Abstract
Objective.
Suppression of local and network alpha and beta oscillations in the human basal ganglia-thalamocortical (BGTC) circuit is a prominent feature of movement, including suppression of local alpha/beta power, cross-region beta phase coupling, and cortical and subcortical phase-amplitude coupling (PAC). We hypothesized that network-level coupling is more directly related to movement execution than local power changes, given the role of pathological network hypersynchrony in movement disorders such as Parkinson disease (PD). Understanding the specificity of these movement-related signals is important for designing novel therapeutics.
Methods.
We recorded globus pallidus internus (GPi) and motor cortical local field potentials during movement execution, passive movement observation and rest in 12 patients with PD undergoing deep brain stimulator implantation.
Results.
Local alpha/beta power is suppressed in the globus pallidus and motor cortex during both action execution and action observation, although less so during action observation. In contrast, pallidocortical phase synchrony and GPi and motor cortical alpha/beta-gamma PAC are suppressed only during action execution.
Conclusions.
The functional dissociation across tasks in pallidocortical network activity suggests a particularly important role of network coupling in motor execution.
Significance.
Network level recordings provide important specificity in differentiating motor behavior and may provide significant value for future closed loop therapies.
Keywords: Parkinson disease, deep brain stimulation, beta oscillations, action observation, motor control
1. Introduction
Human movements are associated with changes in oscillatory neural activity across the basal ganglia-thalamocortical motor (BGTC) network. Movement-related suppression of the power of local field potential (LFP) oscillations in the alpha (α, 8-12 Hz) and beta (β, 13-35 Hz) frequencies and increased power in gamma frequencies (γ, >40 Hz) have been well-established in sensorimotor cortex as well as in the subthalamic nucleus (STN) and globus pallidus internus (GPi) (Brittain and Brown, 2014; Malekmohammadi et al., 2018a; Miller et al., 2012; Pfurtscheller and Lopes da Silva, 1999; van Wijk et al., 2012). In addition, movement is associated with modulation of phase synchrony between alpha and beta signals in the basal ganglia and sensorimotor cortex (Alegre et al., 2010; Kühn et al., 2006; van Wijk et al., 2017) and cross frequency coupling between alpha/beta phase and gamma amplitude in sensorimotor cortex, STN and GPi (AuYong et al., 2018; de Hemptinne et al., 2013; Kato et al., 2016; Kondylis et al., 2016; Malekmohammadi et al., 2018a; Tsiokos et al., 2013; Yanagisawa et al., 2012). While all these changes are recognized as being movement-related, the functional significance of these distinct signals remains incompletely understood, particularly with respect to which of these signals is critical for and specifically associated with actual movement execution. Such insights are important to understanding the pathophysiology of movement disorders that exhibit abnormalities in these oscillations, such as Parkinson disease (PD), as well as identifying viable and meaningful biomarkers for closed-loop brain stimulation therapies.
Alpha and beta oscillations in the BGTC are often referred to as “anti-kinetic” signals, because they are prominent when the motor system is idling or maintaining a motor state (Engel and Fries, 2010; Stolk et al., 2019; van Wijk et al., 2012). The association of suppression in alpha and beta power with movement speed (Gilbertson, 2005), force (Stančák et al., 1997), and corticospinal excitability (Mäki and Ilmoniemi, 2010; Sauseng et al., 2009) and increased beta with movement inhibition (Solis-Escalante et al., 2012; Swann et al., 2009), suggest a pivotal role in motor execution. However, sensorimotor and STN alpha and beta desynchronization is also observed in the absence of overt movement execution, for example during motor imagery (Brinkman et al., 2014; Kühn et al., 2006; Miller et al., 2010; Pfurtscheller and Neuper, 1997), observation of others moving (Alegre et al., 2010; Gastaut and Bert, 1954; Hari, 2006; Marceglia et al., 2009), and passive movement (Arroyo et al., 1993; Neuper and Pfurtscheller, 2001). Thus, while modulation of alpha and beta oscillations in distinct nodes of the BGTC is clearly important in motor processing, suppression of alpha and beta power does not, in itself, indicate movement or even motor preparation. Further work is therefore needed to disentangle the behavioral significance of alpha and beta oscillations and to identify signals that differentiate actual movement execution from other movement-related changes in BGTC signals.
More recent studies of neural oscillations have demonstrated that movement modulates not only local power, but also the synchronization or interaction of signals across network nodes reflecting communication across neuronal populations. For example, subcortical (STN and GPi) and sensorimotor cortical signals are phase synchronized in the beta band in patients with PD and dystonia (Brown et al., 2001; Cassidy et al., 2002; Litvak et al., 2011; Neumann et al., 2015; de Solages et al., 2010) and this synchronization is suppressed during movement (AuYong et al., 2018; Fischer et al., 2019; van Wijk et al., 2017). In addition, phase amplitude coupling (PAC) – a modulation of the amplitude of one frequency by the phase of another – of alpha/beta phase to gamma amplitude in sensorimotor cortex, STN and GPi (de Hemptinne et al., 2015, 2013; Lopez-Azcarate et al., 2010; Tsiokos et al., 2017; Yang et al., 2014) is dynamically modulated by movement (Kondylis et al., 2016; Malekmohammadi et al., 2018a; Miller et al., 2012; Yanagisawa et al., 2012).
In contrast to extensive studies of local alpha and beta power within specific nodes of the BGTC network related to movement, few studies have examined whether changes in network synchrony in the BGTC occurs in the absence of motor execution, as in the case of motor imagery or action observation (Alegre et al., 2010; Kühn et al., 2006; Marceglia et al., 2009). Emerging literature in patients with PD and other movement disorders suggests that network-level synchronization may be particularly relevant to movement execution, as exaggerated cortical-subcortical synchrony and cross-frequency coupling are related to severity of symptoms and are suppressed by therapeutic stimulation (de Hemptinne et al., 2015; Lopez-Azcarate et al., 2010; Malekmohammadi et al., 2018a, 2018b; Oswal et al., 2016; Tsiokos et al., 2017; Whitmer et al., 2012). This is in contrast to local oscillatory power in PD, which is sometimes reported as increased at baseline and suppressed with therapy (Kühn et al., 2008, 2009, 2006; Oswal et al., 2016; Ray et al., 2008; Whitmer et al., 2012) and other times reported as neither abnormally elevated nor modulated by therapy, particularly in motor cortex (de Hemptinne et al., 2015, 2013; Malekmohammadi et al., 2018a, 2018b; Oswal et al., 2016; Swann et al., 2015). In this study, we compare two motor tasks to test the hypothesis that alpha and beta pallidocortical network synchrony, as opposed to local power, may be of particular importance and specific to overt movement execution. Given well-described modulation of the motor system during observation of movements (Fadiga et al., 1995; Gazzola and Keysers, 2009; Hari, 2006; Kilner and Lemon, 2013; Rizzolatti and Sinigaglia, 2016), passive action observation provides an important tool to disentangle signals related to overt movement execution from those related to activation of the motor system in the absence of overt movement. We therefore recorded simultaneous GPi LFPs and sensorimotor cortex electrocorticography (ECoG) during surgical placement of deep brain stimulation electrodes in patients with PD while they executed and passively observed repetitive hand movements.
While studies have shown that in the STN local power and coupling with motor cortex are suppressed during both overt movement and passive action observation or motor imagery (Alegre et al., 2010; Kühn et al., 2006; Marceglia et al., 2009), pallidal activity has never been compared during overt movement and passive motor tasks. The GPi may be particularly important for gating overt movement execution, as it is the primary output nucleus of the basal ganglia and exerts inhibitory control over motor cortex via the thalamus. We therefore hypothesized that, in contrast to the STN, alpha and beta phase coupling and phase amplitude coupling in the pallidocortical network may demonstrate a relative dissociation between motor execution and action observation. In addition, as recent studies suggest functional dissociations between low (~12-20hz) and high (~20-35hz) beta bands in the BGTC network (Tsiokos et al., 2017; van Wijk et al., 2016), we also examined whether movement specificity is differentially represented in specific sub-bands within the alpha/beta range. Characterizing potential differences between BGTC network nodes is important for the refinement of neuromodulation strategies for movement disorders. Though high frequency stimulation of both the STN and GPi are effective in treatment of the cardinal motor signs in PD, differences in cognitive and psychiatric side effects, dopaminergic medication requirements and anti-dyskinetic effects clearly indicate at least partially distinct neurophysiology; improved understanding of differences between neuromodulation targets can inform individualization of future therapies.
2. Materials and Methods
2.1. Patients
Electrophysiological activity from right GPi DBS electrodes and right motor cortex electrocorticoraphy (ECoG) was recorded simultaneously during periods of rest, action execution and action observation in 10 right-handed patients with idiopathic PD. 2 additional right-handed PD patients underwent the same procedure during DBS implantation of the bilateral STN and contributed data only from motor cortex ECoG (Table 1). All recordings were obtained intraoperatively during awake DBS implantation for treatment of PD after overnight withdrawal of dopaminergic medications. Subdural ECoG strips were placed temporarily for research purposes and removed after the task and prior to completion of the surgery. Motor cortical ECoG data from 1 GPi patient was not available due to technical error during signal acquisition. In total, analyses described include GPi signals from 10 patients, motor cortex signals from 11 patients, and simultaneous GPi/motor cortex signals from 9 patients. Prior to the studies, all subjects provided written informed consent, as approved by the institutional review board at the University of California, Los Angeles.
TABLE 1.
Patient demographics
| Subject | Age | Gender | Disease duration (years) | UPDRS (OFF) | Recording Sites* |
|---|---|---|---|---|---|
| 1 | 63 | M | 7 | 32 | Motor cortex, GPi |
| 2 | 78 | F | 14 | na | Motor cortex , GPi |
| 3 | 65 | M | 8 | na | Motor cortex , GPi |
| 4 | 66 | M | 9 | 35 | Motor cortex , GPi |
| 5 | 60 | M | 5 | 22 | Motor cortex , GPi |
| 6 | 70 | M | 12 | 40 | Motor cortex , GPi |
| 7 | 71 | M | 6 | 27 | Motor cortex , GPi |
| 8† | 80 | M | 16 | 28 | Motor cortex , GPi |
| 9† | 61 | M | 5 | 31 | Motor cortex |
| 10† | 59 | F | 16 | 71 | Motor cortex |
| 11† | 68 | F | 10 | 30 | Motor cortex, GPi |
| 12† | 61 | M | 8 | 44 | GPi |
right hemisphere, contralateral to side of movement
na = pre-operative UPDRS scores not available, GPi = Globus pallidus internus
subset of patients who also performed a visual control task
2.2. Behavioral Tasks
All subjects performed each of the execution and observation tasks once, with the order of tasks counterbalanced across participants. Each task involved 30-second blocks of rhythmic left hand opening/closing movements at maximum amplitude with fastest comfortable speed alternating with 30-second rest blocks. Movement/rest periods were cued verbally by the experimenter. In the execution task, patients performed the action with the left hand while wearing a kinematic sensor glove with five piezoelectric sensors that measure finger flexure (5DT data glove 5 Ultra, 5DT Inc., Irvine, CA, USA). In the observation task, patients remained completely still while observing a healthy individual (author NP) perform the same action with the left hand while wearing the kinematic glove. Subjects were instructed to remain as still as possible while keeping their eyes open during rest and observation blocks and were monitored for compliance by the experimenter. Execution and observation tasks consisted of 4 to 6 blocks each of rest and movement.
A subset of patients also performed a control observation task in which the stimulus depicted rhythmic visual motion without any human action to begin to explore whether changes associated with action observation were specific to observation of action or related simply to observation of any moving stimulus (see Table 1; 3 GPi recordings, 4 motor cortex recordings). In this visual control task, patients passively observed 30-second videos of a ball bouncing at similar frequency to the movements in the observation task alternating with 30-second rest blocks in which they looked at a fixation cross. Four blocks each of ball and fixation conditions were presented. The task was performed in counterbalanced order with observation and execution tasks across subjects. Overall, patients performed either 2 (execution, observation) or 3 (execution, observation, visual control) tasks, each lasting 4-6 minutes.
2.3. Neurophysiologic and kinematic signal acquisition
GPi LFP were captured via the four ring electrode contacts on the DBS leads at the target position (Model 3387, 1.27 mm lead body diameter, contact length 1.5mm, inter-contact distance 1.5mm, Medtronic, Inc., Minneapolis, MN, USA). The DBS lead was targeted to motor (ventral posterolateral) GPi using image-guided targeting, 2-4 mm anterior, 19-24 mm lateral and 4-6 mm inferior to the mid-commissural point (accounting for individual anatomy). All trajectories were confirmed with intraoperative microelectrode recordings of neuronal activity (Israel and Burchiel, 2004) and awake macrostimulation testing of therapeutic and side-effect thresholds at each electrode. Unilateral right sensorimotor electrocorticogram (ECoG) recordings were performed via a non-penetrating subdural strip electrode consisting of eight 4 mm platinum contacts with 1 cm inter-contact spacing (Ad-Tech Medical Instruments, Wisconsin, WI, USA). The ECoG electrode strip was introduced through the same burr hole used for DBS electrode implantation and advanced posteriorly past the central sulcus. Ground and reference electrodes were attached to the scalp. Left hand movements synchronized to neural recordings were transduced via the kinematic sensor glove worn by the patient (execution task) or experimenter (observation task). Neurophysiologic and glove signal acquisition was performed using BCI2000 v2 or v3 connected to a standalone amplifier (g.Tec, g.USBamp 2.0) with a sampling rate of 2400 Hz following 0.1 Hz-1000 Hz online band-pass filter.
2.4. Anatomical localization of DBS leads and ECoG electrode strip
In addition to intraoperative neurophysiological localization, anatomical localization of GPi DBS electrode contacts was performed using Lead-DBS software for 8 of 10 subjects (post-operative imaging was not available for the remaining 2 subjects) (Horn et al., 2019). Postoperative CT scans were co-registered to preoperative T1-weighted structural MRI (MPRAGE, slice thickness 1mm, 3T Siemens Skyra) with two-stage linear registration (rigid followed by affine) using the SyN registration approach as implemented in advanced normalization tools (ANTs) (Avants et al., 2008). Automated reconstruction of electrode trajectory and contact locations was performed using the PaCER toolbox (Husch et al., 2018). Electrode locations were then confirmed on the high resolution structural MRI in patient native space. The most ventral pair of electrodes were verified to be located within the GPi and bipolar LFP recordings from these 2 most ventral electrodes were used for all analyses.
Anatomical localization of the ECoG electrode contacts was performed using 2D/3D fusion techniques adapted from Randazzo et al. (Randazzo et al., 2016) by registering the intra-operative fluoroscopic image to the reconstructed cortical surface of the pre-operative MRI. The pair of electrodes immediately anterior to the central sulcus was identified as motor cortex and bipolar ECoG signal from this pair were used for subsequent analyses (Figure 1A). Counting from the most posterior ECoG contact, the M1 bipolar pairs were 4/5 (n=2), 5/6 (n=4) and 6/7 (n=3). For two patients localization was not possible due to missing postoperative imaging or intraoperative fluoroscopy data; for these patients contacts with greatest beta modulation during movement were selected as a proxy for primary motor cortex (Hari and Salmelin, 1997) (contacts 4/5 in both subjects).
Figure 1.
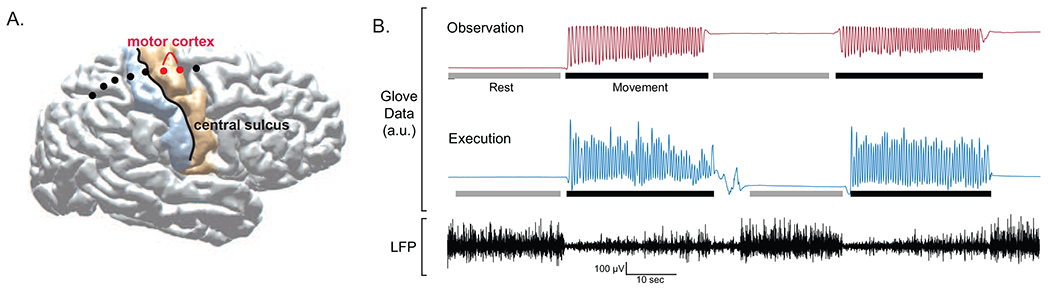
(A) Example of individual subject cortical reconstruction with electrocorticography (ECoG) electrode localization. Motor cortex recordings were obtained from bipolar electrode pair immediately anterior to the central sulcus. (B) Example glove data (top) and local field potential (LFP) recording from motor cortex during execution block. Bars below glove data indicate periods selected for rest (gray) and movement (black) periods. Movement was defined by onset and offset of clear rhythmic finger flexions. Rest periods were defined by absence of movements with brief (~1s) padding before and after rhythmic movement onset to account for transition period during which patient lifted arm from resting position. (a.u. = arbitrary units.)
2.5. Neurophysiologic Signal Processing
Preprocessing
Right motor ECoG and right GPi LFPs were analyzed along with concurrent left hand kinematic data in Matlab (Mathworks Inc., Natick, MA) utilizing the Fieldtrip toolbox (Oostenveld et al., 2011) and custom scripts. Raw ECoG and LFPs were lowpass filtered at 400 Hz and highpass filtered at 1 Hz using onepass zero-phase FIR filter (lowpass transition band width 100, highpass transition band width 2) (Widmann et al., 2015). Line noise at 60 Hz and harmonics were filtered out with bandstop Butterworth filter (order 4). Bipolar re-referencing between adjacent electrodes was then performed to emphasize local voltage changes and minimize common noise. By careful visual inspection of the movement traces recorded by the data glove, electrophysiological data were segmented into rest and movement blocks (Figure 1B). LFP data from selected rest and movement blocks were then visually inspected for electrical artifacts (sudden large amplitude shift) while blinded to condition. Blocks with at least 16 seconds of contiguous artifact-free data were included in analyses. Rest and movement block lengths were then matched for each task condition within subjects by truncating longer blocks to remove any difference in timepoints from contributing to condition differences. This resulted in an average of 115 seconds per condition (range 65-154).
Preprocessed LFP and ECoG data were decomposed into their time-frequency representation by convolution with a set of complex Morlet wavelets. The wavelet family was defined as a set of Gaussian-windowed complex sine waves at 100 logarithmically spaced frequencies between 2 Hz and 300 Hz. For power analyses, wavelet width was 10 cycles for frequencies under 40 Hz and 25 cycles for frequencies above 40 Hz to minimize frequency smoothing and allow for identification of sub-bands within the alpha/beta range. For phase coupling and cross frequency coupling measures requiring instantaneous phase and amplitude values, wavelet width was increased from 3 to 15 cycles in logarithmically spaced steps to emphasize temporal precision (Cohen, 2014). The resulting complex analytic signals provided the input for subsequent power, phase synchronization and phase amplitude coupling analyses.
Power Spectral Density and Peak Estimation
Power spectra for each of the 4 conditions (Execution/Observation x Movement/Rest) within each subject were obtained by taking the squared complex magnitude of the analytic signal and averaging over time within each block, and subsequently over blocks within each of the 4 conditions for each subject. To minimize the inter-electrode and inter-subject baseline power differences, for each task the resulting raw power spectra were normalized by dividing each frequency by the total power at rest between 6 and 200 Hz (excluding line noise and harmonics) and converted to decibel scale(AuYong et al., 2018; Malekmohammadi et al., 2018a, 2018b).
We were interested in potentially dissociable frequency bands within the broader alpha/beta range (8-35 Hz) known to covary with execution and observation tasks. However, as frequency cutoffs used in the motor neurophysiology and PD literatures are variable (common examples are 8-14 Hz and 15-25 Hz in motor physiology and 12-20 Hz and 20-35 Hz in PD), to select relevant sub-bands within this range for use in the ANOVA we used the following data-driven approach similar to previous studies in PD that have identified dissociable “low beta” and “high beta” bands (Tsiokos et al., 2017; van Wijk et al., 2016). Peaks were identified in the power spectra for each subject, task condition and region (9 patients x 4 conditions x 2 regions and 3 patients x 4 conditions x 1 region = 84 spectra) between 8 and 35hz using matlab “peakfind.m” function and confirmed with visual inspection. This was performed across all conditions and brain regions to avoid biasing subsequent statistics. A histogram of the identified peaks showed a bimodal distribution (Figure 2) separating the window from 8-35 Hz into two bands, alpha/low beta (α/low-β) and high beta (high-β), without a clear distinction between alpha and low beta. To identify the specific cut-off frequency between these two bands, a mixture of two Gaussian distributions was fitted using the Matlab curve fitting toolbox and the lowest point between the two distributions (17.9 Hz) used as the boundary between two distinct frequency bands centered at 13.0 Hz (α/low-β, individual subject peaks range 8.7-17.6 Hz) and 23.4 Hz (high-β, individual subject peaks range 18.5-29.2 Hz). Examination of the Akaike information criterion values confirmed that the bimodal mixture model was superior to either single Gaussian distribution or a mixture of 3 Gaussian distributions. Group level frequency band analyses described below were performed on signals averaged over frequencies within the two bands defined by this procedure (same frequency band for all subjects).
Figure 2.
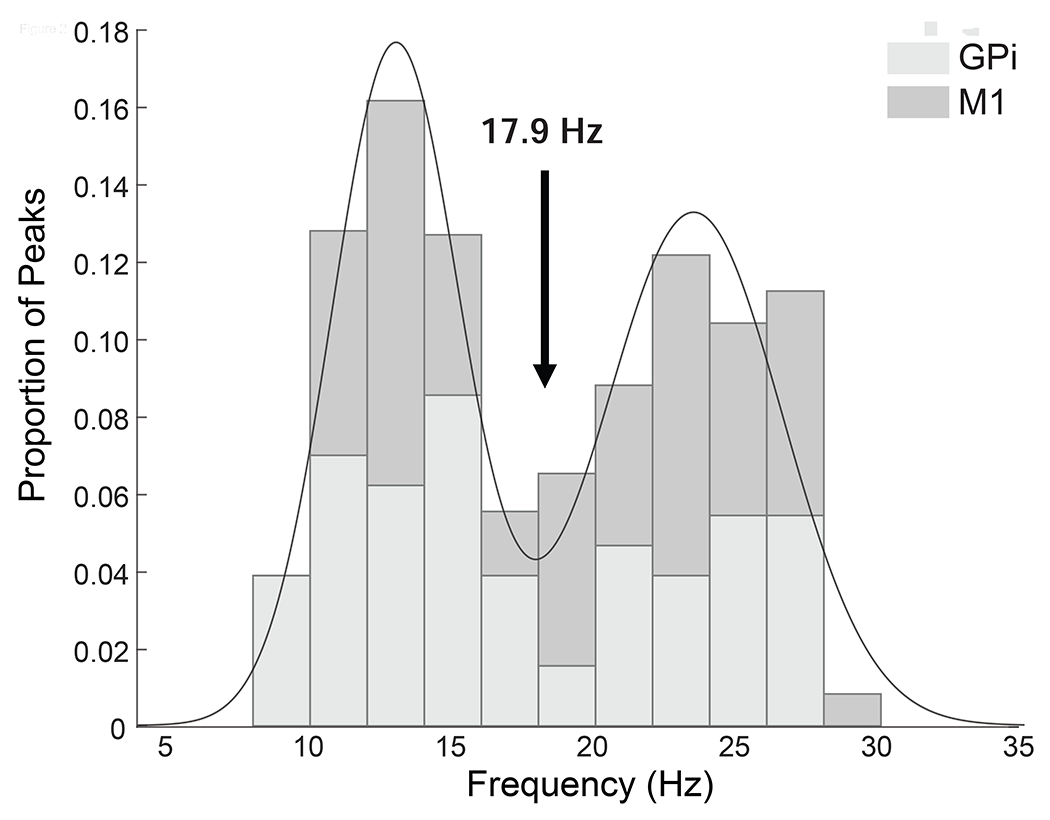
Histogram of individual subject peak frequencies within alpha/beta range (8-35 Hz). Line shows mixture of 2 gaussian models with local minimim between distributions defining border between identified sub-bands. Shading indicates globus pallidus internus (GPi) and motor cortex (M1) contribute to both frequency peaks.
Phase Coupling
Phase based connectivity between GPi and motor cortex was evaluated using the phase locking value (PLV) (Lachaux et al., 1999), which is defined as the magnitude of the mean phase difference between the two signals expressed as a complex unit-length vector according to the formula where is the phase difference between the two signals at time t. If the signals’ phases are completely independent, the relative phase will have a uniform distribution over time and the PLV is zero. Conversely, if the phases of the two signals are strongly coupled then the relative phase (i.e. phase difference) will be clustered and PLV will approach one. PLV was chosen as the measure of interregional coupling, because in contrast to cross-spectral coherence, theoretical studies suggest that changes in PLV are minimally affected by fluctuations in power unless these are close to zero for prolonged periods(Cohen, 2014). Similar to power analyses, PLV was calculated for each frequency in each block, and subsequently averaged over blocks within each of the 4 conditions for each subject.
Cross-frequency phase amplitude coupling
Local cross frequency PAC in GPi and motor cortex was calculated using the debiased PAC (dPAC) method (van Driel et al., 2015; Lin et al., 2006), which is comparable to other commonly used methods but may have higher sensitivity in the presence of noise (van Driel et al., 2015). dPAC describes interactions between activity at different frequencies in which the amplitude of a signal at one frequency is modulated by the phase of a lower frequency signal. In the BGTC network, coupling between alpha/beta phase and gamma amplitude is modulated by movement (AuYong et al., 2018; de Hemptinne et al., 2013; Kato et al., 2016; Kondylis et al., 2016; Tsiokos et al., 2017; Yanagisawa et al., 2012) and we therefore focused on these frequency bands. To calculate the dPAC, instantaneous phase angles were extracted as the angle of the analytic signal at the frequencies of interest for phase (4-40Hz) and corresponding instantaneous amplitudes were extracted as the squared absolute value of the analytic signal at the frequencies of interest for amplitude (30-300 Hz). Vectors in polar space were then defined at each timepoint by the angle of the frequency for phase and the length by the power of the amplitude-modulated frequency. To reduce any potential bias introduced by non-uniformity in the phase angles of frequency for phase, a debiasing term is introduced by subtracting the average vector of the modulating phase angles (Van Driel, 2015). The length of the average of power-adjusted phase angle vectors over time provides the dPAC, which is mathematically defined as where , n is the number of time points, at the amplitude of the modulated frequency at time t and is the phase of the modulating frequency at time t. dPAC is zero if there is no relationship between power and phase and is greater than zero when there is a relationship between power and phase. To normalize across frequencies and patients, dPAC values were transformed into z-values at the single subject level by comparing against surrogate values from 1000 permutations in which the power values were shuffled relative to the phase values (Canolty et al., 2006; Cohen, 2014). For frequency band analyses, gamma frequencies in the amplitude modulated signal were separated into low gamma (50-90 Hz), high gamma (90-200 Hz,) and high frequency oscillations (HFO, 200-300 Hz), motivated by prior studies demonstrating movement-modulated PAC in PD between beta and low gamma/HFO frequencies in the GPi (AuYong et al., 2018; Tsiokos et al., 2017) and between beta and high gamma in motor cortex (de Hemptinne et al., 2013; Kondylis et al., 2016).
2.6. Group Statistical Analyses
We first aimed to define which frequency bands were significantly modulated by movement during observation and execution for each neurophysiological measure (normalized power, PLV, dPACz) across the entire frequency space. Movement and rest blocks were compared within each recording site (GPi, M1) and task (Execution, Observation) using permutation based non-parametric paired tests at the group level with cluster correction for multiple comparisons across frequencies. Implementation of paired tests between movement and rest blocks was accomplished by creating a null distribution of the condition difference by swapping the sign of a subset of pairwise differences in all possible permutations ( permutations for M1, 512 permutations for GPi)(Cohen, 2014; Maris and Oostenveld, 2007; Nichols and Holmes, 2002). P-values were then defined by the probability of the observed condition difference based on the permuted null distribution, with a two-tailed probability of less than 5% considered significant. To correct for multiple comparisons across frequencies, a null distribution of maximum cluster size (number of significant neighboring frequencies based on two-tailed p<0.05) was obtained using the same permutations and only those observed cluster sizes with a probability of less than 5% as compared to the null distribution were considered significant (Maris and Oostenveld, 2007; Nichols and Holmes, 2002).
Next, to determine whether differences between tasks and frequency bands were significant, we performed repeated measures ANOVA for each neurophysiologic measure using the magnitude of signal change (Movement – Rest difference score) averaged over selected frequency bands (α/low-β, 8-17.9 Hz; high-β, 18-35 Hz) as the dependent variable and factors frequency band and task (Execution, Observation) as repeated measures.
Separate ANOVAs were performed for each measure (normalized power, PLV and dPACz) and brain region (M1, GPi). For power and PLV, task (Execution, Observation) and frequency band (α/low-β and high-β) were included as two repeated measures factors. For dPAC three repeated measures factors were task (Execution, Observation), phase encoding frequency band (α/low-β and high-β) and amplitude modulated frequency band (low γ, high γ and HFO). Post-hoc contrasts investigating significant effects were carried out (STATA contrasts command) and p-values corrected for multiple comparisons using Bonferroni method (reported as pcorr). In addition, planned comparisons to determine significant movement suppression within each data-driven frequency band was also assessed by comparing the magnitude of “Movement - Rest” neurophysiologic signal change to zero using one-sample Wilcoxon signed-rank tests (one-tailed given directional hypothesis) with Bonferroni correction for multiple comparisons. These analyses were implemented in Stata 14 software (StataCorp LLC, College Station, TX).
3. Results
3.1. Power is suppressed during action execution and action observation
Comparisons of spectral power between rest and movement during Execution and Observation are shown in Figure 3. Patterns of power modulation were similar in the M1 and GPi with movement-related suppression of alpha and beta power during both Execution and Observation. Non-parametric permutation tests across the frequency space demonstrate that whereas execution led to suppression of a broad band of alpha and beta frequencies (5-35 Hz) in both GPi and motor cortex, observation was associated with significant suppression of a narrower band (8-20 Hz) including alpha and low beta, but not high beta frequencies, in both regions. Results were equivalent when comparing each task’s movement block to a single rest power spectrum (averaged across tasks), confirming that observed differences were due to differences in movement modulation and not rest period activity. Power spectra from immediately adjacent bipolar ECoG contacts (premotor cortex and post-central gyrus) demonstrate that alpha/beta modulation is present but of smaller magnitude in postcentral gyrus and absent in premotor cortex (Supplemental Figure S1), consistent with prior literature demonstrating movement-related suppression in these frequencies is centered over sensorimotor cortex and not due to volume conduction (Crone et al., 1998; Hari et al., 1998; Miller et al., 2010; Ohara et al., 2000).
Figure 3.
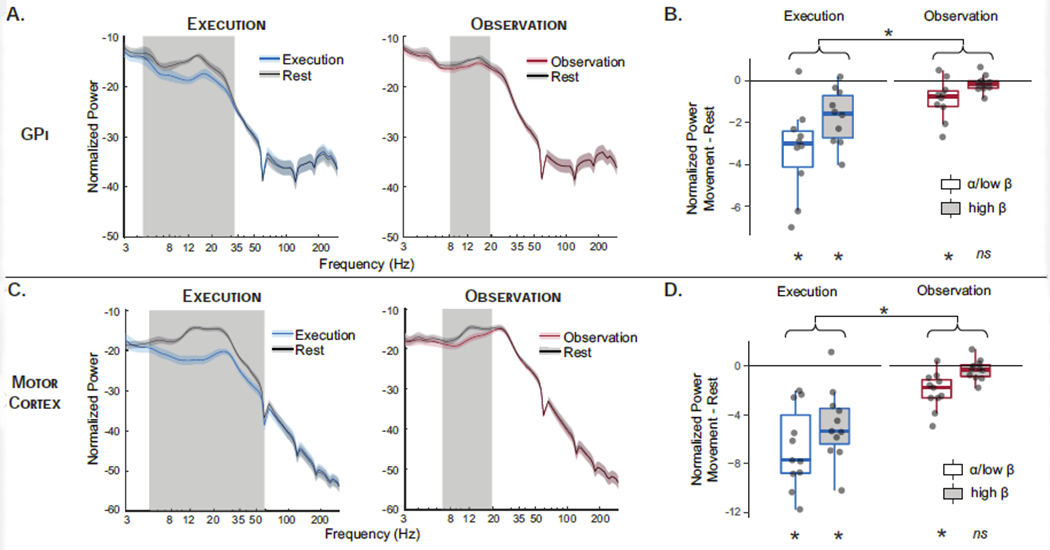
Spectral Power. Normalized spectral power in globus pallidus internus (GPi) (A) and motor cortex (C) across frequencies for movement and rest blocks during execution (blue) and observation (red). Gray shading indicates frequencies in which movement and rest are significantly different by non-parametric permutation testing with cluster correction for multiple comparisons across frequencies. Boxplots show band-averaged power modulation (α/low-β = 8-17.9 Hz; high-β = 18-35 Hz) in GPi (B) and motor cortex (D) during movement relative to rest (movement – rest change score) for execution (blue) and observation (red) tasks. Significant modulation by movement (e.g. significant difference from zero by Wilcoxon signed rank tests) is indicated at bottom. Main effect of task is indicated at top; main effect of frequency band was also significant but not shown for readability. Given the absence of a two-way interaction, simple effects were not tested. (* p<0.05 Bonferroni corrected, ns non-significant.)
Similar effects were demonstrated in statistical tests of band-averaged power, extracted from α/low-β (8-17.9 Hz) and high-β (18-35 Hz) frequency bands (Figure 3B/D). In motor cortex, significant movement-related suppression (i.e. Movement - Rest < 0 by one-sample Wilcoxon signed rank test) was observed during execution in both α/low-β (z=2.93, pcorr=0.002) and high-β (z=2.845, pcorr=0.004) whereas during observation only α/low-β (z=2.845, pcorr=0.004) was significantly suppressed (high-β, z=1.33, pcorr=0.41). The same pattern was observed in the GPi with significant movement-related suppression in all frequency bands except high-β (Execution: α/low-β z=2.70, pcorr=0.008; high-β z=2.70, pcorr=0.008. Observation: α/low-β z=2.40, pcorr=0.03; high-β z=1.27, pcorr=0.46).
2x2 repeated measures ANOVA directly comparing the effects of frequency band (α/low-β, high-β) and task (Execution, Observation) on Movement-Rest difference score were significant in both regions (GPi F12=5.0, p<0.001; M1 F13=6.39, p<0.001). Similar patterns were observed in GPi and M1 with significant main effects of frequency band (GPi F1,9=10.1, p=0.004; M1 F1,10=8.2, p=0.008) and task (GPI F1,9= 28.6, p<0.001; M1 F1,10=53.2, p<0.001), reflecting greater movement-related power suppression for execution compared to observation (GPi t=3.18, p=0.004; M1 t=7.29, p<0.001) and for α/low-β compared to high-β (GPi t=5.34, p<0.001; M1 t=2.86, p=0.008). The interaction between frequency band and task was not significant, indicating that the absence of modulation of high-β power during observation may have resulted from the overall smaller magnitude of power changes during observation as compared to execution. Similar effects are observed when using 4 hz frequency bands centered on subject-specific peaks defined by Rest power spectra (Supplemental Figure S2). In GPi, peaks were present in 9/10 patients in the α/low-β band and 6/10 patients in the high-β band.
Qualitative comparison of power during action observation and ball observation in the subset of patients who performed the visual control task (n=3 GPi, n=4 M1) shows minimal suppression of alpha/beta power during observation of a moving ball as compared to during action observation in these subjects (Figure 4).
Figure 4.
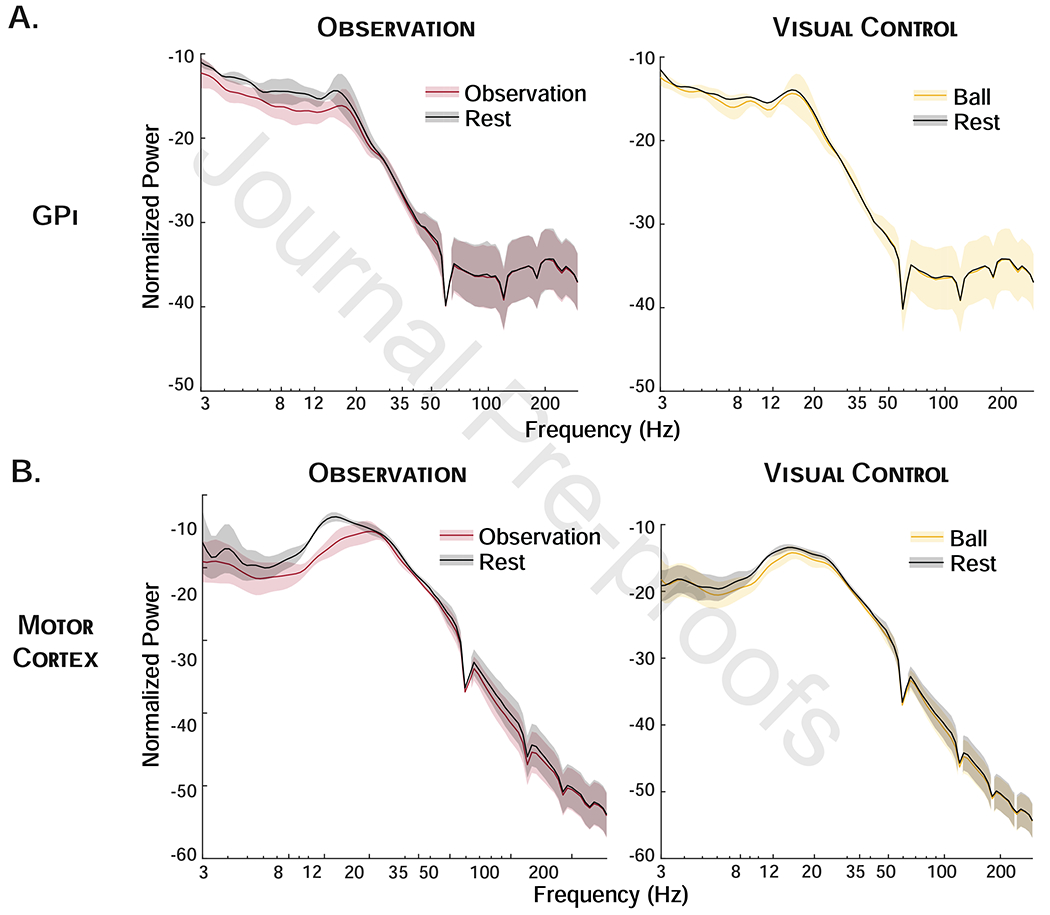
Visual Control Task. Normalized spectral power in globus pallidus internus (GPi) (A) and motor cortex (B) across frequencies for movement and rest blocks during observation (red) and ball observation (visual control, yellow) in subset of patients performing both tasks show greater suppression during action observation than ball observation.
3.2. GPi-M1 phase coupling is suppressed during action execution but not action observation
Significant suppression in phase coupling (PLV) between GPi and M1 was observed in α/low-β (9-14 Hz) during action execution. In contrast, during the action observation task there was no significant suppression of pallidocortical phase coupling (Figure 5A). Again, there were no significant differences between rest conditions in the two tasks and results were equivalent when comparing each task’s movement block to the same rest data obtained by averaging rest periods across tasks.
Figure 5.
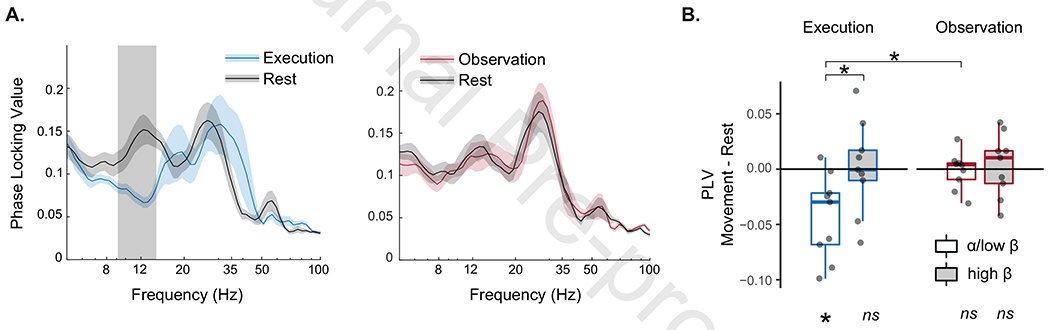
Globus pallidus internus – motor cotex (GPi-M1) phase locking value (PLV). (A) PLV across frequencies for execution and observation tasks shows significant movement-modulated PLV only during execution limited to the α/low-β band. Gray shading indicates frequencies in which movement and rest are significantly different by non-parametric permutation testing with cluster correction for multiple comparisons across frequencies. (B) Boxplot shows GPi-M1 PLV modulation during movement relative to rest (movement – rest change score) during execution (blue) and observation (red). Significant modulation by movement (e.g. significant difference from zero) is shown at bottom, and is present only in the α/low-β band during execution. Paired contrasts from significant task x frequency band interaction are shown at top. (* p<0.05 bonferroni corrected; ns = non-significant.)
Planned comparisons of band extracted PLV corroborate these results, with significant modulation of phase coupling present only in the α/low-β band during movement execution (one-sample Wilcoxon signed rank test, z=2.43. pcorr=0.02) and no significant modulation of the high-β band during execution, or any α/β modulation during observation (all pcorr=1) (Figure 5B).
The 2x2 repeated measures ANOVA examining the effect of frequency band (α/low-β, high-β) and task (Execution, Observation) was significant (F11=3.36, p=0.006). There was a significant interaction between condition and frequency band (F1,8=4.942, p=0.046). Post-hoc contrasts demonstrate greater suppression in α/low-β than high-β during execution (t=3.35, pcorr=0.02) and no difference between frequency bands during observation (t=0.38, pcorr=1); comparisons between tasks also demonstrated greater suppression of α/low-β coupling during execution than observation (t=3.15, pcorr=0.03) and no difference in suppression of high-β (t=0.17, pcorr=1). Importantly, the PLV measure is independent of power (as long as power is not close to zero for prolonged periods)(Cohen, 2014). Furthermore, changes observed in the power spectrum and PLV dissociated, in that suppression of high beta power during action execution was not associated with a change in phase coupling. Therefore, these findings cannot be explained by changes in power.
3.3. Motor cortex and GPi alpha/beta-gamma PAC are suppressed during action execution but not action observation
Paired comparisons of dPAC between movement and rest for each recording site and condition across the entire frequency space using non-parametric permutation testing demonstrated significant suppression of coupling between alpha/beta phase and low gamma and HFO amplitude in GPi, and with low and high gamma in motor cortex during movement (Figure 6A/C). In contrast, there was no significant movement-related change in dPAC during action observation in the GPi or M1.
Figure 6.
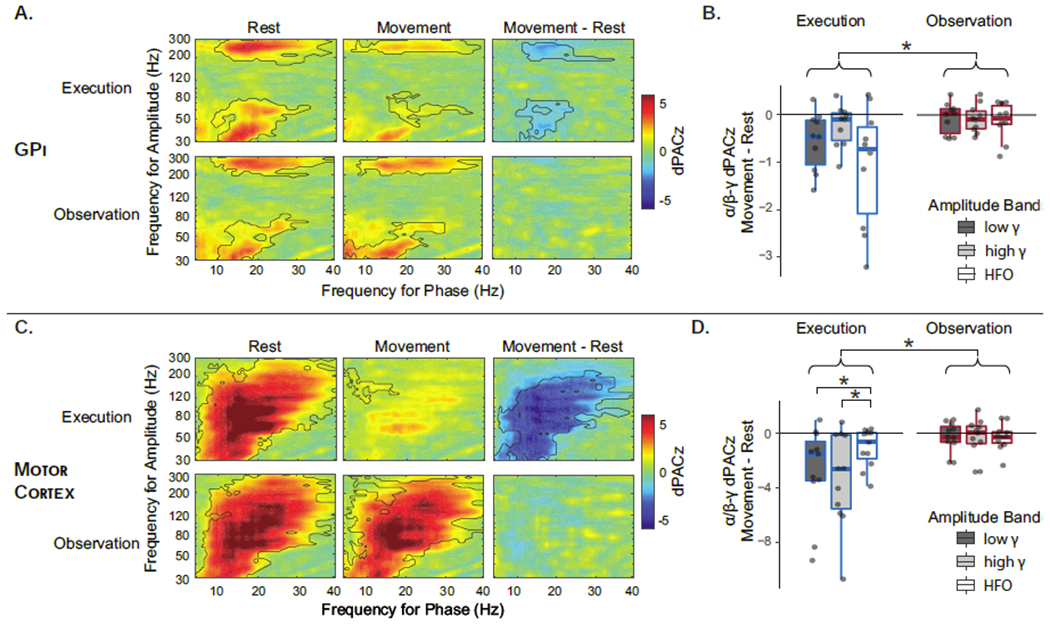
Phase amplitude coupling between alpha/beta phase and gamma/HFO (high frequency oscillation) amplitude. Debiased phase amplitude coupling z-score (dPACz) in globus pallidus internus (GPi). (A) and motor cortex (C) during movement, rest and their difference demonstrate significant coupling during rest that is suppressed by execution and not by observation. Black outlines indicate significant clusters by non-parametric permutation testing. Boxplots show dPACz modulation in GPi (B) and motor cortex (D) during execution (blue) and observation (red), stratified by amplitude band only as the effect of phase frequency band was not significant for either region. Significant post-hoc contrasts are indicated at top (main effect of task for both regions and trend in task x amplitude band interaction for motor cortex only). (*= p<0.05 post-hoc contrast, Bonferroni corrected.)
3-way repeated measures ANOVA examining the effects of phase encoding frequency band (α/low-β, high-β), amplitude modulated frequency band (low-γ, high-γ, HFO) and task (execution, observation) on movement-related modulation were significant in both M1 (F21=4.69, p<0.001) and GPi (F20=2.27, p=0.004)(Figure 6B/D). In motor cortex, there was a significant main effect of task (F1,10=32.9, p<0.001) and trends toward significance in the main effect of amplitude band (F2,10=3.19, p=0.056) and the interaction between task and amplitude band (F2,10=3.06, p=0.064). Post-hoc contrasts indicate that suppression was greater during Execution than Observation (t=5.44, pcorr<0.001) and differences in suppression across amplitude frequency bands was present only during Execution (F2,10=6.23, pcorr=0.003) and not during Observation (F2,10=0.02, pcorr=0.98); during Execution, suppression was greater in the high-γ and low-γ bands as compared to HFO (t=3.02, pcorr=0.009 and t=3.09, pcorr=0.008, respectively). In the GPi, only the main effect of task was significant (F1,9=12.69, p<0.001), again due to greater suppression during Execution than Observation (t=3.56, p<0.001). A trend toward significance was present for the main effect of amplitude modulated frequency band (F2,9=3.03, p=0.053) with greater movement suppression for HFO compared to high gamma (t=2.43, p=0.05). Interactions between task and frequency bands were not significant in the GPi. This is may be due to lack of power for the relatively smaller effect sizes, as comodulograms (Figure 6A/C) suggest predominance of suppression in the low-γ and HFO frequencies in the GPi as previously described (AuYong et al., 2018; Tsiokos et al., 2017).
4. Discussion
Several previous studies indicate that alpha and beta oscillations in motor cortex and STN are modulated by motor tasks that do not involve overt movement execution (Alegre et al., 2010; Brinkman et al., 2014; Gastaut and Bert, 1954; Hari et al., 1998; Kuhn et al., 2006; Marceglia et al., 2009; Miller et al., 2010; Pfurtscheller and Neuper, 1997), suggesting that these signal changes are not specific to actual movement execution. Given report of similarities across tasks, there remains an outstanding question of neurophysiological mechanisms differentiating action execution and action observation, which has implications both for our understanding of motor control and for identifying biomarkers for therapeutic development. Understanding basal ganglia contributions to motor control requires characterization of activity across nodes that are known to be differentially connected and likely have at least partially separable functions. A notable gap in prior work is the absence of studies assessing GPi activity in motor tasks in the absence of overt movement execution. In contrast to previous observations in the STN, we hypothesized that alpha/beta network oscillations in the pallidocortical circuit may be modulated specifically by movement execution, given the GPi’s unique position as the primary output nucleus of the basal ganglia which exerts inhibitory control over the motor system. Our results demonstrate that, in the pallidocortical network, suppression of both local cross frequency coupling and interregional phase coupling occurred only during overt movement, whereas both overt execution and passive action observation were associated with power suppression, although with different magnitudes. In addition, we show for the first time that power suppression in alpha/low beta frequencies during action observation extends to the GPi node of the motor system.
Suppression of local cross frequency and inter-regional phase coupling is specific to movement execution
The dissociation of action execution and observation by pallidocortical phase coupling suggests that pallidocortical network interactions are more closely related to movement execution than is power modulation within the individual network nodes. Of note, this observation is in contrast to STN power and STN-cortical interactions described in previous studies, which were suppressed across both active and passive motor tasks including action observation (Alegre et al., 2010; Marceglia et al., 2009) and motor imagery (Fischer et al., 2017; Kühn et al., 2006). We posit that this reflects the unique role of the GPi as the final common output of the basal ganglia, providing a potential mechanism in which gating of actions represented in motor cortex is released through suppression of functional connectivity between GPi and motor cortex. This is consistent with therapeutic suppression of pallidocortical phase coupling which is associated with decreased rigidity and more fluid movements in patients with PD (Malekmohammadi et al., 2018b).
Similar to cross region coupling, local alpha/beta-gamma PAC in both GPi and motor cortex is suppressed during execution but not during action observation. Previous studies in patients without movement disorders (epilepsy patients) have also demonstrated suppression of motor cortex alpha/beta-gamma PAC with movement (Miller et al., 2012; Yanagisawa et al., 2012), and it is suggested that PAC in the motor cortex may represent a mechanism for controlling movement execution, in which pro-kinetic gamma signals are released from the constraint of alpha/beta phase to allow motor processing (de Hemptinne et al., 2013; Yanagisawa et al., 2012). The excessive PAC observed in PD, may therefore be one mechanism for reduced motor output due to failure of release of pro-kinetic processing (de Hemptinne et al., 2015, 2013). Our results are in line with this view, given the specificity of PAC suppression to active motor states.
The specificity of coupling measures to motor execution in this task is also supportive of the view that it may be the heightened network coupling in the β band that contributes to motor symptoms in PD, rather than the degree of local β power. The use of a phase-based measure of coupling largely insensitive to power changes, as well as distinct patterns of suppression of PLV and PAC across bands and tasks relative to power changes, argue against these effects simply being epiphenomena of local power modulation. We were unable to identify relationships between network coupling and movement phase or kinematics during execution in the present study, though this is limited by a lack of manipulations of kinematic features in the present task design. An alternative explanation for execution-specific changes is that they reflect the sensory reafference present only during overt execution. However, this seems less likely given previous observations that movement speed is correlated with pallidocortical coupling (van Wijk et al., 2017) and PAC (Malekmohammadi et al., 2018a).
Action mirroring in the basal ganglia
Similar activity in neural systems for movement when actions are performed and when they are passively observed has been proposed to provide an efficient mechanism for understanding others’ actions through simulation – observed actions are matched to the observers own motor representation of that action (Rizzolatti and Sinigaglia, 2016). Despite a central role of BGTC loops in motor processing and a recent interest in action observation therapies for a variety of motor disorders with prominent basal ganglia dysfunction including PD (Buccino, 2014; Caligiore et al., 2017; Pelosin et al., 2018, 2010), only few studies have examined whether shared mechanisms for observation and execution of actions extend to subcortical motor regions. Several meta-analyses of neuroimaging studies have suggested that activity during action observation is limited to cortical regions, in contrast to motor execution and motor imagery which also activate subcortical areas (Caspers et al., 2010; Hardwick et al., 2018). However, direct recordings from human STN (Alegre et al., 2010; Marceglia et al., 2009), and now our current data from the GPi, suggest that there may indeed be modulation not detectable by non-invasive neuroimaging methods and that other mechanisms are responsible for differentiating execution and observation.
In this study, we provide the first evidence that spectral power in the GPi is modulated similarly during action observation and execution in parallel to the power modulations previously observed in sensorimotor cortex and STN. Several hypotheses have been put forth regarding the potential role for the basal ganglia during action observation. For example, the basal ganglia may act to reduce or suppress the activity of pyramidal tract neurons to prevent motor output when the motor system is activated by action observation (Bonini, 2017), though the suppression of beta power observed here is less consistent with this view given beta suppression is associated with pro-kinetic functions. Alternatively it has been proposed that the basal ganglia may select between multiple potential action representations evoked by an observed action (Caligiore et al., 2013). Finally, as the motor GPi output is likely to be involved in the scaling or vigor of actions (Thura and Cisek, 2017; Turner and Anderson, 1997), activity in the GPi during action observation may reflect the representation of the dynamics of observed action, which have been shown to be represented in motor cortical beta power modulation (Press et al., 2011). We were unable to identify kinematic-related changes in power during observation in this study. This may be due to low variability in the amplitude and cycle speed present in our task, and future studies directly manipulating kinematic features will be informative in this regard. Nonetheless, our work adds to increasing evidence that the basal ganglia, including the GPi, is indeed modulated during action observation (Alegre et al., 2010; Marceglia et al., 2009). Further studies are required to disentangle the above hypotheses regarding specific functional significance during action observation, to understand the potentially differential contributions of GPi and STN to mirroring mechanisms, and to confirm whether the GPi power modulations are specific to observation of actions and not to non-specific observation of visual stimuli, as previously described in the sensorimotor cortex and STN.
Dissociation between alpha/low-beta and high beta
Studies in PD have identified functionally distinct bands within the beta frequency range, which classically spans 12-35 Hz oscillations (Oswal et al., 2016; Tsiokos et al., 2017; van Wijk et al., 2017, 2016). Our results provide further support for functionally dissociable bands with 2 distinct peaks (around 13 and 23 Hz in this sample) and distributions, which are differentially modulated by execution and observation. Phase coupling modulation by execution was observed only in the alpha/low-beta band and not the high beta band. In addition, power modulation of alpha/low beta was observed during action observation in the absence of high beta modulation, though whether this is attributable to the overall relatively small magnitude of power modulation by action observation or a true band-specific effect remains unclear, as the suppression of power was overall lower in the observation task and no significant frequency band by task interaction was observed.
In previous non-invasive studies of healthy adults, action observation in motor cortex has been shown to be associated with suppression of power in both a lower alpha rhythm (~7-15Hz) and a higher beta rhythm (~15-30 Hz) (Avanzini et al., 2012; Hari, 2006; Kilner et al., 2009; Press et al., 2011). It is possible that the relative lack of suppression of the high beta band during action observation in the present study is attributable to PD pathology. Beta synchronization in the BGTC network is excessive in PD, and some studies have proposed high beta synchronization in particular is pathologic (AuYong et al., 2018; Malekmohammadi et al., 2018a). The absence of movement-related suppression of high beta oscillations may be related to network-wide hypersynchrony in this band. However, this is speculative, as the pathological nature of bands is difficult to assess given invasive recordings are limited to clinical populations and others have argued instead that low β synchrony is more closely related to PD pathology (Oswal 2016, Wijk 2016, Litvak 2011). Regardless, clear dissociations between low and high beta bands emerge in our comparison of active and passive motor tasks that warrant further consideration in studies of motor neurophysiology in PD.
4.1. Limitations
Invasive recordings are necessarily driven by clinical indications, which limits the populations available for study and therefore the generalizability to the healthy state as well as the sample size. However, similar movement-related modulation of cortical PAC in healthy populations and patients without movement disorders (Babiloni et al., 2016; Hari et al., 1998; Yanagisawa et al., 2012), and of pallidocortical coupling in patients with dystonia (Tsiokos et al., 2017; van Wijk et al., 2017), suggests that the movement specificity of these coupling measures may well extend beyond the Parkinsonian state. We acknowledge that this work does not exclude the possibility that modulation of phase coupling and phase amplitude coupling in the pallidocortical network may be observed in the absence of motor execution in other tasks (e.g. motor imagery) and future studies to test this hypothesis further are warranted. In addition, we cannot completely exclude the possibility that absence of changes in network coupling during action observation is due to insufficient power to detect small effects. However, we must bear in mind that previous studies have identified such changes in other regions (Alegre et al., 2010; Marceglia et al., 2009) suggesting the methods are sensitive to these effects when present. The current data demonstrate movement specificity as compared to observation that has not previously been observed in the STN-cortical network, where changes in network coupling were similar during overt movement and action observation. In addition, future work is necessary to determine whether pallidal alpha/beta suppression is specific to observation of actions; although we observe smaller effects during observation of moving objects, the small sample size in this visual control condition limits statistical inference. Finally, we acknowledge that estimation of phase can be affected by changes in band power, in particular if band power is near zero. However, the analytic techniques have specifically been chosen to minimize this effect (PLV vs coherence) and we highlight that if phase-based results (PLV and PAC) were simply due to underlying changes in power, one would expect results of power, PLV, and PAC to precisely parallel, which they do not. In fact, the dissociation of power and phase-based results highlight the novelty and significance of the current results.
4.2. Conclusions
While motor execution and observation differentially modulate local spectral power in the motor cortex and GPi, both tasks result in discernible and significant modulation of local power. In contrast, coupling in the form of pallidocortical phase locking and local cross frequency coupling is specific to motor execution, suggesting a potentially more direct role of alpha/beta network coupling in motor output. In addition, we provide the first evidence of modulation of GPi activity by passive observation of actions in the form of alpha/low beta power suppression.
Supplementary Material
Highlights.
Alpha and beta power are suppressed in the globus pallidus and motor cortex during movement execution and action observation.
Pallidocortical alpha and beta network oscillations differentiate movement execution from action observation.
Network coupling signals should be considered for closed loop deep brain stimulation in Parkinson disease.
Acknowledgments
Conflict of Interest Statement
MM is currently employed by Boston Scientific; work was done while she was employed at UCLA. NP receives grant support from Medtronic and Boston Scientific and consultant fees from Abbott and Boston Scientific.
Financial Support
This work was supported by the National Institute of Neurological Disorders and Stroke [R01NS097782] and [U01NS098961]. Dr. Cross is supported by a fellowship from the National Institute of Neurologic Disorders and Stroke [R25NS065723].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alegre M, Rodríguez-Oroz MC, Valencia M, Pérez-Alcázar M, Guridi J, Iriarte J, et al. Changes in subthalamic activity during movement observation in Parkinson’s disease: Is the mirror system mirrored in the basal ganglia? Clin Neurophysiol 2010;121:414–25. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Lesser RP, Gordon B, Uematsu S, Jackson D, Webber R. Functional significance of the mu rhythm of human cortex: an electrophysiologic study with subdural electrodes. Electroencephalogr Clin Neurophysiol 1993;87:76–87. [DOI] [PubMed] [Google Scholar]
- AuYong N, Malekmohammadi M, Ricks-Oddie J, Pouratian N. Movement-Modulation of Local Power and Phase Amplitude Coupling in Bilateral Globus Pallidus Interna in Parkinson Disease. Front Hum Neurosci 2018;12:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini P, Fabbri-Destro M, Volta RD, Daprati E, Rizzolatti G, Cantalupo G. The Dynamics of Sensorimotor Cortical Oscillations during the Observation of Hand Movements: An EEG Study. PLoS One 2012;7:e37534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Vecchio F, Sebastiano F, Di Gennaro G, Quarato PP, et al. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin Neurophysiol 2016;127:641–54. [DOI] [PubMed] [Google Scholar]
- Bonini L The Extended Mirror Neuron Network: Anatomy, Origin, and Functions. Neurosci 2017;23:56–67. [DOI] [PubMed] [Google Scholar]
- Brinkman L, Stolk A, Dijkerman HC, de Lange FP, Toni I. Distinct Roles for Alpha- and Beta-Band Oscillations during Mental Simulation of Goal-Directed Actions. J Neurosci 2014;34:14783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Brown P. Oscillations and the basal ganglia: Motor control and beyond. Neuroimage 2014;85:637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci 2001;21:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G Action observation treatment: a novel tool in neurorehabilitation. Philos Trans R Soc B Biol Sci 2014;369:20130185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiore D, Mustile M, Spalletta G, Baldassarre G. Action observation and motor imagery for rehabilitation in Parkinson’s disease: A systematic review and an integrative hypothesis. Neurosci Biobehav Rev 2017;72:210–22. [DOI] [PubMed] [Google Scholar]
- Caligiore D, Pezzulo G, Miall RC, Baldassarre G. The contribution of brain sub-cortical loops in the expression and acquisition of action understanding abilities. Neurosci Biobehav Rev 2013;37:2504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science 2006;313:1626–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010;50:1148–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Lazzaro V Di, et al. Movement-related changes in synchronization in the human basal ganglia. Brain 2002;125:1235–46. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data. Cambridge, MA: MIT Press; 2014. [Google Scholar]
- Crone N, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain 1998;121:2271–99. [DOI] [PubMed] [Google Scholar]
- van Driel J, Cox R, Cohen MX. Phase-clustering bias in phase-amplitude cross-frequency coupling and its removal. J Neurosci Methods 2015;254:60–72. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol 2010;20:156–65. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 1995;73:2608–11. [DOI] [PubMed] [Google Scholar]
- Fischer P, Pogosyan A, Cheeran B, Green AL, Aziz TZ, Hyam J, et al. Subthalamic nucleus beta and gamma activity is modulated depending on the level of imagined grip force. Exp Neurol 2017;293:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, Pogosyan A, Green AL, Aziz TZ, Hyam J, Foltynie T, et al. Beta synchrony in the cortico-basal ganglia network during regulation of force control on and off dopamine. Neurobiol Dis 2019;127:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaut HJ, Bert J. EEG changes during cinematographic presentation (Moving picture activation of the EEG). Electroencephalogr Clin Neurophysiol 1954;6:433–44. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The Observation and Execution of Actions Share Motor and Somatosensory Voxels in all Tested Subjects: Single-Subject Analyses of Unsmoothed fMRI Data. Cereb Cortex 2009;19:1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson T Existing Motor State Is Favored at the Expense of New Movement during 13-35 Hz Oscillatory Synchrony in the Human Corticospinal System. J Neurosci 2005;25:7771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Caspers S, Eickhoff SB, Swinnen SP. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci Biobehav Rev 2018;94:31–44. [DOI] [PubMed] [Google Scholar]
- Hari R Action-perception connection and the cortical mu rhythm. Prog Brain Res 2006;159:253–60. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc Natl Acad Sci U S A 1998;95:15061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci 1997;20:44–9. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A 2013;110:4780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci 2015;18:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Li N, Dembek TA, Kappel A, Boulay C, Ewert S, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, V. Petersen M, Gemmar P, Goncalves J, Hertel F. PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Burchiel KJ. Microelectrode Recording in Movement Disorder Surgery. New York, NY: Thieme Medical Publishers; 2004 [Google Scholar]
- Kato K, Yokochi F, Iwamuro H, Kawasaki T, Hamada K, Isoo A, et al. Frequency-Specific Synchronization in the Bilateral Subthalamic Nuclei Depending on Voluntary Muscle Contraction and Relaxation in Patients with Parkinson’s Disease. Front Hum Neurosci 2016;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Lemon RN. What We Know Currently about Mirror Neurons. Curr Biol 2013;23:R1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Marchant JL, Frith CD. Relationship between Activity in Human Primary Motor Cortex during Action Observation and the Mirror Neuron System. PLoS One 2009;4:e4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondylis ED, Randazzo MJ, Alhourani A, Lipski WJ, Wozny TA, Pandya Y, et al. Movement-related dynamics of cortical oscillations in Parkinson’s disease and essential tremor. Brain 2016;139:2211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn A a, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci 2008;28:6165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Doyle L, Pogosyan A, Yarrow K, Kupsch A, Schneider GH, et al. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson’s disease. Brain 2006;129:695–706. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol 2009;215:380–7. [DOI] [PubMed] [Google Scholar]
- Lachaux J, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp 1999;8:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-C, Gervasoni D, Nicolelis MAL. Fast Modulation of Prefrontal Cortex Activity by Basal Forebrain Noncholinergic Neuronal Ensembles. J Neurophysiol 2006;96:3209–19. [DOI] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain 2011; 134:359–74. [DOI] [PubMed] [Google Scholar]
- Lopez-Azcarate J, Tainta M, Rodriguez-Oroz MC, Valencia M, Gonzalez R, Guridi J, et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci 2010;30:6667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin Neurophysiol 2010;121:492–501. [DOI] [PubMed] [Google Scholar]
- Malekmohammadi M, AuYong N, Ricks-Oddie J, Bordelon Y, Pouratian N. Pallidal deep brain stimulation modulates excessive cortical high β phase amplitude coupling in Parkinson disease. Brain Stimul 2018a;11:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekmohammadi M, Shahriari Y, AuYong N, O’Keeffe A, Bordelon Y, Hu X, et al. Pallidal stimulation in Parkinson disease differentially modulates local and network β activity. J Neural Eng 2018b;15:056016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceglia S, Fiorio M, Foffani G, Mrakic-Sposta S, Tiriticco M, Locatelli M, et al. Modulation of beta oscillations in the subthalamic area during action observation in Parkinson’s disease. Neuroscience 2009;161:1027–36. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007;164:177–90. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey NF, Knight RT, et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol 2012;8:e1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Schalk G, Fetz EE, Den Nijs M, Ojemann JG, Rao RPN. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc Natl Acad Sci U S A 2010;107:4430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann W, Jha A, Bock A, Huebl J, Horn A, Schneider G, et al. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain 2015;138:1894–906. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol 2001;43:41–58. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 2002;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Ikeda A, Kunieda T, Yazawa S, Baba K, Nagamine T, et al. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain 2000;123:1203–15. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Beudel M, Zrinzo L, Limousin P, Hariz M, Foltynie T, et al. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain 2016;139:1482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosin E, Avanzino L, Bove M, Stramesi P, Nieuwboer A, Abbruzzese G. Action Observation Improves Freezing of Gait in Patients With Parkinson’s Disease. Neurorehabil Neural Repair 2010;24:746–52. [DOI] [PubMed] [Google Scholar]
- Pelosin E, Barella R, Bet C, Magioncalda E, Putzolu M, Di Biasio F, et al. Effect of Group-Based Rehabilitation Combining Action Observation with Physiotherapy on Freezing of Gait in Parkinson’s Disease. Neural Plast 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842–57. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett 1997;239:65–8. [DOI] [PubMed] [Google Scholar]
- Press C, Cook J, Blakemore S-J, Kilner J. Dynamic modulation of human motor activity when observing actions. J Neurosci 2011;31:2792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo MJ, Kondylis ED, Alhourani A, Wozny TA, Lipski WJ, Crammond DJ, et al. Three-dimensional localization of cortical electrodes in deep brain stimulation surgery from intraoperative fluoroscopy. Neuroimage 2016;125:515–21. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol 2008;213:108–13. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nat Rev Neurosci 2016;17:757–65. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, Hummel FC. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 2009;47:284–8. [DOI] [PubMed] [Google Scholar]
- de Solages C, Hill BC, Koop MM, Henderson JM, Bronte-Stewart H. Bilateral symmetry and coherence of subthalamic nuclei beta band activity in Parkinson’s disease. Exp Neurol 2010;221:260–6. [DOI] [PubMed] [Google Scholar]
- Solis-Escalante T, Müller-Putz GR, Pfurtscheller G, Neuper C. Cue-induced beta rebound during withholding of overt and covert foot movement. Clin Neurophysiol 2012;123:1182–90. [DOI] [PubMed] [Google Scholar]
- Stančák A, Riml A, Pfurtscheller G. The effects of external load on movement-related changes of the sensorimotor EEG rhythms. Electroencephalogr Clin Neurophysiol 1997;102:495–504. [DOI] [PubMed] [Google Scholar]
- Stolk A, Brinkman L, Vansteensel MJ, Aarnoutse E, Leijten FSS, Dijkerman CH, et al. Electrocorticographic dissociation of alpha and beta rhythmic activity in the human sensorimotor system. Elite 2019;8:e48065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, et al. Intracranial EEG Reveals a Time- and Frequency-Specific Role for the Right Inferior Frontal Gyrus and Primary Motor Cortex in Stopping Initiated Responses. J Neurosci 2009;29:12675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, De Hemptinne C, Aron AR, Ostrem JL, Knight RT, Starr PA. Elevated synchrony in Parkinson disease detected with electroencephalography. Ann Neurol 2015;78:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thura D, Cisek P. The Basal Ganglia Do Not Select Reach Targets but Control the Urgency of Commitment. Neuron 2017;95:1160–1170.e5. [DOI] [PubMed] [Google Scholar]
- Tsiokos C, Hu X, Pouratian N. 200-300Hz movement modulated oscillations in the internal globus pallidus of patients with Parkinson’s Disease. Neurobiol Dis 2013;54:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokos C, Malekmohammadi M, AuYong N, Pouratian N. Pallidal low β-low γ phase-amplitude coupling inversely correlates with Parkinson disease symptoms. Clin Neurophysiol 2017;128:2165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Pallidal Discharge Related to the Kinematics of Reaching Movements in Two Dimensions. J Neurophysiol 1997;77:1051–74. [DOI] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci 2012;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann A, Schröger E, Maess B. Digital filter design for electrophysiological data - a practical approach. J Neurosci Methods 2015;250:34–46. [DOI] [PubMed] [Google Scholar]
- van Wijk BCM, Beek PJ, Daffertshofer A. Neural synchrony within the motor system: what have we learned so far? Front Hum Neurosci 2012;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Beudel M, Jha A, Oswal A, Foltynie T, Hariz MI, et al. Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson’s disease. Clin Neurophysiol 2016;127:2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Neumann WJ, Schneider GH, Sander TH, Litvak V, Kühn AA. Low-beta cortico-pallidal coherence decreases during movement and correlates with overall reaction time. Neuroimage 2017;159:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T, Yamashita O, Hirata M, Kishima H, Saitoh Y, Goto T, et al. Regulation of motor representation by phase-amplitude coupling in the sensorimotor cortex. J Neurosci 2012;32:15467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AI, Vanegas N, Lungu C, Zaghloul KA. Beta-Coupled High-Frequency Activity and Beta-Locked Neuronal Spiking in the Subthalamic Nucleus of Parkinson’s Disease. J Neurosci 2014;34:12816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


