric restriction diet as described above, while the rest were maintained on an AL diet. Depletion was maintained after tumor challenge for the duration of the experiment with administration of anti-CD8 antibody every 3 days. CD8 T-cell depletion was confirmed via flow cytometry. Splenocytes were stained with CD3 e450 (Clone 17A2, eBioscience), CD4 FITC (Clone RM4–5, eBioscience), and CD8 APC-Cy7 (Clone 53–6.7, Life Technologies). Samples were sorted on a BD Biosciences LSR II flow cytometer and analyzed using FlowJo v10.6.2.
Intracellular cytokine staining
PBMC were collected from mice fed either a CR or AL diet and treated either with or without RT, as previously described. Cells were restimulated in vitro for 5 hours with eBioscience cell stimulation cocktail (phorbol 12-myristate 13-acetate and ionomycin, eBioscience # 00-4970-93) in the presence of Brefeldin A (eBioscience, #B7450). Cells were collected and spun down in V-bottom 96 well plates (Corning) and ICS was performed according to the manufacturer’s protocol using the eBioscience intracellular fixation and permeabilization buffer set (eBioscience, 88-8824). Cells were stained with conjugated antibodies for CD3 e450 (Clone 17A2, eBioscience) and interferon (IFN)-y PE-Cy7 (Clone XMG1.2, eBioscience). Samples were sorted on a BD Biosciences LSR II flow cytometer and analyzed using FlowJo v10.6.2.
Flow cytometry
Single cell suspensions were spun down in V-bottom 96 well plates (Corning) and Foxp3 staining performed according to manufacturer protocol using the eBioscience Foxp3/Transcription factor staining (#00-5523-00). Cells were stained with CD3 e450 (Clone 17A2, eBioscience), CD4 FITC (Clone RM4–5, eBioscience), CD8α APC-Cy7 (Clone 53–6.7, Life Technologies), FoxP3 PE(Clone FJK-16s, Ebiosciences), CD25 PE-Cy5.5(Clone PC61.5, eBiosciences), PD-1 APC (Clone RMP1–30, Biolegend), and Fix Aqua (Thermofisher). Samples were sorted on a BD Biosciences LSR II flow cytometer and analyzed using FlowJo v10.6.2.
Immunofluorescence microscopy
Tumor sections were cut into 10µm sections using a CryoStat (Leica CM1850). Sections were fixed in acetone at −20C for 10 minutes then rehydrated with Phosphate Buffered Saline (PBS) for 5 minutes at room temperature. Sections were blocked with blocking buffer (PBS, 10% heat inactivated fetal bovine serum (FBS), and 0.1% Tween 20)) for 30 minutes. All sections were stained with antibodies for CD3 (Clone 17A2, Biolegend #100203), CD8 (Clone 53–6.7, Biolegend #100707), PD-1 (Clone RMP1–30, Biolegend #109111) and with 4’,6-diamidino-2-phenylindole (DAPI) in blocking buffer for 2 hours at room temperature. Samples were imaged using a Nikon A1R fluorescent confocal microscope. Settings for each series of images was determined using single color splenic controls and maintained throughout imaging. Composites of confocal images (4–6 planes per section) were made using maximum intensity projection in ImageJ and PD-1+ CTLs were counted if they stained positive for CD3, CD8, and PD-1. Six images were taken per tumor from three tumors per treatment condition across two independent experiments. All images were taken at 20x magnification (635.4µm by 635.4µm).
Human Samples
Human serum samples were obtained on an IRB approved protocol at National Cancer Institute (02-C-0064) and Thomas Jefferson University (12G.616) according to the protocol by Khoury et al.21. After signing consent, 36 patients were accrued from 2013–2016 and enrolled on a study evaluating the compliance of Stage 0 and I breast cancer patients to undergo a caloric restriction diet (25% reduction of their baseline diet) during 60 Gy dose of radiation. The patients had venipuncture to collect whole blood at baseline before starting the diet. They then proceeded with 2 weeks lead in of caloric restriction followed by the combination of diet during radiation which consisted of 50 Gy to the whole breast with a 10 Gy boost followed by an additional 2 weeks of caloric restriction. Whole blood was then collected on the last day of caloric restriction. In addition, control samples was obtained at National Institute of Health (NIH) on a serum collection protocol and consisted of 11 consecutive early stage breast cancer patients for which whole blood collected on the initial day of radiation just prior to their first dose and after a dose of 50–60 Gy of radiation to the breast and/or regional lymph nodes upon completion of radiation. The treatment serum was collected at Thomas Jefferson University using the same protocol as NCI (02-C-0064): whole blood was spun at 2500 rpm for 10 minutes and serum was collected and aliquoted. Cytokine levels were measured using quantitative multiplexed sandwich ELISA arrays using a cytokine-antibody-biotin complex which was visualized using a streptavidin-conjugated Cy3 dye.
Statistics
GraphPad Prism v8.4.2 was used to perform the following statistical analysis of the data: ordinary one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test (Figure 1B–E; Figure 3C); unpaired Student T-test (Figure 1F; Figure 2E); mixed effects analysis of group progression (Figure 2C); log-ranked (Mantel-Cox) test (Figure 2D); ordinary one-way ANOVA with Holm-Sidak’s multiple comparisons test (Figure 3B); and unpaired, nonparametric Mann-Whitney test (Figure 4B). Significance set to a p-value less than 0.05.
Figure 3. PD-1 expression increases after CR+RT on CD8+ tumor infiltrating lymphocytes.
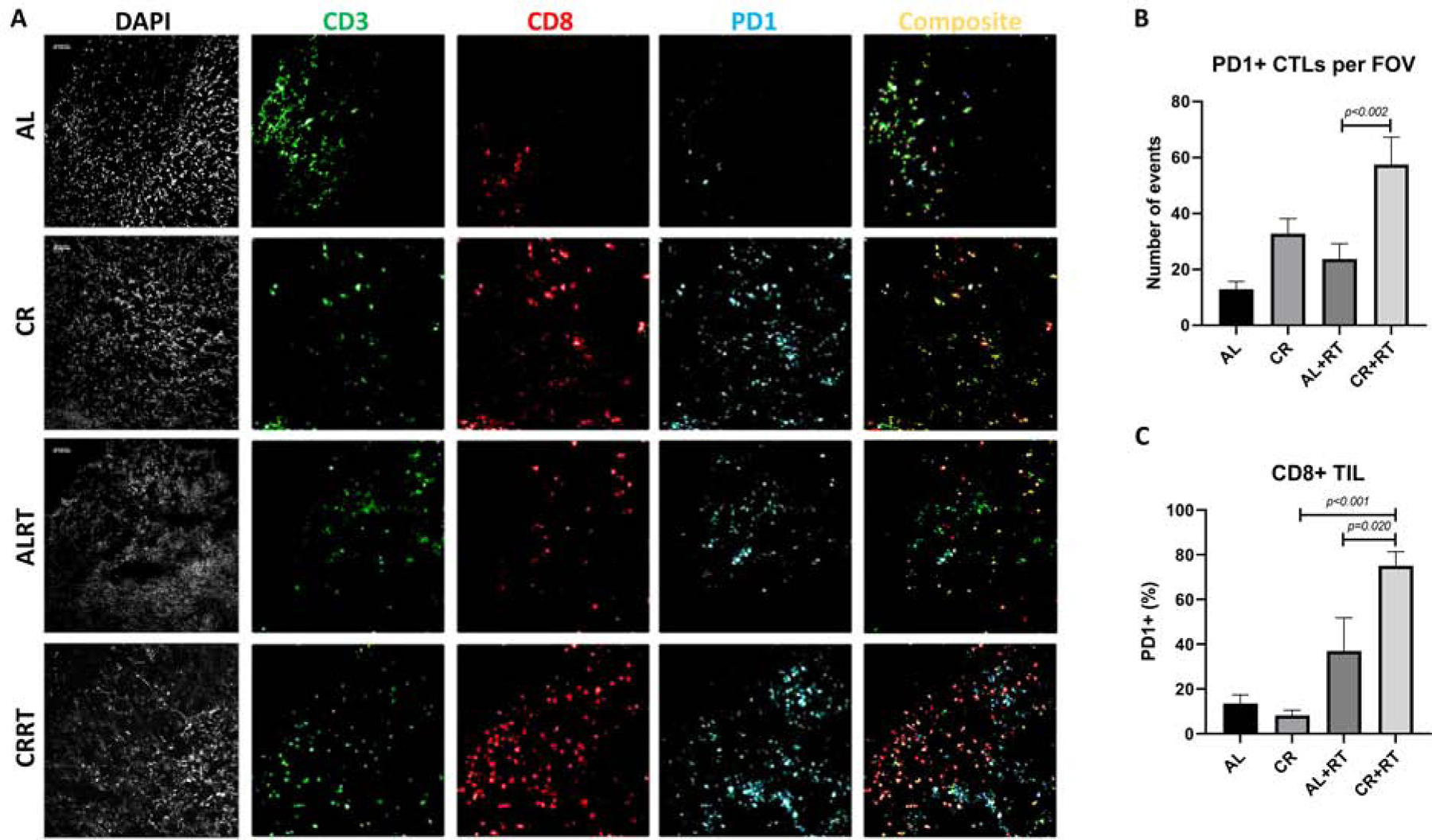
A) Representative images of staining from each tumor condition. B) Microscopic counting of CD3+CD8+PD-1+ triple stained cells from each tumor condition. C) Flow Cytometric analysis of CD3+CD8+PD-1+ cells of tumor infiltrating lymphocytes
Figure 2. CD8 Tcell Depletion.
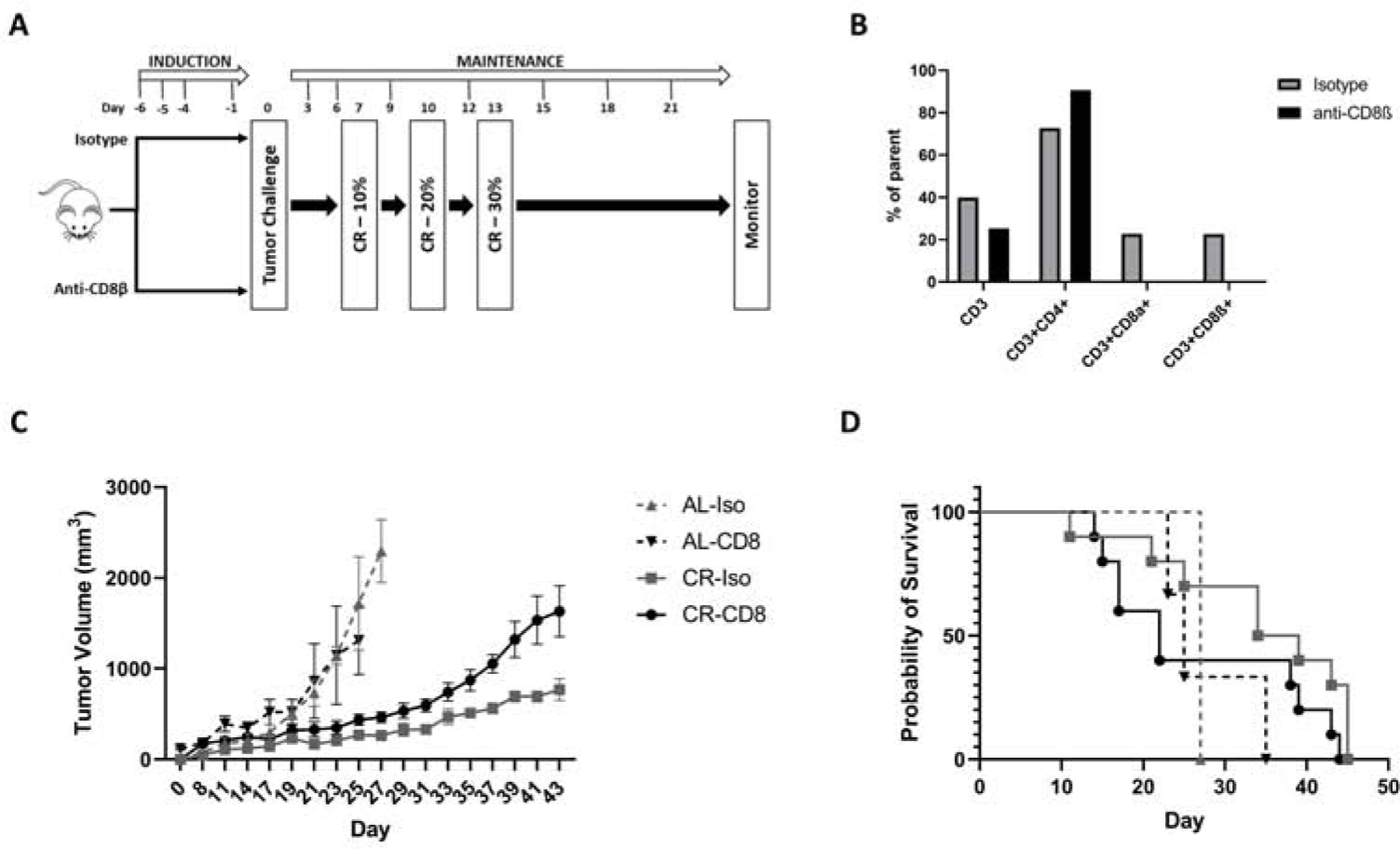
A) Experimental design. B) Validation of CD8 depletion. C) Changes in tumor growth over time in animals fed either an AL or a CR diet and treated with either isotype control or CD8 depleting antibody. D) Kaplan Meir survival curve of animals fed either an AL or a CR diet and treated with either CD8 depleting antibody or isotype control. Median survival of AL-Iso, AL-CD8, CR-Iso, and CR-CD8 was 27, 25, 36.5, and 22 days, respectively.
Figure 4. Breast cancer patients undergoing RT while on CR exhibit decreased levels of anti-inflammatory serum cytokine levels.
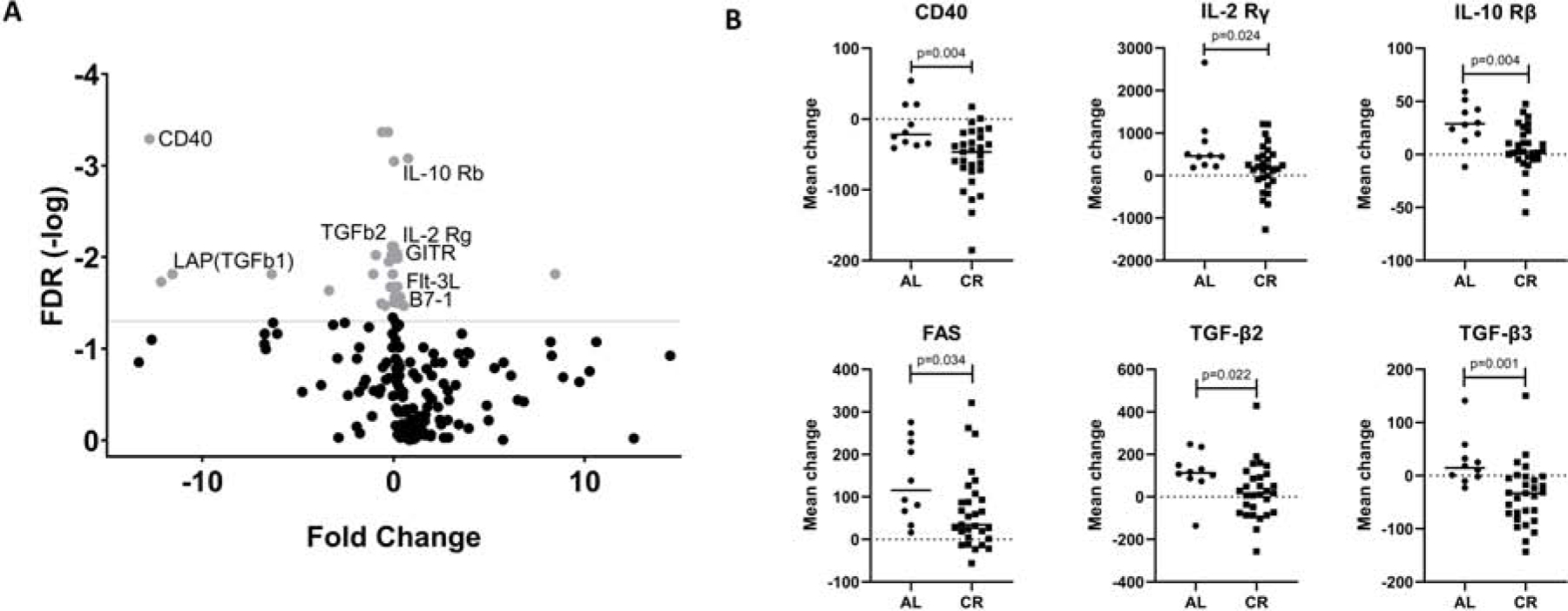
A) Volcano plot demonstrating fold change in cytokine levels vs false discovery rate (FDR). Gray dots represent cytokines whose change corresponds to a FDR with p<0.01. B) Mean change in serum cytokine levels before and after radiation in patients on either an AL or CR diet.
RESULTS
CR reduces intratumoral Tregs and increases effector T cells after RT.
Animals from each group, were sacrificed on day 21 at which time spleen, dMLN and tumors were harvested for downstream assays. The change in the Treg population, defined as CD3+CD4+ CD25+Foxp3+ lymphocytes, in these tissue compartments was assessed using flow cytometry (Figure 1B). In spleen, the number of Tregs was not significantly different between dietary conditions (9.05% in AL vs 6.7% in CR, p=0.15) but was significantly decreased after RT in the AL (9.05% in AL alone vs 1.54% in AL+RT, p<0.001) and CR (6.7% in CR alone vs 0.79% in CR-RT, p<0.001) fed animals (Figure 1C). There was no significant difference in Treg values across any conditions in dMLN (Figure 1D). The percent of Tregs within tumor infiltrating lymphocytes (TIL) was significantly increased in AL-fed animals after radiation (4.62% in AL alone vs 12.9% in AL+RT, p=0.012). This was not the case in CR-fed mice in which Tregs remained at baseline levels after RT (7.74% in CR alone vs 5.8% in CR+RT, p=0.8). Compared with AL+RT, CR+RT resulted in a significant decrease within TILs (12.93% in AL+RT vs 5.8% in CR+RT, p=0.04)(Figure 1E). The presence of intratumoral CD8+ T-cells predicts improved survival and response to neoadjuvant chemotherapy and the ratio of effector T-cells to Tregs have previously been shown to be predictive of treatment outcomes in breast cancer patients22–25. Compared with AL+RT, the ratio of effector T-cells to Tregs in TIL was increased roughly 4 fold in animals maintained on a CR diet (1.11 in AL+RT vs 4.3 in CR+RT, p<0.002)(Figure 1F).
Depletion of CD8+ T cells abrogates the effects of CR.
CD8+ effector T cells have tumoricidal activity and their presence within the tumor microenvironment has been is associated with better prognosis in several cancers22,26–29. To determine that the anti-tumor effects of CR are mediated by CD8+ T cells, Balb/c mice were treated with either an anti-CD8β depleting antibody or isotype control for 3 consecutive days (Figure 2A). To assess efficacy of CD8 T cell depletion, an animal from each group was sacrificed on day 5 and splenocytes were harvested using flow cytometry. Over 95% of CD8 T-cells were successfully depleted using this strategy (Supplemental Figure 2). Depletion was confirmed with antibodies against both the alpha and beta chains of CD8. CD3 and CD3+CD4+ lymphocytes were unaffected by CD8 depletion (Figure 2B).
Mice were challenged with 4T1 tumor cells and CR was started once tumors became palpable on day 7 as previously described. CD8 depletion was sustained with repeated antibody injections every 3 days. Tumor growth was significantly delayed in mice kept on a CR diet and treated with isotype control compared with CD8 depleted mice (Figure 2C) at all timepoints. Tumor growth in CR-fed animals was delayed compared with AL-fed animals regardless of CD8 depletion. The median survival of CD8 depleted CR mice was 22 days compared with 36.5, 27, and 25 days for CR-isotype, AL-isotype, and AL-CD8 depleted controls, respectively (Figure 2D).
PD-1 expression by CD8+ tumor infiltrating lymphocytes is increased after CR+RT.
PD-1 expression by CD8 T-cells occurs after activation and is often exploited by tumors as a way to evade T-cell mediated killing. Li et al have previously demonstrated that PD-1 expression significantly increases after RT30. We therefore wanted to determine whether PD-1 expression is impacted by CR. To assess this, we performed flow cytometry on TIL collected from animals treated with either AL, AL+RT, CR, or CR+RT and found that PD-1 expression on CD3+CD8+ T cells was significantly increased in CR+RT (75.05%) versus AL+RT (36.98%) treated mice (p=0.02)(Figure 3C). We confirmed these findings with immunofluorescence microscopy and indeed found that the mean number of PD-1+ CD3+CD8+ lymphocytes per field of view was significantly higher in tumors of CR+RT mice (57.53 events) compared with AL+RT mice (23.78 events), p<0.002 (Figure 3A, 3B).
Breast cancer patients undergoing RT while on CR exhibit decreased levels of anti-inflammatory serum cytokine levels.
In order to determine whether CR also impacts immunity in humans, the delta of serum cytokine changes before and after treatment was assessed for early-stage breast cancer patients treated with caloric restriction and radiation and compared to those who only underwent radiation with patient characteristics as per Table 1.
Table 1.
Patient Characteristics
| Patient Characteristics | Control patients: Radiation only n=10 | Radiation and Caloric Restriction n=28 |
|---|---|---|
| Mean Age at Radiation Initiation (range) | 50 (34–65) | 56.3 (44–69) |
| Race | ||
| - African American | 2 (20%) | 14 (50%) |
| - Caucasian | 7 (70%) | 12 (43%) |
| - Other | 1 (10%) | 2 (7%) |
| Tumor Stage | ||
| - T1 | 6 (60%) | 24 (85%) |
| - ≥T2 | 4 (40%) | 4 (15) |
| Estrogen positivity | 6 (60%) | 24 (85%) |
| Radiation dose median (range) | 60.2 Gy (50–60.4Gy) | 60 Gy (50–60Gy) |
| Weight Change during radiation therapy | +2.33 lbs | −9.2lbs |
Analysis of these samples revealed significant changes in cytokines and related markers after RT and CR compared with changes after RT alone as depicted by the volcano plot (Figure 4A). Individual analysis of select cytokines revealed significant decreases in interleukin (IL)-2 receptor gamma (p=0.024), IL-10 receptor β(p=0.004), transforming growth factor (TGF)-β2 (p=0.022) and TGF-β3 (p=0.001) (Figure 4B). Taken together these findings suggest CR results in a migration away from an immunosuppressive phenotype on a systemic level after RT in humans.
DISCUSSION
Caloric restriction is a well-established method of delaying tumor growth and augmenting the efficacy of RT and chemotherapy in a TNBC mouse model, but it’s effects on the immune response is ill defined15–17. Preclinical studies with the CR mimetic, hydroxycitrate, suggest that one possible mechanism of immune modulation may be through the depletion of Tregs31. We therefor set out to determine whether the increased efficacy of RT in CR-fed mice was mediated by changes in Tregs. Indeed, we found that intratumoral Tregs are suppressed after RT in animals maintained on a CR diet. This was in stark contrast to animals fed an AL diet, after which Tregs are significantly increased within tumors after RT (Figure 1E). It is worth noting that an increase in intratumoral Tregs after RT is consistent with previously published findings and is hypothesized to be a way to curtail RT induced inflammation32,33.
An increase in the CD8 T-cell to Treg ratio is currently believed to be one of the most critical factors in predicting treatment outcomes34. We found that the decrease in Tregs coincides with a significant increase in the number of effector T-cells within the tumor microenvironment in CR+RT mice compared to AL+RT mice (Figure 1F). The importance of CD8 T cells in CR is highlighted by an increase in tumor growth (Figure 2C) and a decrease in median survival (Figure 2D) after CD8 depletion compared with our isotype treated and AL-fed controls. Interestingly, tumor growth in CR-CD8 mice was not on par with the growth observed in AL-fed mice suggesting that other mechanisms may be contributing to the delays in tumor growth seen with CR. Taken together, these findings show that CR alters intratumoral immunity, by reducing Tregs and enhancing the CD8/Treg ratio, and that CD8 T-cells are, at least in part, responsible for mediating the anti-tumor effects observed with CR in a murine TNBC model.
Increasing anti-tumor immunity via metabolic alterations can also improve functional activation of T-cells. Since PD1 is upregulated by T-cells after recognition of their cognate antigen, we sought to assess the impact of CR on T-cell activation. Using immunofluorescent microscopy on mouse tumors we found that after RT, CR animals had a significantly higher number of PD1 expressing CD3+CD8+ T effector cells within the tumor compared with AL+RT mice (Figure 3A, 3B). Similarly, the number of CD8+PD1+ TILs increased significantly after CR+RT compared to either AL, CR, or AL+RT groups as ascertained by flow cytometry (Figure 3C). Restimulation of PBMCs with phorbol myristate acetate and ionomycin as part of an intracellular cytokine staining assay also revealed increased interferon-у production by effector T cells collected from CR-fed mice compared with those from AL-fed mice (Supplemental Figure 3). These findings suggest that CR combined with RT increases effector T-cell activation and activity within the tumor microenvironment. PD1 binding however can result in decreased cytotoxic activity and T cell exhaustion34. Recently, Ajona et al demonstrated that intermittent short-term starvation sensitizes tumors to PD-1 blockade in a preclinical non-small cell lung cancer model35. Our discovery that CR combined with RT increases the number of PD1+ CD8 T cells within the tumor lends support to the use of anti-PD1 directed therapy in our model to further improve treatment response.
Although the positive effects of CR have been known for years, its use in humans in the formal clinical trial setting is limited. Our group recently completed the first clinical trial assessing the safety and feasibility of a 25% reduction in daily caloric intake in patients with early stage breast cancer undergoing breast conservation therapy. Analysis of serum cytokine levels taken from patients before and after CR+RT revealed significant changes in numerous cytokines consistent with an alteration in systemic immunity. When these changes were compared to changes in serum cytokine levels in patients undergoing only RT, we found a significant change in the secretion of nearly 39 cytokines. Specifically, we saw significant decreases in immune-suppressive markers such as IL-2 receptor gamma, IL-10 receptor beta, and TGF-beta 2 and 3. Since each patient served as their own internal control, changes seen after radiation compared to before radiation should be an effect of treatment with or without caloric restriction, however since serum was evaluated two weeks after radiation, it is possible that reduction of immune suppressive cytokines might be an indirect effect. In addition, this trial consisted of a heterogeneous patient population with varying hormone receptor subtypes and tumor stages, the fact that our preclinical findings in a murine TNBC model are echoed in these patients suggests that they may be generalizable to a wider population of breast cancer patients. Unfortunately, the sample size of this study is too small to address specific tumor characteristics in this pilot study but it warrants further investigation in a future clinical trial.
In determining clinical trial design in the future and envisioning a trial including caloric restriction used in combination with immune modulators such as PD-1 inhibitors, it is necessary to ensure translation is safe without increased normal tissue toxicity caused by the dietary intervention. Although there are no other currently published clinical trials using caloric restriction in combination with radiation we expect decreased toxicity due to a differential effect noted with increased tumor kill but protection of normal tissues, notably without fatigue or wound healing issues36. To date, several preclinical studies using caloric restriction have shown to increase efficacy of radiation and chemotherapy while having a protective effect on normal tissues such as decreasing genetic mutations induced by radiation and preventing cardiotoxicity in doxorubicin-treated rats37–39. There is currently an accruing clinical trial called the CREATE study that is randomizing caloric restriction and exercise will determine if these lifestyle interventions will protect against cardiac toxicity from anthracyclines which should add to the body of literature40.
A limitation of our study was the use of a single animal model. This model was chosen because it is a well-delineated syngeneic model of breast cancer that metastasizes from local disease in a manner similar to breast cancer in humans. Few syngeneic mouse models exist, and these experiments require a mouse model with an intact immune system. Since differences in all three treatment groups were noted to be significantly altered compared with the control group, the interventions themselves are responsible for the changes noted and not any characteristic held constant in these experiments. The other two syngeneic breast cancer models available, E0771 and EMT6 have characteristics that make them unsuitable for these experiments. They are poorly metastatic or fail to generate metastases, and the EMT6 model (balb/c) has minimal CD4+ and CD8+ T cell infiltrates with suggestion of an immunosuppressive microenvironment.41–42 Future studies should investigate other models as they become available.
In summary, we show for the first time that combining CR with RT alters intratumoral immunity by decreasing Tregs and increasing effector T cells/Treg ratio in a murine TNBC model. Additionally, we demonstrated that the immunogenic effects of CR are, at least in part mediated by CD8+ T cells and that number of PD1+ CD8+ effector T-cells increases significantly within the tumor microenvironment after CR+RT. The immune mediating effects of CR are also seen in breast cancer patients undergoing radiation and CR. Future studies should include investigation into the underlying mechanism of CR on T cell immunity, modulation of other immune cell types such as Th17, Th1, Th2, and macrophages, and its role in augmenting treatment outcomes in the clinical setting, possibly in combination with PD-1 inhibitors.
Supplementary Material
Acknowledgements
This work was supported by R01CA227479 (NLS), The Bloomberg~Kimmel Institute for Cancer Immunotherapy (JZ), Prostate Cancer Foundation Young Investigator award (JZ), National Institutes for Health/National Cancer Institute K22 CA237623 award (JZ), and the NCI Cancer Center Grant P30CA056036 for supporting the Flow Cytometry, Histology, and Lab Animals core facilities.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Data Sharing: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer Vol 363.; 2010. www.nejm.org. Accessed April 13, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63(1):11–30. doi: 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 3.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California Cancer Registry. Cancer 2007;109(9):1721–1728. doi: 10.1002/cncr.22618 [DOI] [PubMed] [Google Scholar]
- 4.Moran MS. Radiation therapy in the locoregional treatment of triple-negative breast cancer. Lancet Oncol 2015;16(3):e113–e122. doi: 10.1016/S1470-2045(14)71104-0 [DOI] [PubMed] [Google Scholar]
- 5.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol 2018;8. doi: 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manukian G, Bar-Ad V, Lu B, Argiris A, Johnson JM. Combining radiation and immune checkpoint blockade in the treatment of head and neck squamous cell carcinoma. Front Oncol 2019;9(MAR):122. doi: 10.3389/fonc.2019.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Chen W, Xu ZP, Gu W. PD-L1 distribution and perspective for cancer immunotherapy—blockade, knockdown, or inhibition. Front Immunol 2019;10(AUG). doi: 10.3389/fimmu.2019.02022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 10.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 12.Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib keynote-012 study. J Clin Oncol 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 14.De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzúa P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis 2011;32(9):1381–1387. doi: 10.1093/carcin/bgr107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleh AD, Simone BA, Palazzo J, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 2013;12(12):1955–1963. doi: 10.4161/cc.25016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simone BA, Dan T, Palagani A, et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle 2016;15(17):2265–2274. doi: 10.1080/15384101.2016.1160982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simone BA, Palagani A, Strickland K, et al. Caloric restriction counteracts chemotherapy-induced inflammation and increases response to therapy in a triple negative breast cancer model. Cell Cycle 2018;17(13):1536–1544. doi: 10.1080/15384101.2018.1471314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol 2016;16(9):553–565. doi: 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajona D, Ortiz-Espinosa S, Lozano T, et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat Cancer 2020;1:75–85. doi: 10.1038/s43018-019-0007-9 [DOI] [PubMed] [Google Scholar]
- 20.Buettner M, Bode U. Lymph node dissection - understanding the immunological function of lymph nodes. Clin Exp Immunol 2012;169(3):205–212. doi: 10.1111/j.1365-2249.2012.04602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury S, Ajuyah P, Tran N. Isolation of small noncoding RNAS from human serum. J Vis Exp 2014;88(88):51443. doi: 10.3791/51443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud SMA, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 23.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Gruosso T, Zuo D, et al. Infiltration of CD8 + T cells into tumor cell clusters in triple-negative breast cancer. Proc Natl Acad Sci U S A 2019;116(9):3678–3687. doi: 10.1073/pnas.1817652116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng GL, Li L, Guo YW, et al. CD8+ cytotoxic and FoxP3+ regulatory T lymphocytes serve as prognostic factors in breast cancer. Am J Transl Res 2019;11(8):5039–5053. [PMC free article] [PubMed] [Google Scholar]
- 26.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (80- ) 2006;313(5795):1960–1964. doi: 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 27.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007;104(10):3967–3972. doi: 10.1073/pnas.0611618104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carstens JL, De Sampaio PC, Yang D, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun 2017;8(1):1–13. doi: 10.1038/ncomms15095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Chen R, Wang Y-W, Fornace AJ, Li H-H. Prior irradiation results in elevated programmed cell death protein 1 (PD-1) in T cells. Int J Radiat Biol 2018;94(5):488–494. doi: 10.1080/09553002.2017.1400192 [DOI] [PubMed] [Google Scholar]
- 31.Pietrocola F, Pol J, Vacchelli E, et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016;30(1):147–160. doi: 10.1016/j.ccell.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kachikwu EL, Iwamoto KS, Liao Y-P, et al. Radiation Enhances Regulatory T Cell Representation. Int J Radiat Oncol 2011;81(4):1128–1135. doi: 10.1016/J.IJROBP.2010.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu Y, Jin S, Zhang A, et al. Gamma-Ray Resistance of Regulatory CD4 + CD25 + Foxp3 + T Cells in Mice. Radiat Res 2010;173(2):148–157. doi: 10.1667/RR0978.1 [DOI] [PubMed] [Google Scholar]
- 34.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol May 2020:1–18. doi: 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajona D, Ortiz-Espinosa S, Lozano T, et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat Cancer 2020;1(1):75–85. doi: 10.1038/s43018-019-0007-9 [DOI] [PubMed] [Google Scholar]
- 36.Nicklas BJ, Brinkley TE, Houston DK, et al. Effects of Caloric Restriction on Cardiorespiratory Fitness, Fatigue, and Disability Responses to Aerobic Exercise in Older Adults with Obesity: A Randomized Controlled Trial. Journals Gerontol - Ser A Biol Sci Med Sci 2019;74(7):1084–1090. doi: 10.1093/gerona/gly159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Icard P, Ollivier L, Forgez P, et al. Perspective: Do Fasting, Caloric Restriction, and Diets Increase Sensitivity to Radiotherapy? A Literature Review. Adv Nutr 2020;11(5):1089–1101. doi: 10.1093/advances/nmaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakomi S, Nakayama T, Shang Y, et al. The effects of short-term calorie restriction on mutations in the spleen cells of infant-irradiated mice. J Radiat Res 2020;61(2):187–196. doi: 10.1093/jrr/rrz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall SE, Smuder AJ, Hayward R. Effects of Calorie Restriction and Voluntary Exercise on Doxorubicin-Induced Cardiotoxicity. Integr Cancer Ther 2019;18. doi: 10.1177/1534735419843999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkham AA, Paterson DI, Prado CM, et al. Rationale and design of the Caloric Restriction and Exercise protection from Anthracycline Toxic Effects (CREATE) study: A 3-arm parallel group phase II randomized controlled trial in early breast cancer. BMC Cancer 2018;18(1). doi: 10.1186/s12885-018-4778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Howard HY, et al. Immunocompetent mouse allograft models for development of therapies to target breast cancer metastasis. Oncotarget 2017; 8(19):30621–30643. doi: 10.18632/oncotarget.15695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piranlioglu Raziye,1 EunMi Lee,1 Maria Ouzounova. Primary tumor-induced immunity eradicates disseminated tumor cells in syngeneic mouse model. Nat Commun 2019; 10: 1430. doi: 10.1038/s41467-019-09015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


