Abstract
Disseminated candidiasis is a hospital-acquired infection that results in high degree of mortality despite antifungal treatment. Autopsy studies revealed that kidneys are the major target organs in disseminated candidiasis and death due to kidney damage is a frequent outcome in these patients. Thus, the need for effective therapeutic strategies to mitigate kidney damage in disseminated candidiasis is compelling. Recent studies have highlighted the essential contribution of kidney-specific immune response in host defense against systemic infection. Crosstalk between kidney-resident and infiltrating immune cells aid in the clearance of fungi and prevent tissue damage in disseminated candidiasis. In this review, we provide our recent understanding on antifungal immunity in the kidney with an emphasis on IL-17-mediated renal defense in disseminated candidiasis.
Keywords: Kidney, Candida albicans, disseminated candidiasis, innate, adaptive, IL-17
Introduction
Candida albicans is both a commensal and an opportunistic fungal pathogen of humans. Multiple manifestations of C. albicans infection can occur when there is a defect in the antifungal immunity [1]. Mucocutaneous forms of the disease include oropharyngeal, vaginal, and cutaneous candidiasis [2]. Disseminated candidiasis is the most severe and the third most common healthcare-associated infection with high mortality rate (~40%) [3].
Death due to fungal sepsis is an inevitable outcome in disseminated candidiasis [4]. Additionally, fungal hyphae invade and damage solid organs like kidneys, liver, spleen, lung and brain. The principal target organ involved in disseminated candidiasis are kidneys. This was evident in a review of 45 autopsies of patients with disseminated candidiasis, where 89% of patients had evidence of renal pathology [5]. Kidney infections can occur via hematogenous routes or from ascending spread from the bladder or urethra [6]. A mouse model of disseminated candidiasis has been established in which mice develop renal failure and septic shock following systemic spread [4,5,7–9]. Following intra-venous infection, Candida invades the kidney and forms 20- to 25-μm–long filaments within 2 hours post infection [10]. Interestingly, only kidneys show continuously increasing fungal burden, whereas fungal load declines in other organs [9]. Moreover, Candida filamentation, a key virulence factor, is seen in kidneys but not in the liver and spleen, indicating renal micro-environment plays a major role in the fungal virulence [9]. C. albicans hyphae proliferate in tubular space during infection and form cortical and medullary abscesses causing pyelonephritis, interstitial edema, and renal insufficiency [3,8,11]. Here, we list the recent advancement over the past 5 years in kidney-specific immunity with a focus on IL-17-driven renal defense against disseminated candidiasis.
Local antifungal immunity in the kidney
The kidney is an organ particularly susceptible to damage caused by infections and autoinflammatory conditions. Even so, renal immunology remains remarkably understudied by immunologists. Several kidney-specific factors including poor regenerative capacity of the nephrons, uremia, hypoxia and blood pressure associated changes, make it extremely challenging to study immune response in the kidney. Under homeostatic conditions, the kidneys contain a varied network of immune and non-immune cells that are ideally positioned to sense and respond to fungi [12].
1. Fungal recognition in the kidney:
Immune and non-immune cells of the kidney recognize pathogen-associated molecular patterns (PAMPs) of Candida yeast and hyphae by various pattern recognition receptors (PRRs). These PRRs include Toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide binding oligomerization domain (NOD)-like receptors, retinoid-inducible gene 1 protein (RIG1)-like receptors and complement receptors (CR). Among TLRs, TLR2 senses phospholipidomannan on the fungal surface [13], and TLR4 recognizes O-linked mannosyl chains in the fungal cell wall [14]. CLRs include Dectin-1, Dectin-2, and macrophage-inducible C-type lectin, DC-Sign, and mannose receptor. CLRs sense carbohydrate moieties found in the C. albicans cell wall, including mannans and β-glucan [3]. Accordingly, Dectin-1, Dectin-2 or Dectin-3 knockout mice showed impairment in renal fungal clearance and increased susceptibility to disseminated candidiasis [15–17]. TLR2 and TLR4 have also been implicated in renal immunity against systemic C. albicans infection [18]. However, recognition of fungal RNA by TLR7 has been shown to play a non-redundant role in renal antifungal activity [19]. Studies reported NLRP3 inflammasome activation by C. albicans hyphae [20]. Accordingly, NLRP3 and NLRP10-deficient mice showed increased susceptibility to disseminated candidiasis [21]. The melanoma differentiation-associated protein 5 (MDA5) senses C. albicans and polymorphisms in this receptor influence susceptibility to disseminated candidiasis in humans [22]. Galectin-3 recognizes β-mannans from C. albicans and mice lacking galectin-3 succumb to disseminated candidiasis [23]. CR3 on neutrophils recognizes β-glucans and play a role in phagocytosis of C. albicans [24].
2. Kidney-resident cells:
The kidney-resident myeloid cell populations comprise macrophages and dendritic cells (DCs). Phenotypically, human renal tissue-resident macrophages are CD14+CD11b+CD11c+CD64+CD68− [25]. The macrophages, which reside in the medullary region, phagocytose C. albicans yeast within the first hour after infection and produce pro-inflammatory mediators [3]. In CX3CR1-deficient mice, reduced accumulation of monocyte-derived macrophages in the kidney leads to renal failure in disseminated candidiasis. Increased susceptibility to disseminated candidiasis was also noted in patients with a polymorphism resulting in diminished CX3CR1 function [10].
Human kidneys house tissue-resident lymphocytes. Among CD4+ T and CD8+ T cells, the main subsets are CD69+CCR7−CD45RA− and CD69+CCR7−CD45RA+, respectively [26]. NK cells in the kidney exhibit dual expression of γ- and δ-T cell receptors [12,27]. The B cell populations in kidney include IgM−, IgG−, and IgA− cells [12]. Compared to resident myeloid cells, the role of kidney-resident lymphoid cells in antifungal immunity is less clear.
The renal tubular epithelial cells (RTECs) constitute around 80% of the total non-hematopoietic kidney-resident cells [28]. The crosstalk between RTECs and immune cells is essential for antimicrobial defense in the kidney (Fig 1). RTECs express various pathogen recognition receptors (PRRs) that sense fungal PAMPS and upregulate inflammatory cytokine and chemokine gene expression. Studies from our group showed that RTECs produce cytokines and chemokines in response to IL-17, necessary for the recruitment of innate immune effectors [29]. Consequently, mice with conditional deletion of IL-17RA in RTECs showed more severe renal damage and reduced survival during disseminated candidiasis [30]. We also demonstrated that hyphal invasion of the kidney parenchyma drives RTECs apoptosis and subsequently renal damage and dysfunction. Recently, we showed an unexpected kidney tissue protective role of IL-17 via activating the Kallikrein-Kinin System (KKS) [8]. IL-17 acts on RTECs to induce the expression of nephro-protective Kallikrein 1. Kallikrein 1 cleaves kininogen to produce kidney protective bradykinin. Consequently, therapeutic manipulation of IL-17-KKS pathway in mice restored kidney function and improved survival following disseminated candidiasis [8,30]. Thus, kidney-resident hematopoietic and non-hematopoietic cells form a very important network of antifungal immune defense in the kidney.
Fig 1: Antifungal immunity in the kidney.
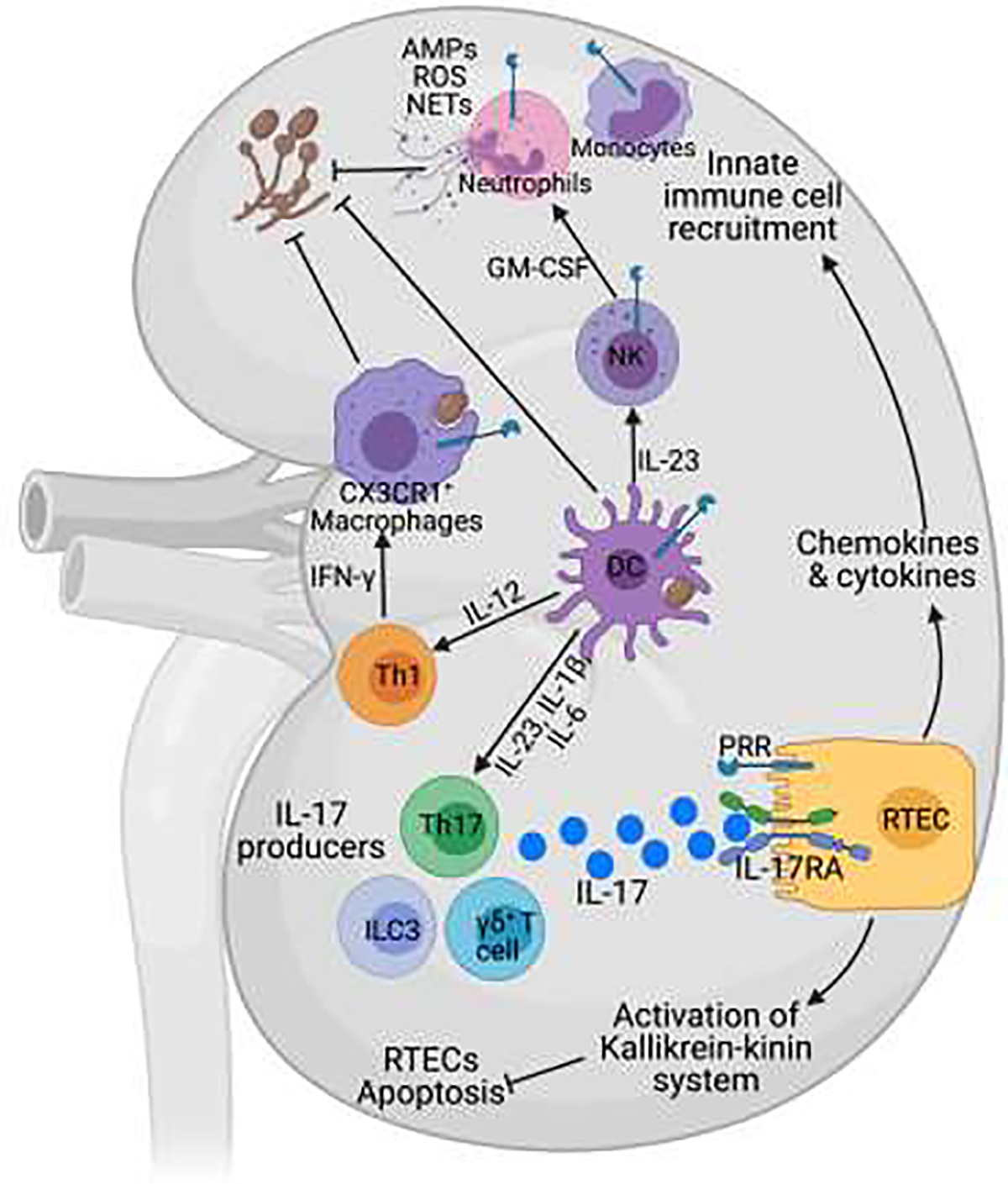
Following fungal invasion, kidney-resident immune and non-immune cells recognize the fungi via pathogen recognition receptors (PRRs). The tissue-resident-macrophages phagocytose and clear the fungus. Dendritic cells (DCs) process fungal antigens and present it to naïve CD4+ T cells to develop adaptive T helper (Th) cell responses. Th1 cells produce IFN-γ, which potentiates the phagocytic activity of macrophages. In response to C. albicans infection, kidney infiltrating γδ-T cells, Th17, and ILC3s are the major producers of IL-17. IL-17, in turn, binds to its receptor (IL-17RA/RC) on renal tubular epithelial cells (RTECs), activating downstream signaling events leading to expression of IL-17-responsive cytokines and chemokines genes. Innate immune cells including neutrophils recruited in response to IL-17-induced signals facilitate fungal clearance by producing antimicrobial peptides (AMPs), reactive oxygen species (ROS) and neutrophil extra-cellular traps (NETs). IL-17 also induces activation of the Kallikrein-kinin system in RTECs, which subsequently prevents apoptosis and controls tissue damage. IL-23 secreted by DCs acts on natural killer cells to produce GM-CSF, which enhances the candidacidal activity of neutrophils.
3. Kidney infiltrating immune effectors:
Innate immune cells
Neutrophils
During disseminated candidiasis, an innate response dominated by neutrophils is the major driver of fungal clearance in the kidney [1]. Accordingly, neutropenia is a risk factor for infection in humans and mice depleted of neutrophils are highly susceptible to disseminated candidiasis [1,31,32]. Indeed, delayed trafficking of neutrophils into kidney is associated with increased fungal evasion in the renal parenchyma [9]. A recent study revealed the role for IL-33 in limiting the rapid CXCL2 elevation and neutrophil aggregation resulting in impaired C. albicans clearance from the kidneys [33]. Moreover, Type I interferon-dependent IL-15 production from splenic monocytes drives GM-CSF production from NK cells, aiding kidney neutrophils to control fungal growth [34].
Neutrophils kill C. albicans by non-oxidative and oxidative mechanisms [24]. After phagocytosis of C. albicans, phagosomes fuse with lysosomes and neutrophil granules containing proteolytic enzymes and antimicrobial peptides (AMPs). On the other hand, activated nicotinamide adenine dinucleotide phosphate oxidase generates reactive oxygen species (ROS), which, along with other oxidants, kill fungi [35]. Additionally, neutrophils produce neutrophil extracellular traps (NETs) to kill pathogenic hyphal form [36]. The NADPH required for ROS generation by neutrophils is produced by the breakdown of glucose via glycolysis and the pentose phosphate pathway [37]. Consistently, glycolytic inhibition in neutrophils and monocytes decreased C. albicans killing [32,38]. We showed that kidney disease and associated uremia inhibit glucose uptake in neutrophils, which is upstream of and essential for ROS production and fungal killing in the kidney [32]. Although neutrophils are crucial for the host defense against disseminated candidiasis, but they can also drive immunopathology in the infected kidney. Studies have indicated the role of Ccr1+ neutrophils in causing immunopathology in infected kidneys during later course of C. albicans infection. Accordingly, genetic deficiency of Ccr1 or pharmacological inhibition with the Ccr1-selective antagonist ameliorated kidney tissue damage during disseminated candidiasis [39,40]. In line with these reports, neutrophil-mediated immunopathology has been reported in individuals with renal candidiasis [41].
Monocytes and macrophages:
The murine kidney-resident and kidney-infiltrating macrophages are F4/80highCD11blow and F4/80lowCD11bhigh, respectively [42]. The kidney-resident macrophages have unique functions in maintaining tissue homeostasis and resolving inflammation. One study indicated that CD169+ kidney-resident macrophages protect the kidney during fungal infection by promoting IFNγ-dependent host resistance and neutrophil ROS activity [43]. Recently, murine kidney-resident macrophages are also implicated in cyst formation in the kidney [44]. The monocytes and macrophages also possess significant Candida killing capacity. Macrophage-depleted mice showed accelerated fungal proliferation in kidney [45]. Similarly, deficiency of CCR2, which is essential for monocyte recruitment to inflamed tissues, contributes to enhanced susceptibility to disseminated candidiasis [46]. The deficiency of CBLB, a E3 ubiquitin ligase that controls CLR signaling in macrophages and DCs, resulted in increased inflammasome activation, enhanced reactive oxygen species production and improved survival of mice during disseminated candidiasis. C. albicans competes for glucose with macrophages and triggers cell death and supplementation of glucose delayed macrophage cell death [47].
DCs:
The renal DCs are present in the tubulointerstitial space and function as sentinels in homeostasis, local injury, and infection [48]. Classical myeloid DCs (cDCs) in human kidneys are CD11c+MHCII+CD14− and they have the ability to present antigens to T cells [48]. The renal cDCs can be divided into cDC1 and cDC2 [49]. cDC1 (CD103+) constitute less than 5% of renal DCs, whereas majority of kidney DCs population is cDC2 [50]. Mice lacking cDC1 showed comparable susceptibility and renal fungal load in disseminated candidiasis [51]. CX3CR1 is a kidney-specific homing receptor for DCs and CX3CR1+ DCs in the kidney cortex are mainly involved in mediating adaptive immune responses [52]. DCs are important for host defense during disseminated candidiasis via the production of inflammatory mediators and antigen presentation to the T cells [53,54]. Studies have indicated that DCs are involved in priming of the anti-Candida activity of neutrophils through an IL− 23–GM− CSF pathway, which involves NK cells [55–57]. Accordingly, GM− CSF therapy resulted in reduced mortality in patients with allogeneic hematopoietic stem cell transplant and suffering from disseminated candidiasis [58].
Adaptive immune cells:
CD4+ T cells are the central organizers of adaptive immune response against disseminated candidiasis [1,59].
Th1 cells:
Th1 cells exert a protective role during disseminated candidiasis by virtue of IFNγ production. Hence, mice lacking IFNγ receptor are highly susceptible to infection [60]. Additionally, mice devoid of IL-18, which augments IFNγ production, are also susceptible to infection [61]. IFNγ induces nitric oxide production from neutrophils and macrophages and immunoglobulin production in C. albicans infection [62]. The therapeutic potential of recombinant IFNγ and IL-18 therapies have been demonstrated in mouse model and human patients [63–66].
Th2 cells:
Th2 cytokines, such as IL-4 and IL-10, inhibit Th1 development and suppress phagocytic cells; therefore Th2 response favors fungal infection [67]. Overexpression of GATA-3, master transcription factor of Th2 cells, restricts IFNγ production and impairs antifungal host defense [68]. Interestingly, IL-13 is reported to increase host tolerance to C. albicans kidney infection by enhancing the antimicrobial function of innate cells [69].
Th17 cells:
The IL-17A mRNA is produced in kidneys during the early stage of infection [30,70]. We identified that TCRγδ+ T cells are the primary source of IL-17 in C. albicans-infected kidneys [30]. Considerable evidence suggests a role for Th17 and other innate IL-17 (IL-17A) producing cells in immunity against disseminated candidiasis. Mice deficient in IL-17RA, IL-17RC, RORγt, and IL-17A exhibit higher renal fungal load and heightened susceptibility [8,56,71,72]. Mice heterozygous for MCPIP1, a feedback inhibitor of IL-17 receptor signaling, showed enhanced resistance to disseminated candidiasis [73]. Furthermore, adenovirus or C. albicans-mediated overexpression of IL-17 protected mice from disseminated candidiasis [71,74]. IL-23 expression is induced in response to C. albicans via the CLR pathway and regulates IL-17 production by innate lymphoid cells [75]. IL-23 also protects against disseminated candidiasis by preventing myeloid cell death in infected kidneys [76]. Unlike IL-17, IL-17C or IL-17F have limited impact on survival of mice to disseminated candidiasis [77,78]. Instead, mice lacking IL-17C exhibited increased survival during disseminated candidiasis [79].
IL-17 regulates antifungal immunity through induction of a signature gene profile including antimicrobial peptides (β-defensins, calprotectin, and mucins) and neutrophil recruiting chemokines (CXCL1, CXCL5, and G-CSF). The neutrophil recruitment in kidneys of Il17ra−/− mice is impaired during infection [72]. One report study suggests that IL-17–dependent signaling in candidiasis does not occur in the kidney. Instead IL-17 signaling in NK cell drives GM-CSF production, which increases candidacidal activity of neutrophils in the kidney [56]. In contrast, data from our group showed that RTECs specific deletion of IL-17 signaling exacerbates kidney damage without impacting renal fungal load during disseminated candidiasis [8]. This data indicates a kidney-specific and tissue-protective role of IL-17 signaling in antifungal host defense. Another study showed that intestinal colonization with C. albicans drives systemic expansion of fungal-specific Th17 CD4+ T cells and increased IL-17 responsiveness by neutrophils, which synergistically protect against C. albicans infection [80].
Conclusion
Host antifungal defense in the context of kidney is a highly neglected area of inquiry. Although it has been known that kidney-specific immune response promote protection to disseminated infection, the mechanisms by which it impacts the kidney are largely undefined. Exploring the mechanisms of local immunity in the kidney will discover downstream mediators that could act as novel drug targets for preventing kidney damage. Moreover, it may also be used to decide whether targeting these targets could be a safer and effective therapeutic option in combination with antifungal drugs. Identifying renal therapeutic targets may reveal new therapeutic strategies to counter kidney damage while sparing other vital organs from unwanted side effects.
Highlights.
During disseminated candidiasis C. albicans invade and damage kidney
No approved antifungal vaccines to prevent renal damage in disseminated candidiasis
Kidney-resident and infiltrating immune cells control fungal burden in the kidney
IL-17 play a renal tissue protective role in disseminated candidiasis
Acknowledgments
This work was supported by a Rheumatology Research Foundation grant and NIH grants AI145242, DK104680 and AI142354 to PSB. We would like to thank Biswas lab members for helpful discussion and suggestions.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Netea MG, van der Meer JWM, Kullberg BJ, van de Veerdonk FL: Immune defence against Candida fungal infections. Nature Reviews Immunology 2015, 15:630–642. [DOI] [PubMed] [Google Scholar]
- 2.Mengesha BG: The Role of IL-17 in Protection against Mucosal Candida Infections. J Fungi (Basel) 2017, 3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas PG, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ: Invasive candidiasis. Nature Reviews Disease Primers 2018, 4:18026. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Edwards JE Jr, Filler SG: Mice with disseminated candidiasis die of progressive sepsis. Journal of Infectious Diseases 2005, 192:336–343. [DOI] [PubMed] [Google Scholar]
- 5.Lehner T: SYSTEMIC CANDIDIASIS AND RENAL INVOLVEMENT. Lancet 1964, 2(7348):1414–1416. [DOI] [PubMed] [Google Scholar]
- 6.Hammond NA, Miller FH: Infectious and inflammatory diseases of the kidney. Radiol Clin North Am. 2012, 20:259–270. [DOI] [PubMed] [Google Scholar]
- 7.Kullberg BJ: Invasive Candidiasis. New England Journal of Medicine 2015, 373:1445–1456. [DOI] [PubMed] [Google Scholar]
- 8.Ramani K, Jawale CV, Conti HR, Whibley N, Jackson EK, Shiva SS, Horne W, Kolls JK, Gaffen SL, Biswas PS: The Kallikrein-Kinin System: A Novel Mediator of IL-17-Driven Anti-Candida Immunity in the Kidney. PLOS PATHOGENS 2016, 12:e1005952.• These findings highlight the unexpected role of IL-17 in inducing the Kallikrein-Kinin System in C. albicans-infected kidney, which is important in preventing renal tissue damage
- 9.Lionakis MS, Lee CC, Murphy PM: Organ-specific innate immune responses in a mouse model of invasive candidiasis. Journal of Innate Immunity 2011, 3:180–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lionakis MS, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee CC, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao JL, Kullberg BJ, Netea MG, Murphy PM: CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. Journal of Clinical Investigation 2013, 123:5035–5051.• This study highlighted the role of CX3CR1 in antifungal host defense. Cxcr1 deficiency in mice and mutant CX3CR1 allele CX3CR1-T276 in human results in impaired neutrophil degranulation and fungal killing
- 11.Jae-Chen S, Young-Joo J, Seon-Min P, Kang Seok S, Jung-Hyun S, & Jung-Il C: Mechanism underlying renal failure caused by pathogenic Candida albicans infection. Biomedical reports 2015, 3:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart BJ, Young MD, Mitchell TJ, Loudon KW, Riding AM, Richoz N, Frazer GL, Staniforth JUL, Vieira Braga FA, Botting RA, Popescu DM, Vento-Tormo R, Stephenson E, Cagan A, Farndon SJ, Polanski K, Efremova M, Green K, Del Castillo Velasco-Herrera M, Guzzo C, Collord G, Mamanova L, Aho T, Armitage JN, Riddick ACP, Mushtaq I, Farrell S, Rampling D, Nicholson J, Filby A, Burge J, Lisgo S, Lindsay S, Bajenoff M, Warren AY, Stewart GD, Sebire N, Coleman N, Haniffa M, Teichmann SA, Behjati S, Clatworthy MR: Spatiotemporal immune zonation of the human kidney. Science 2019, 365:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jouault T, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D: Candida albicans phospholipomannan is sensed through toll-like receptors. Journal of Infectious Diseases 2003, 188:165–172. [DOI] [PubMed] [Google Scholar]
- 14.Netea MG, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ: Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. Journal of Clinical Investigation 2006, 116:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. : Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007, 8:31–38.•• This study showed the mechanisms of Dectin-1 mediated recognition of fungal ligand alpha-mannans in mounting an effective anti-C. albicans immunity against disseminated candidiasis.
- 16.Ifrim DC, Quintin J, Courjol F, Verschueren I, van Krieken JH, Koentgen F, Fradin C, Gow NA, Joosten LA, van der Meer JW, et al. : The Role of Dectin-2 for Host Defense Against Disseminated Candidiasis. J Interferon Cytokine Res 2016, 36:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X: C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013, 39:324–334. [DOI] [PubMed] [Google Scholar]
- 18.Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ: The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis 2002, 185:1483–1489. [DOI] [PubMed] [Google Scholar]
- 19.Biondo C, Malara A, Costa A, Signorino G, Cardile F, Midiri A, Galbo R, Papasergi S, Domina M, Pugliese M, et al. : Recognition of fungal RNA by TLR7 has a nonredundant role in host defense against experimental candidiasis. Eur J Immunol 2012, 42:2632–2643. [DOI] [PubMed] [Google Scholar]
- 20.Gross O, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J: Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459:433–436. [DOI] [PubMed] [Google Scholar]
- 21.Joly S, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS: Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. Journal of Immunology 2012, 189:4713–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaeger M, Cheng SC, Johnson MD, Kumar V, Ng A, Plantinga TS, Smeekens SP, Oosting M, Wang X, Barchet W, Fitzgerald K, Joosten LAB, Perfect JR, Wijmenga C, van de Veerdonk FL, Huynen MA, Xavier RJ, Kullberg BJ, Netea MG The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur J Clin Microbiol Infect Dis 2015, 34:963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden JR, Laforce-Nesbitt SS, Bliss JM. : Galectin-3 plays an important role in protection against disseminated candidiasis. Medical Mycology 2013, 51:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazendam RP, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, Liese JG, van de Veerdonk FL, Roos D, van den Berg TK, Kuijpers TW: Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 2014, 124:590–597. [DOI] [PubMed] [Google Scholar]
- 25.Berry MR, Ferdinand JR, Jing C, Loudon KW, Wlodek E, Dennison TW, Kuper C, Neuhofer W, Clatworthy MR.: Renal Sodium Gradient Orchestrates a Dynamic Antibacterial Defense Zone. Cell 2017, 170:860–874.e819. [DOI] [PubMed] [Google Scholar]
- 26.Park JG, Kim MG, Park SH, Lee HJ, Kim DK, Kwak C, Kim YS, Chang S, Moon KC, Lee DS, Han SS: Immune cell composition in normal human kidneys. Scientific Reports 2020, 10:15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner JE, Healy H, Kassianos AJ: Natural Killer Cells in Kidney Health and Disease. Frontiers in Immunology 2019, 10:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong S, Healy H, Kassianos AJ: The Emerging Role of Renal Tubular Epithelial Cells in the Immunological Pathophysiology of Lupus Nephritis. Front Immunol 2020, 11:578952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramani K, Pawaria S, Maers K, Huppler AR, Gaffen SL, Biswas PS: An essential role of interleukin-17 receptor signaling in the development of autoimmune glomerulonephritis. J Leukoc Biol 2014, 96:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramani K JC, Verma AH, Coleman BM, Kolls JK, Biswas PS: Unexpected kidney-restricted role for IL-17 receptor signaling in defense against systemic Candida albicans infection. JCI Insight 2018, 3:e98241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filler S: Insights from human studies into the host defense against candidiasis. Cytokine 2012, 58:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jawale CV RK, Li DD, Coleman BM, Oberoi RS, Kupul S, Lin L, Desai JV, Delgoffe GM, Lionakis MS, Bender FH, Prokopienko AJ, Nolin TD, Gaffen SL, Biswas PS: Restoring glucose uptake rescues neutrophil dysfunction and protects against systemic fungal infection in mouse models of kidney disease. Science Translational Medicine 2020, 12:eaay5691.•• This study identified the mechanisms of uremia-induced neutrophil dysfunction, leading to enhanced susceptibility to disseminated candidiasis in kidney disease. The paper showed GSK3β inhibition as a potential translatable tharapeutic strategy to restore defect in neutrophil function and treat disseminated candidiasis in kidney disease.
- 33.Abe Y, Nakamura K, Arai K, Sakurai C, Hatsuzawa K, Ogura Y, Iseki K, Tase C, Kanemitsu K: IL-13 attenuates early local CXCL2-dependent neutrophil recruitment for Candida albicans clearance during a severe murine systemic infection. Immunobiology 2019, 224:15–29. [DOI] [PubMed] [Google Scholar]
- 34.Domínguez-Andrés J, Minguito de la Escalera M, González L, López-Bravo M, Ardavín C: Inflammatory Ly6Chigh Monocytes Protect against Candidiasis through IL-15-Driven NK Cell/Neutrophil Activation. Immunity 2017, 46:1059–1072.e1054.•• This study reported that spleen Ly6Chigh inflammatory monocytes as cellular source of IL-15, which drives GM-CSF release by spleen NK cells, a necessary event to boost the Candida killing potential of kidney neutrophils.
- 35.Mayadas TN, Lowell CA: The multifaceted functions of neutrophils. Annual Review of Pathology 2014, 9:181–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu SY, Weng CL, Jheng MJ, Kan HW, Hsieh ST, Liu FT, Wu-Hsieh BA: Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog 2019, 15:e1008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azevedo EP, Guimarães-Costa AB, de Souza-Vieira TS, Ganilho J, Saraiva EM, Palhano FL, Foguel D: A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. Journal of Biological Chemistry 2015, 290:22174–22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domínguez-Andrés J, Ter Horst R, Gresnigt MS, Smeekens SP, Ratter JM, Lachmandas E, Boutens L, van de Veerdonk FL, Joosten LAB, Notebaart RA, Ardavín C, Netea MG: Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog 2017, 13:e1006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM: Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog 2012, 8:e1002865.• The report identified the role of Ccr1 signaling in exaggerated recruitment of neutrophils leading to renal immunopathology in the later stages of systemic C. albicans infection.
- 40.Lionakis MS, Albert ND, Swamydas M, Lee CR, Loetscher P, Kontoyiannis DP: Pharmacological Blockade of the Chemokine Receptor CCR1 Protects Mice from Systemic Candidiasis of Hematogenous Origin. Antimicrob Agents Chemother 2017, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, Lortholary O: Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis 2008, 46:696–702. [DOI] [PubMed] [Google Scholar]
- 42.Park JG, Na M, Kim MG, Park SH, Lee HJ, Kim DK, Kwak C, Kim YS, Chang S, Moon KC, et al. : Immune cell composition in normal human kidneys. Sci Rep 2020, 10:15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo YJ, Ng SL, Mak KW, Setiagani YA, Chen Q, Nair SK, Sheng J, Ruedl C: Renal CD169(+) resident macrophages are crucial for protection against acute systemic candidiasis. Life Sci Alliance 2021, 4:e202000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman KA, Song CJ, Li Z, Lever JM, Crossman DK, Rains A, Aloria EJ, Gonzalez NM, Bassler JR, Zhou J, et al. : Tissue-Resident Macrophages Promote Renal Cystic Disease. J Am Soc Nephrol 2019, 30:1841–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian Q, Van Rooijen N, Cutler JE: Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. Journal of immunology 1994, 152:5000–5008. [PubMed] [Google Scholar]
- 46.Ngo LY, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM: Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. Journal of Infectious Diseases 2014, 209:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tucey TM, Harrison PF, Snelgrove SL, Lo TL, Scherer AK, Barugahare AA, Powell DR, Wheeler RT, Hickey MJ, Beilharz TH, Naderer T, Traven A. Glucose Homeostasis Is Important for Immune Cell Viability during Candida Challenge and Host Survival of Systemic Fungal Infection. Cell Metabolism 2018, 27:988–1006.e1007.• This study was the first to discover the importance of maintaining host glucose homeostasis to prevent systemic C. albicans infection. This study describes the Candida-macrophage interaction with regard to upregulation of glycolysis in both host and pathogen, setting up glucose competition.
- 48.Guilliams M, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S: Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature Reviews Immunology 2014, 14:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, Dennison TW, Kuper C, Neuhofer W, Clatworthy MR: Renal Sodium Gradient Orchestrates a Dynamic Antibacterial Defense Zone. Cell 2017, 170:860–874 e819. [DOI] [PubMed] [Google Scholar]
- 50.Kurts C, Ginhoux F, Panzer U: Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat Rev Nephrol 2020, 16:391–407. [DOI] [PubMed] [Google Scholar]
- 51.Break TJ, Hoffman KW, Swamydas M, Lee CC, Lim JK, Lionakis MS: Batf3-dependent CD103(+) dendritic cell accumulation is dispensable for mucosal and systemic antifungal host defense. Virulence 2016, 7:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hochheiser K, Heuser C, Krause TA, Teteris S, Ilias A, Weisheit C, Hoss F, Tittel AP, Knolle PA, Panzer U, et al. : Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J Clin Invest 2013, 123:4242–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Netea MG GK, Coolen N, Verschueren I, Figdor C, Van der Meer JW, Torensma R, Kullberg BJ.: Human dendritic cells are less potent at killing Candida albicans than both monocytes and macrophages. Microbes Infect. 2004, 6:985–989. [DOI] [PubMed] [Google Scholar]
- 54.Pathakumari B, Liu W: Immune defence to invasive fungal infections: A comprehensive review. Biomedicine & Pharmacotherapy 2020, 130:110550. [DOI] [PubMed] [Google Scholar]
- 55.del Fresno C, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavín C: Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 2013, 38:1176–1186. [DOI] [PubMed] [Google Scholar]
- 56.Bar E, Whitney PG, Moor K, Reis e Sousa C & LeibundGut-Landmann S: IL- 17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014, 40:117–127.• This report identified the role of IL-17 in regulating the functional competence of NK cells and its contribution in antifungal immunity during disseminated candidiasis.
- 57.Whitney PG, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis E, Sousa C: Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog 2014, 10:e1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan L, Lai Y, Jiang M, Song Y, Zhou J, Zhang Z, Duan X, Fu Y, Liao L, Wang C: Effect of Granulocyte-Macrophage Colony-Stimulating Factor on Prevention and Treatment of Invasive Fungal Disease in Recipients of Allogeneic Stem-Cell Transplantation: A Prospective Multicenter Randomized Phase IV Trial. J Clin Oncol. 2015, 33:3999–4006. [DOI] [PubMed] [Google Scholar]
- 59.Speakman EA, Salazar R, Brown GD: T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends in Immunology 2020, 41:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balish E, Vazquez-Torres A, Pierson C, Warner T: Candidiasis in interferon-gamma knockout (IFN-gamma−/−) mice. The Journal of infectious diseases 1998, 178:478–487. [DOI] [PubMed] [Google Scholar]
- 61.Netea MG, van den Hoven M, Verschueren I, Joosten LA, van Krieken JH, van den Berg WB, Van der Meer JW, Kullberg BJ: Differential role of IL-18 and IL-12 in the host defense against disseminated Candida albicans infection. European journal of immunology 2003, 33:3409–3417. [DOI] [PubMed] [Google Scholar]
- 62.Kaposzta R, Marodi L, Gordon S: Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infection and Immunity 1998, 66:1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kullberg BJ, Hoogstraten C, van Furth R: Recombinant interferon-gamma enhances resistance to acute disseminated Candida albicans infection in mice. Journal of Infectious Diseases 1993, 168:436–443. [DOI] [PubMed] [Google Scholar]
- 64.Stuyt RJ, van Krieken JH, van der Meer JW, Kullberg BJ: Recombinant interleukin-18 protects against disseminated Candida albicans infection in mice. Journal of Infectious Diseases 2004, 189:1524–1527. [DOI] [PubMed] [Google Scholar]
- 65.Delsing CE, Leentjens J, Preijers F, Frager FA, Kox M, Monneret G, Venet F, Bleeker-Rovers CP, van de Veerdonk FL, Pickkers P, Pachot A, Kullberg BJ, Netea MG: Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infectious Diseases 2014, 14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buddingh EP, van der Lugt J, Dik WA, Gresnigt MS, Netea MG, Pickkers P, Driessen GJ: Interferon-gamma immunotherapy in a patient with refractory disseminated candidiasis. Pediatr Infect Dis 2015, 34:1391–1394. [DOI] [PubMed] [Google Scholar]
- 67.Romani L: Immunity to fungal infections. Nature Reviews Immunology 2011, 11:275–288. [DOI] [PubMed] [Google Scholar]
- 68.Haraguchi N, Morishima Y, Yoh K, Matsuno Y, Kikuchi N, Sakamoto T, Takahashi S, Hizawa N: Impairment of host defense against disseminated candidiasis in mice overexpressing GATA-3. Infection and Immunity 2010, 78:2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran VG, Kim J, Kang SW, Moon UJ, Cho HR, Kwon B: IL-33 Enhances Host Tolerance to Candida albicans Kidney Infections through Induction of IL-13 Production by CD4+ T Cells. Journal of Immunology 2015, 194:4871–4879. [DOI] [PubMed] [Google Scholar]
- 70.Cua DJ: Innate IL-17-producing cells: the sentinels of the immune system. Nature Reviews Immunology 2010, 10:479–489. [DOI] [PubMed] [Google Scholar]
- 71.Huang W, Fidel PL, Schwarzenberger P: Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. The Journal of infectious diseases 2004, 190.• This is the first report showing the essential contribution of IL-17 signaling in protection against systemic infection with C. albicans.
- 72.van de Veerdonk FL, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, Netea MG: Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock 2010, 34:407–411. [DOI] [PubMed] [Google Scholar]
- 73.Garg AV, Chen K, Cruz JA, Grover P, Whibley N, Conti HR, Hernandez Mir G, Sirakova T, Childs EC, Smithgall TE, Biswas PS, Kolls JK, McGeachy MJ, Kolattukudy PE, Gaffen SL: MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity. Immunity 2015, 43:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huppler AR, Woolford CA, Childs EE, He J, Biswas PS, McGeachy MJ, Mitchell AP, Gaffen SL: A Candida albicans Strain Expressing Mammalian Interleukin-17A Results in Early Control of Fungal Growth during Disseminated Infection. Infection and Immunity 2015. 83:3684–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGeachy MJ, Gaffen SL: The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50:892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nur S, Lemberg C, Guiducci E, Schweizer TA, Zwicky P, Becher B: IL-23 supports host defense against systemic Candida albicans infection by ensuring myeloid cell survival. PLoS Pathog 2019, 15:e1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saijo S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y: Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010, 32:681–691.•• This study showed the mechanisms of Dectin-2 mediated recognition of fungal ligand alpha-mannans in mounting an effective anti-C. albicans immunity against disseminated candidiasis.
- 78.Conti HR, Coleman BM, Garg AV, Jaycox JR, Gaffen SL: Signaling through IL-17C/IL-17RE Is Dispensable for Immunity to Systemic, Oral and Cutaneous Candidiasis. Plos ONE 2015, 10:e0122807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang J, Hong S, Lin X, Jin W, Dong C: IL-17C is required for lethal inflammation during systemic fungal infection. Cell Mol Immunol. 2016, 13:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao TY, Jiang TT, Huang FS, Andersen H, Kinder JM, Pham G, Burg AR, Ruff B, Gonzalez T, Khurana Hershey GK, Haslam DB, Way SS: Commensal Candida albicans Positively Calibrates Systemic Th17 Immunological Responses. Cell Host Microbe. 2019, 25:404–417.e406.• This study highlighted the essential role of intestinal C. albicans colonization in protecting against systemic C. albicans infection. These results indicate that intestinal colonization with C. albicans drives systemic expansion of fungal-specific Th17 T cells and IL-17 responsiveness by circulating neutrophils, which protect against systemic C. albicans infection.


