Abstract
Objective:
Improvements to bladder cancer risk stratification guidelines are needed to better tailor post-operative surveillance and adjuvant therapy to individual patients. We previously identified STAG2 as a commonly mutated tumor suppressor gene in bladder cancer and an independent predictor of progression in NMIBC. Here we test the value of combining STAG2 immunostaining with other risk stratification biomarkers in NMIBC, and as an individual biomarker in MIBC.
Materials and Methods:
STAG2 immunohistochemistry was performed on a progressor-enriched cohort of tumors from 297 patients with NMIBC, and on tumors from 406 patients with MIBC from Aarhus University Hospital in Denmark. Survival analysis was performed using Kaplan-Meier survival analysis, the log rank test, and Cox proportional hazards models.
Results:
STAG2-negative low-grade NMIBC tumors were 2.5 times less likely to progress to muscle invasion than STAG2-positive low-grade NMIBC tumors (Log-rank test, P = 0.008). In a composite group of patients with AUA intermediate and high-risk NMIBC tumors, STAG2-negative tumors were less likely to progress (Log-rank test, P = 0.02). In contrast to NMIBC, we show that STAG2 is not useful as a prognostic biomarker in MIBC.
Conclusions:
STAG2 immunostaining can be used to subdivide low grade NMIBC tumors into two groups with substantially different risks of disease progression. Furthermore, STAG2 immunostaining may be useful to enhance NMIBC risk stratification guidelines, though larger cohorts are needed to solidify this conclusion in individual risk groups. STAG2 is not useful as a biomarker in MIBC. Further study of the use of STAG2 immunostaining as a biomarker for predicting the clinical behavior in NMIBC is warranted.
Keywords: Bladder cancer, STAG2, risk stratification guidelines, bladder cancer recurrence and progression
1. Introduction
Over 60,000 cases of non-muscle invasive bladder cancer (NMIBC) are diagnosed in the United States every year [1]. After transurethral resection (TUR), as many as 70% of these tumors will recur, and ~20% of these recurrences will progress to muscle invasion [2]. Because of this risk, intensive regimens of post-resection surveillance by cystoscopy are often performed. However, such intensive surveillance is inconvenient and uncomfortable for these often-elderly patients. It is also extremely expensive; the clinical management of NMIBC imposes higher per patient costs on the US health care system than the management of any other cancer type [3,4,5].
Another challenge in the management of NMIBC is the decision whether to treat patients with post-operative adjuvant intravesical immunotherapy and/or chemotherapy. While these treatments can be effective, they are sometimes avoided because of the potential for side effects.
Recommendations for post-operative surveillance and adjuvant therapy are based primarily on tumor grade and/or on risk stratification guidelines published by groups such as the American Urology Association (AUA) [2]. There is an ongoing effort to develop additional biomarkers that can be incorporated into these risk stratification guidelines so urologists can more effectively target post-operative surveillance and adjuvant therapy to individual patients.
In 2013, we and others discovered that 35-40% of all NMIBC tumors and ~10% of all MIBC tumors harbor truncating mutations of the STAG2 gene [6,7,8,9]. STAG2 encodes a component of a chromatin-bound protein complex known as cohesin, which plays important roles in sister chromatid cohesion, chromatin structure, gene expression, and DNA repair [10].
The difference between the STAG2 mutation frequency in NMIBC and MIBC suggested the hypothesis that STAG2-mutant NMIBC tumors may be less likely to progress to MIBC than STAG2-wild type NMIBC tumors. To test this, we and others developed a simple, robust, and binary STAG2 immunostaining assay that identifies the STAG2 mutational status of tumors [6]. In this assay, the absence of staining indicates the presence of a truncating mutation in the STAG2 gene, which account for ~90% of all tumor-derived mutations in the gene. We then used this assay to demonstrate that tumors harboring STAG2 mutations were 2-3x less likely to progress to muscle invasion than tumors with wild-type STAG2 genes [11,12], indicating that STAG2 might be useful as a biomarker for risk stratification of NMIBC. However, these studies tested STAG2 as a single biomarker; it was unclear whether combining STAG2 with other currently-used biomarkers would enhance their predictive power.
Here we show that adding STAG2 immunostaining to tumor grade and risk stratification guidelines in NMIBC enhances their prognostic capabilities. We also show that the value of STAG2 as a biomarker is limited to NMIBC, as it provides no prognostic discrimination in MIBC tumors.
2. Materials and methods
Patient cohort and tumor material
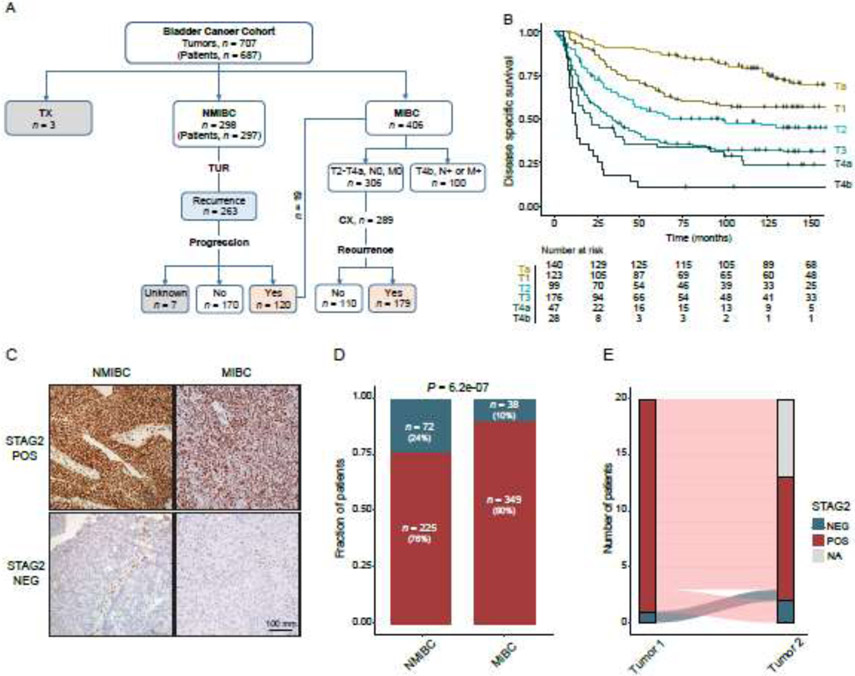
A total of 707 bladder tumors (stage pTa-T4b) collected from 687 patients treated with TUR at Aarhus University Hospital, Aarhus, Denmark between 1979-2013 were included in this retrospective study (Fig. 1A). The median follow-up was 74 months. Details regarding criteria used for patient selection are described in refs. [13,14]. Informed written consent was obtained from all patients, and the study was approved by the National Committee on Health Research Ethics (#1706291).
Figure 1.
Disease-specific survival and STAG2 expression in the bladder cancer cohort studied. (A) Overview of the bladder cancer (BC) cohort. Tumors were grouped based on pathological stage and stratified by clinical outcome. 20 patients had cores taken from more than one tumor; hence, the entire cohort consisted of 707 bladder tumors from 687 patients with BC. One of the 20 patients had two NMIBC tumors, whereas the remaining patients had both NMIBC and MIBC tumors. TX: tumor of unknown pathological stage. (B) Kaplan Meier curve depicting disease-specific survival by pathological stage. (C) Examples of STAG2 staining of NMIBC and MIBC tumors. (C) Frequency of maintenance (red) and loss (blue) of STAG2 expression in NMIBC and MIBC in this cohort. (D) Concordance of STAG2 status in patients with two tumors represented in the cohort. 20 patients had two tumors in the cohort; in 19 patients they were tumors from an initial NMIBC and a subsequent MIBC, in one patient they were two independent NMIBC tumors. Unfortunately, in only 11 of these patients could STAG2 status be determined for both tumors of the pair. STAG2 staining was discordant in 3/11 of these pairs (Fig. 1E); each of the three discordant pairs were comprised of one NMIBC tumor and one MIBC tumor.
In this intentionally progressor-enriched cohort, 90% of patients with NMIBC recurred (263/297), 40% (120/297) of patients with NMIBC progressed to MIBC, and 62% (179/289) recurred following cystectomy (CX). 25% (100/406) of MIBC patients were diagnosed with advanced BC (T4b, N+ or M+) at the time of diagnosis. As such, this cohort is enriched for patients whose tumors ultimately progressed to muscle invasion or developed metastatic disease, and is, therefore, particularly well-suited for biomarker validation.
In this cohort, recurrence of NMIBC tumors was defined as local, regional, or distant recurrence, or progression to muscle invasive disease, as determined by pathology or computed tomography (CT). Recurrence of MIBC tumors was defined as local, upper urinary tract, or distant recurrence after cystectomy, as determined by CT [15]. Progression of NMIBC tumors was defined as pathologically-verified muscle-invasive disease, or the development of metastasis as determined by CT.
For NMIBC tumors, progression-free survival (PFS) was measured from the date of TUR to the development of MIBC, metastasis, or the end of follow-up. NMIBC patients who underwent CX before pathological evidence of progression or who did not develop metastatic disease were censored at the time of CX. Recurrence-free survival (RFS) was measured separately for NMIBC and MIBC tumors, and was measured from the date of TUR (NMIBC) or CX (MIBC) to either the date of recurrence or the end of follow-up. Disease-specific survival (DSS) was measured from the date of TUR to either the date of death from BC or the end of follow-up. Survival data was updated June 2020.
NMIBC tumors were risk stratified based on the 2016 AUA/SUO guidelines [2]. Eighteen patients were excluded from risk stratification due to a lack of needed clinical information. TNM staging was based on cross-sectional imaging and pathological assessment of the TUR or CX specimen and regional lymph nodes.
Formalin-fixed paraffin-embedded (FFPE) tissue and corresponding hematoxylin-eosin stained sections from TUR specimens were retrieved for all patients. Tumor stage and grade were determined by experienced genitourinary pathologists in accordance with the 2004 WHO Classification of Tumors of the Urinary System and Male Genital Organs [16] while blinded to outcome. The most representative tumor areas with a high carcinoma cell percentage were identified for each tumor. Tissue micro arrays (TMA) were constructed from 0.6 to 1mm core biopsies from each tumor area using the manual tissue-microarray (Beecher Instruments, Sun Prairie, WI) or the automated TMA-GRAND Master (3DHISTECH Ltd, Budapest, Hungary) as described by Kononen and colleagues [17].
Immunohistochemistry and TMA scoring
Immunohistochemistry was performed in the Georgetown University Medical Center Histopathology and Tissue Shared Resource. Sections measuring 5 mm from FFPE tissues were deparaffinized with xylene and rehydrated through a graded alcohol series. Heat-Induced Epitope Retrieval (HIER) was performed by immersing the tissue sections at 98°C for 20 minutes in Target Retrieval Solution, high pH (Dako). Immunohistochemical staining was performed using the VectaStain Kit from Vector Labs according to manufacturer’s instructions. Briefly, slides were treated with 3% hydrogen peroxide for 10 minutes. Endogenous biotin was blocked using an avidin/biotin blocking kit from Invitrogen. The slides were then treated with 10% normal goat serum for 10 minutes and exposed to STAG2 antibodies (1:50, Santa Cruz Biotechnology, sc81852) for 1 hour at room temperature. Slides were then exposed to biotin-conjugated mouse secondary antibody (Vector Labs), Vectastain ABC reagent, and DAB chromagen (Dako). Slides were counterstained with hematoxylin (Fisher, Harris Modified Hematoxylin) at a 1:8 dilution for 2 minutes at room temperature, blued in 1% ammonium hydroxide for 1 minute at room temperature, dehydrated, and mounted with Acrymount.
TMA cores were read as either positive or negative for nuclear STAG2 staining as determined by three observers (B.H., T.W., Y.R.P.). The STAG2 staining profile of a subset of the NMIBC tumors in this cohort has been described previously [11]. Since STAG2 is a nuclear protein and the STAG2 antibody has a high degree of sensitivity and specificity, there was generally no cytoplasmic or membrane staining with STAG2 antibodies. This STAG2 immunostaining assay sensitively and specifically identifies tumors harboring mutations of STAG2 because (i) ~90% of tumor-derived mutation of STAG2 mutations are truncating, (ii) the epitope for the STAG2 monoclonal antibody used for immunohistochemistry (Santa Cruz Biotechnology sc81852) is at the very carboxyl terminus of the encoded protein, and is therefore absent in all tumors harboring truncating mutations of STAG2, and (iii) STAG2 is on the X chromosome and is therefore a functionally single copy gene, so there is no possibility of a complicating intermediate heterozygote effect.
8/707 cores had regions of both STAG2 positivity and STAG2 negativity. Since we previously showed that such mosaic tumors harbor inactivating mutations of STAG2 in their STAG2 non-expressing cells [6] and recur at a similarly low frequency as tumors with uniform loss of STAG2 expression [11], the eight mosaic tumors were considered as STAG2 negative for statistical analysis.
Statistical analysis
Statistical analyses were conducted using R version 3.6.1 (RStudio Team (2019). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA). Assessment of statistical significance included Fisher’s exact test or Chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables. Demographic information is presented as the mean for continuous variables and counts for categorical variables. Cumulative survival analysis was performed using the Kaplan-Meier survival method, and the log rank test was used to compare curves (R packages survminer and survival). Hazard ratios (HR), with 95% confidence intervals (CI), were calculated using Cox proportional hazards models. Covariates with a p < 0.05 in univariate cox-regression models were included in the multivariable cox-regression model and included age, stage, grade, carcinoma in situ (CIS), and STAG2 staining. Logistic regression models were built to predict progression (R packages glmnet) and the predicted probabilities were used as variables in the receiver operating characteristic (ROC) analysis. The R package pROC was used for data visualization, as well as to calculate area under the curve (AUC) and associated 95% CI (computed with 2,000 stratified bootstrap replicates). Delong’s test was used to compare AUCs. Statistical significance was set at p ≤ 0.05. In all analyses, only one tumor per patient was included (taken from the earliest time point).
3. Results
Clinical and pathological correlates of STAG2 mutation in bladder cancer
STAG2 immunostaining was performed on a progressor-enriched cohort of 707 bladder tumors – 298 NMIBC tumors (patients, n = 297; pathological stage Ta-T1b), 406 MIBC tumors (patients, n = 406; pathological stage T2-T4b), and 3 tumors of unknown pathological stage (Fig. 1A). The number of tumors is greater than the number of patients because one patient had two distinct NMIBCs included in the TMAs, and 19 patients had both a NMIBC and MIBC tumor included in the TMAs. The clinical and pathological characteristics of this cohort are described in Table 1, and disease-specific survival outcomes by pathological stage are shown in Fig. 1B. Representative examples of STAG2 immunostaining are shown in Fig. 1C).
Table 1.
Clinical and pathological characteristics
| NMIBC Tumors (n = 297) | MIBC Tumors (n = 406)* | |||||
|---|---|---|---|---|---|---|
| Variable | STAG2 NEG (n = 72) |
STAG2 POS (n = 225) |
p-value** | STAG2 NEG (n = 40) |
STAG2 POS (n = 359) |
p-value** |
| Mean Age (SD) | 64 (10) | 65 (10.6) | 0.424 | 63 (7.1) | 62 (8.4) | 0.455 |
| Range | 41 – 84 | 32 – 87 | 46 – 75 | 29 – 83 | ||
| Sex | 0.018 | 0.007 | ||||
| Female | 23 (31.9%) | 42 (18.7%) | 17 (42.5%) | 83 (23.1%) | ||
| Male | 49 (68.1%) | 183 (81.3%) | 23 (57.5%) | 276 (76.9%) | ||
| T-stage | 0.007 | 0.418 | ||||
| Ta | 49 (68.1%) | 112 (49.8%) | - | - | ||
| T1 | 23 (31.9%) | 113 (50.2%) | - | - | ||
| T2-T4a | - | - | 35 (87.5%) | 328 (91.4%) | ||
| T4b | - | - | 5 (12.5%) | 31 (8.6%) | ||
| N-stage | 0.574 | 0.036 | ||||
| N+ | 0 (0.0%) | 1 (0.4%) | 8 (20.5%) | 130 (37.5%) | ||
| N0 | 71 (100.0%) | 224 (99.6%) | 31 (79.5%) | 217 (62.5%) | ||
| Unknown | 1 | 12 | ||||
| M-stage | 0.425 | 0.643 | ||||
| M+ | 0 (0.0%) | 2 (0.9%) | 4 (10.0%) | 44 (12.5%) | ||
| M0 | 71 (100.0%) | 223 (99.1%) | 36 (90.0%) | 307 (87.5%) | ||
| Unknown | 1 | 0 | 0 | 8 | ||
| Grade | 0.011 | 0.291 | ||||
| High grade | 23 (31.9%) | 110 (49.1%) | 39 (100.0%) | 349 (97.2%) | ||
| Low grade | 49 (68.1%) | 114 (50.9%) | 0 (0.0%) | 10 (2.8%) | ||
| Unknown | 0 | 1 | 1 | 0 | ||
| Concomitant CIS | 0.049 | 0.745 | ||||
| Yes | 17 (23.9%) | 140 (63.3%) | 2 (8.0%) | 20 (10.1%) | ||
| No | 54 (76.1%) | 81 (36.7%) | 23 (92.0%) | 179 (89.9%) | ||
| Unknown | 1 | 3 | 15 | 160 | ||
STAG2 status was not assessable in 7 out of 406 MIBC tumors. For the seven patients STAG2 status was assessable in a previous NMIBC tumor. P-values were calculated using ANOVA test for continuous variables and Chi-square test for categorical variables.
As previously observed [6,11], loss of STAG2 was more common in NMIBC (24%; 72/297) than in MIBC (10%; 38/387; Chi-Square Test, P = 6.2e-07; Fig. 1D). Of note, this 24% frequency of observed STAG2 loss in this NMIBC cohort underestimates the true frequency of STAG2 loss in NMIBC (35-40%) because this NMIBC cohort is enriched for progressors, which as we show here tend to have wild-type STAG2. In NMIBC tumors, loss of STAG2 was correlated with pathological stage (Ta), pathological grade (low), and the absence of concomitant CIS (Chi-Square Test, P = 0.007-0.049; Table 1). In MIBC tumors, loss of STAG2 was significantly correlated with the absence of lymph node metastasis (Chi-Square Test, P = 0.036; Table 1). Taken together, these correlations suggest that STAG2-negative bladder cancer tends to be less aggressive overall than STAG2-positive bladder cancer, especially in NMIBC.
Loss of STAG2 was significantly more common in female patients than male patients (Chi-Square Test, NMIBC, P = 0.027; MIBC, P = 0.013; Table 1; Supplementary Fig. 1). The specific mechanism for this difference is unknown, though it seems most likely related to the fact that the STAG2 gene is on the X chromosome.
20 patients had two tumors in the cohort; in 19 patients they were tumors from an initial NMIBC and a subsequent MIBC, in one patient it was two independent NMIBC tumors. Unfortunately, in only 11 of these patients could STAG2 status be determined for both tumors of the pair. STAG2 staining was concordant in eight of these pairs (Fig. 1E).
STAG2 as an individual prognostic biomarker in NMIBC and MIBC
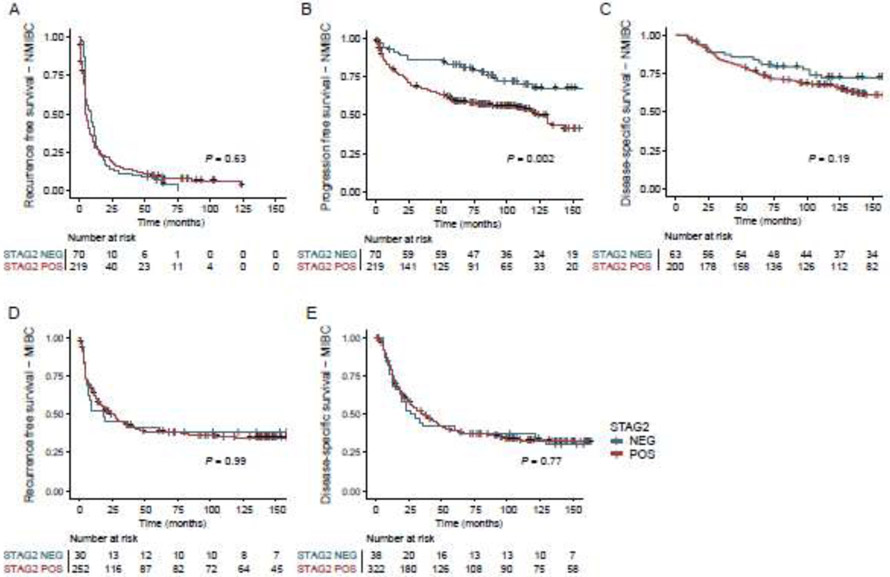
We next determined the prognostic value of STAG2 immunostaining as an individual biomarker in NMIBC and MIBC tumors using Kaplan-Meier survival analysis (Fig. 2A-E). NMIBC tumors with negative STAG2 immunostaining were less likely to progress to muscle invasion than NMIBC tumors with intact STAG2 immunostaining (Log-rank test, P = 0.002; Fig. 2B) and in univariate Cox regression analysis (HR = 2.1, P = 0.002; Table 2). Consistent with our previous findings [11], the prognostic value of STAG2 remained significant in multivariable Cox regression analysis after adjustment for clinicopathologic factors (age, stage, grade, CIS; HR = 1.7, P = 0.029; Table 2). In contrast, there was no significant prognostic value for STAG2 in predicting recurrence-free survival (fig. 2, A,D) or disease-specific survival (Fig. 2C,E) in NMIBC or MIBC.
Figure 2.
Predictive value of STAG2 staining as an individual biomarker in NMIBC (A-C) and MIBC (D-E) on recurrence-free survival (A,D), progression-free survival (B) and disease-specific survival (C,E).
Table 2.
Univariate and multivariate Cox proportional hazard regression analysis for prediction of progression in NMIBC
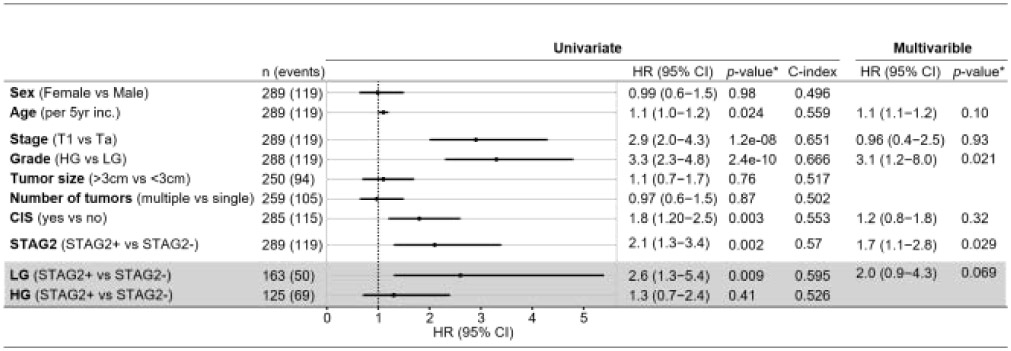 |
Cox proportional hazards regression. Covariates with a p < 0.05 in the univariate models were included in the final multivariable model. Grey box: subanalyses of low or high grade tumors and STAG2 expression. Covariants; Age, stage, CIS and LG (p<0.05) were included in the final multivariable model. HR, hazard ratio; CI, Confidence interval; C-index, Harrell’s concordance index. LG, low grade; HG, high grade.
Addition of STAG2 immunostaining enhances the prognostic value of tumor grade in NMIBC
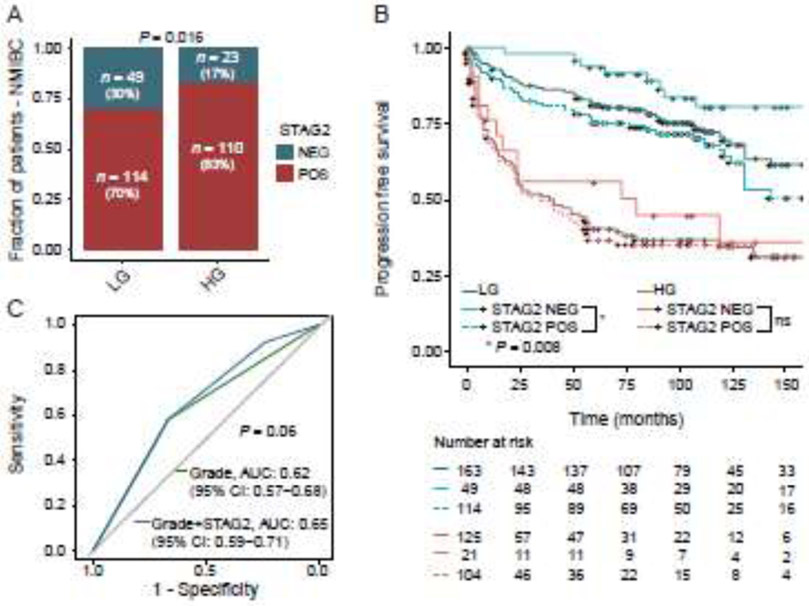
In NMIBC, tumor grade is often used as the primary determinant for determining the frequency of post-TUR surveillance and the need for adjuvant intravesical therapy. In this cohort, 30% (49/163) of low grade NMIBC tumors were STAG2-negative, and 17% (23/133) of high grade NMIBC tumors were STAG2 negative (Chi-Square Test, P = 0.016, Fig. 3A). This is consistent with previous findings indicating that low grade NMIBC tumors are more likely to harbor mutations in STAG2 than high grade tumors [9]. In this cohort, tumor grade was the strongest independent prognostic factor for PFS in a multivariable Cox regression analysis after adjustment for age, T-stage, CIS and STAG2 (HR = 3.1, P = 0.021, Table 2). STAG2 was the second strongest prognostic factor (HR = 1.7, P = 0.029, Table 2). Therefore, wondered whether a two-biomarker combination of tumor grade and STAG2 would outperform the use of tumor grade alone in predicting progression of NMIBC to muscle invasion.
Figure 3.
STAG2 expression and tumor grade. (A) STAG2 expression compared to tumor grade (n = 296). For one patient tumor grade could not be determined. (B) Predictive value of a combination of STAG2 staining and tumor grade on progression-free survival in NMIBC. LG, low grade; HG, high grade. (C) Receiver operating characteristics (ROC) curve for predicting progression using logistic regression models (n = 289).
To test this, we divided our cohort of NMIBC tumors into high grade and low grade subgroups, then performed Kaplan Meier survival analysis to determine whether STAG2 status could enhance prediction of progression within these subgroups. We found that low grade STAG2-negative NMIBC tumors were significantly less likely to progress to muscle invasion than low grade STAG2-positive NMIBC tumors (Log-rank test, P = 0.008; Fig. 3B). At five years after TUR, only 6% (3/49) of STAG2-negative low-grade tumors had progressed to muscle invasion, whereas 25% (28/114) of STAG2-positive low-grade tumors had progressed to muscle invasion. We then performed a univariate Cox proportional hazard regression analysis on low grade tumors using STAG2 staining as a variable (Table 2). STAG2-negative low grade NMIBC tumors were 2.6 times less likely to progress to muscle invasion than STAG2-positive low grade NMIBC tumors (P = 0.009). Next, we performed a receiver operating characteristics (ROC) analysis using logistic regression models (Fig. 3C). Combining grade with STAG2 positivity increased the area under the curve (AUC) from 0.62 to 0.65 (DeLong’s test, P = 0.06). Taken together, these data indicate that a biomarker combination of tumor grade and STAG2 immunostaining can aid in the prediction of progression of NMIBC, particularly in low-grade tumors.
Addition of STAG2 immunostaining may improve AUA guidelines for NMIBC risk stratification
In NMIBC, risk stratification guidelines such as those published by the American Urology Association (AUA) are often used to determine the course of post-operative surveillance and treatment that can best maximize efficacy while preserving quality of life for each patient. These guidelines rely on a weighted consideration of tumor multiplicity, tumor size, prior recurrence, pathological stage, pathological grade, and the presence or absence of CIS [2].
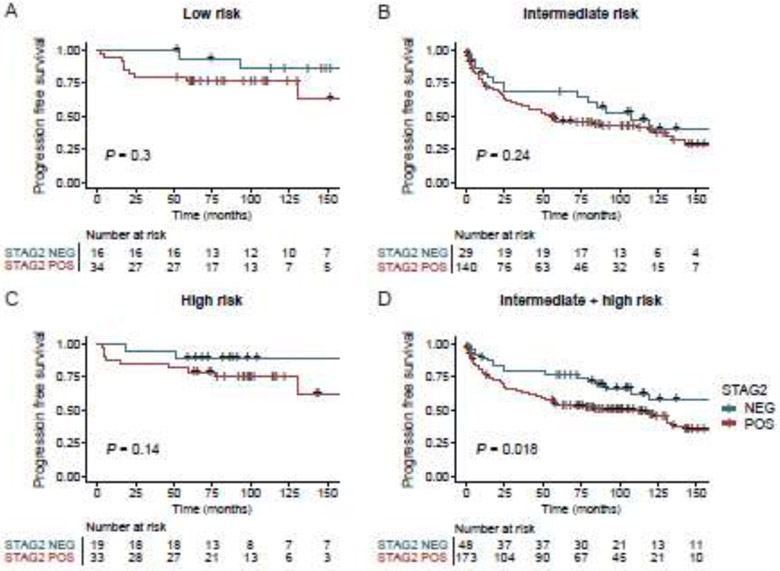
To assess whether adding STAG2 staining could improve the predictive power of the AUA guidelines, we first applied the guidelines to our cohort, and then assessed whether STAG2 staining improved their prognostic discrimination. There were 50 low-, 52 intermediate-, and 169 high-risk NMIBC tumors; 18 patients were excluded in risk stratification due to lack of sufficient clinical information. In each of these subgroups, Kaplan Meier survival analysis indicated that STAG2-negative tumors appeared less likely to progress to MIBC than STAG2-positive tumors (Fig. 4 A-C); however, the STAG2 effect did not reach p<0.05 for individual subgroups, likely at least in part due to sample size considerations. Therefore, we next performed Kaplan-Meier analysis on a composite (and therefore larger) group of intermediate- and high-risk tumors. This analysis revealed that STAG2-negative intermediate+high risk tumors were significantly less likely to progress to MIBC than STAG2-positive intermediate+high risk tumors (Log-rank test, P = 0.018; Fig. 4D). In addition, we performed a ROC analysis for predicting progression using logistic regression models (Supplementary Fig. 2). Combining STAG2 with AUA risk scores increased the AUC from 0.57 (95% CI 0.52-0.61) to 0.60 (95% CI 0.54-0.65); however this did not reach statistical significance (Delong’s test, P = 0.10). Additional studies with larger cohorts are required to confirm this with sufficient statistical power for individual risk groups.
Figure 4.
Predictive value of STAG2 staining on progression-free survival in NMIBC risk groups based on the American Urological Association risk stratification guidelines.
4. Discussion
In this study we show that STAG2 immunostaining can be used to stratify patients with low grade NMIBC tumors into groups with different risks of disease progression. We also show that in a composite group of patients with intermediate and high-risk tumors, STAG2-negative tumors had better clinical outcomes. Finally, we show that these findings were specific for NMIBC; STAG2 immunostaining provided no prognostic discrimination in MIBC.
One of the most important aspects of the STAG2 immunostaining assay for NMIBC is its simplicity and ease of evaluation and interpretation. As described previously [11], STAG2 immunostaining is binary – it is either present or absent; there are no intermediate levels of staining to generate ambiguity in the STAG2 calls. In fact, in a previous study we showed that inter-observer agreement for STAG2 immunostaining was >99% [11]. Furthermore, the assay is simple to perform; it is a standard immunohistochemistry assay using a commercially-available antibody that can easily be performed in any department of pathology. Especially considering the inter-observer variability in the assignment of tumor grade in NMIBC [18,19,20], we believe that adding STAG2 immunostaining could improve the prognostic discrimination of current biomarkers in a meaningful and useful way.
It is worth emphasizing that the NMIBC cohort studied here was intentionally enriched for patients whose tumors progressed to MIBC. A progressor-enriched cohort was used because in the current clinical setting of extremely aggressive post-operative surveillance by cystoscopy, relatively few patients progress to muscle invasion. Therefore, in order to generate sufficient statistical power to test the prognostic value of a biomarker on progression, it is necessary to use a cohort that is enriched for progressors. Since the NMIBC cohort was enriched for progressors, the frequency of STAG2 mutations in the NMIBC cohort (24%) is less than would be predicted by previous studies (35-40%). This is expected, since we know STAG2-mutant NMIBC tumors are less like likely to progress to muscle invasion than STAG2 wild-type NMIBC tumors.
It was surprising to us that STAG2 immunostaining seemed particularly useful in two somewhat contrasting groups of NMIBC tumors – (i) low grade tumors, and (ii) tumors in a composite risk group of intermediate and high-risk tumors (most of which are high grade). We believe that this is due to the relatively small number of tumors in our cohort that are in the low and intermediate AUA risk groups (52 and 50 tumors, respectively). Based on the Kaplan Meier curves shown in Fig. 4, we believe that with larger cohorts, there would be a statistically significant STAG2 effect in the AUA low and intermediate risk groups. Future experiments with larger cohorts are needed to confirm this.
It is also arguably counterintuitive that a cancer-causing mutation in a tumor suppressor gene like STAG2 would define a tumor subtype with a lower risk (instead of a higher risk) of progression. However, this should not be surprising since it is by now well known that cancer is driven by mutations in a panoply of oncogenes and tumor suppressor genes that individually and in combination define cancer subtypes with varying clinical behavior. For example, NMIBCs harboring oncogenic mutations in FGFR3 have better clinical outcomes than equivalent NMIBCs with wild-type FGFR3 genes [21,22,23]. We believe it is likely that STAG2 mutations define a previously unrecognized, relatively well-differentiated form of NMIBC.
The mechanism through which mutations of STAG2 and other components of the cohesin complex drive cancer pathogenesis remains unknown. Initially, it was thought that STAG2 mutations caused aneuploidy in cancer. However, this hypothesis was convincingly challenged when it became clear that STAG2 mutations were most common in tumor types that were very often not aneuploid, such as NMIBC. More recently, cohesin research has shifted and now focuses on the role of cohesin in controlling cellular differentiation by generating, maintaining and regulating the intra-chromosomal DNA looping events that modulate 3D genome organization and gene expression [10].
One limitation of the current study is the size of the NMIBC cohort, which lessened the statistical power of the analysis for individual AUA risk groups. Therefore, despite the promise of these findings, additional studies are needed with additional, larger cohorts to further validate STAG2 immunostaining as an effective prognostic biomarker in NMIBC.
5. Conclusion
In previous studies, we and others have reported that STAG2 is a potentially useful individual biomarker for prediction of recurrence and progression in NMIBC [11,12]. Here we show that STAG2 immunostaining can be used to stratify patients with low grade NMIBC tumors into groups with different risks of disease progression. If confirmed by additional studies, this finding has the potential to improve the predictive power of risk stratification in NMIBC, enabling urologists to better optimize regimens of post-TUR surveillance and adjuvant therapy for individual patients.
Supplementary Material
Highlights.
STAG2 is an independent prognostic biomarker in non-muscle invasive bladder cancer
STAG2 immunostaining is a simple, binary assay that detects STAG2 mutation status
STAG2 is particularly useful as a biomarker in low grade tumors
Acknowledgments
Grant Support: Supported by NCI/NIH grants R01CA169345 (TW), P30CA051008 (DB, KC, BH), T32CA009686 (YP, TW), the Hyundai Hope on Wheels Foundation (TW), and Aarhus University (AT, LD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].National Cancer Institute, National Institutes of Health. Cancer Stat Facts: Bladder Cancer https://seer.cancer.gov/statfacts/html/urinb.html; [accessed 19 January 2021].
- [2].Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO Guideline. J Urol 2016;196:1021–9. doi: 10.1016/j.juro.2016.06.049 [DOI] [PubMed] [Google Scholar]
- [3].James AC, Gore JL. The costs of non-muscle invasive bladder cancer. Urol Clin North Am 2013;40:261–9. doi: 10.1016/j.ucl.2013.01.004 [DOI] [PubMed] [Google Scholar]
- [4].Mossanen M and Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol 2014;24: 487–91. doi: 10.1097/MOU.0000000000000078 [DOI] [PubMed] [Google Scholar]
- [5].Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014;66: 253–62. 10.1016/j.eururo.2014.01.006 [DOI] [PubMed] [Google Scholar]
- [6].Solomon DA, Kim JS, Bondaruk J, Shariat SF, Wang ZF, Elkahloun AG, et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat Genet 2013;45:1428–30. doi: 10.1038/ng.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet 2013;45:1459–63. doi: 10.1038/ng.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Balbás-Martínez C, Sagrera A, Carrillo-de-Santa-Pau E, Earl J, Marquez M, Vazquez M, et al. Recurrent inactivation of STAG2 in bladder cancer is not associated with aneuploidy. Nat Genet 2013;45:1464–9. doi: 10.1038/ng.2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taylor CF, Platt FM, Hurst CD, Thygesen HH, Knowles MA. Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum Mol Genet 2014;23:1964–74. doi: 10.1093/hmg/ddt589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Waldman T Emerging themes in cohesin cancer biology. Nat Rev Cancer 2020;20:504–15. doi: 10.1038/s41568-020-0270-1 [DOI] [PubMed] [Google Scholar]
- [11].Lelo A, Prip F, Harris BT, Solomon D, Berry DL, Chaldekas K, et al. STAG2 Is a Biomarker for Prediction of Recurrence and Progression in Papillary Non-Muscle-Invasive Bladder Cancer. Clin Cancer Res 2018;24:4145–53. doi: 10.1158/1078-0432.CCR-17-3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Qiao Y, Zhu X, Li A, Yang S, Zhang J. Complete loss of STAG2 expression is an indicator of good prognosis in patients with bladder cancer. Tumour Biol 2016;37:10279–86. doi: 10.1007/s13277-016-4894-4 [DOI] [PubMed] [Google Scholar]
- [13].Fristrup N, Ulhøi BP, Birkenkamp-Demtröder K, Mansilla F, Sanchez-Carbayo M, Segersten U, et al. Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am J Pathol 2012;180:1824–34. doi: 10.1016/j.ajpath.2012.01.023 [DOI] [PubMed] [Google Scholar]
- [14].Taber A, Christensen E, Lamy P, Nordentoft I, Prip F, Lindskrog SV, et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat Commun 2020;11:4858. doi: 10.1038/s41467-020-18640-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam 2020. ISBN 978-94-92671-07-3. EAU Guidelines Office, Arnhem, The Netherlands. http://uroweb.org/guidelines/compilations-of-all-guidelines/; [accessed 19 January 2021]. [Google Scholar]
- [16].Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016;70:106–19. 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- [17].Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–7. doi: 10.1038/nm0798-844 [DOI] [PubMed] [Google Scholar]
- [18].Tosoni I, Wagner U, Sauter G, Egloff M, Knönagel H, Alund G, et al. Clinical significance of interobserver differences in the staging and grading of superficial bladder cancer. BJU Int 2000;85:48–53. doi: 10.1046/j.1464-410x.2000.00356.x [DOI] [PubMed] [Google Scholar]
- [19].Bol MG, Baak JP, Buhr-Wildhagen S, Kruse AJ, Kjellevold KH, Janssen EA, et al. Reproducibility and prognostic variability of grade and lamina propria invasion in stages Ta, T1 urothelial carcinoma of the bladder. J Urol 2003;169:1291–4. doi: 10.1097/01.ju.0000055471.78783.ae. [DOI] [PubMed] [Google Scholar]
- [20].Bosschieter J, Hentschel A, Savci-Heijink CD, Patrick van der Voorn J, Rozendaal L, Vis AN, et al. Reproducibility and prognostic performance of the 1973 and 2004 World Health Organization classifications for grade in non-muscle-invasive bladder cancer: a multicenter study in 328 bladder tumors. Clin Genitourin Cancer 2018;16: e985–92. doi: 10.1016/j.clgc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- [21].Billerey C, Chopin D, Aubriot-Lorton MH, Ricol D, Gil Diez de Medina S, Van Rhijn B, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol 2001;158:1955–9. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Burger M, van der Aa MN, van Oers JM, Brinkmann A, van der Kwast TH, Steyerberg EC, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur Urol 2008;54:835–43. doi: 10.1016/j.eururo.2007.12.026 [DOI] [PubMed] [Google Scholar]
- [23].van Rhijn BW, van der Kwast TH, Liu L, Fleshner NE, Bostrom PJ, Vis AN, et al. The FGFR3 mutation is related to favorable pT1 bladder cancer. J Urol 2012;187:310–4. doi: 10.1016/j.juro.2011.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.