Abstract
Aim
Our aim was to analyse 12-month outcomes of children who were prenatally exposed to the Zika virus and asymptomatic at birth.
Methods
This was an observational, exploratory study of infants exposed to the Zika virus during gestation and born between March 2016 and April 2017 without congenital Zika syndrome. They were followed until the age of 22 months. The outcome measure was neurodevelopment at 12 months of life, which was evaluated with the Bayley Scales of Infant and Toddler Development, Third edition (Bayley-III). The scores were adjusted for maternal education and prematurity.
Results
A total of 96 infants were included in the study and 35.4% scored below the normal range in at least one Bayley-III domain. The majority (91.2%) of the infants with delayed scores presented with language delay, which was not associated with the gestational age at exposure. Receptive language was more affected by exposure than expressive language (27.0% versus 19.8%). There was a direct, and significant, association between the head circumference Z-score at birth and language delay.
Conclusion
Language delay was associated with a smaller head circumference at birth in infants prenatally exposed to the Zika virus and born asymptomatic. This may indicate future learning difficulties.
Keywords: Children, congenital infection, language, neurodevelopment, Zika virus
INTRODUCTION
The first outbreak of the Zika virus infection in Brazil in 2015 was associated with an increased number of microcephaly cases and this was confirmed by the World Health Organization in 2016 (WHO) (1,2). This associated pathological condition is called congenital Zika syndrome and several studies have described its clinical spectrum since that outbreak (3-5).
In some congenital infections the outcome and severity of the fetal infection mainly depend on the gestational period in which the maternal infection occurred. These include toxoplasmosis, rubella and the parvovirus. Newborn infants exposed to these infectious agents during pregnancy may be born asymptomatic, show late manifestations during childhood or even develop symptoms during adulthood (6). It has been shown that asymptomatic children exposed to the Zika virus exhibit language delay during the first year of life (7-9). However, there is little information on which components of language function are affected. Research is also lacking on the relationship between early impairment, children who were asymptomatic at birth and the gestational period in which exposure occurred. We hypothesised that exposure during early gestation would be associated with neurodevelopmental abnormalities, even in asymptomatic newborn infants.
This study aimed to evaluate the neurodevelopment of infants who were exposed to the Zika virus, but born without congenital Zika syndrome, and the gestational period in which the exposure occurred. It also aimed to explore which components of the developmental domains were most affected and whether these impairments were associated with head circumference at birth.
METHODS
Study design and cohort
This was an observational, exploratory study that followed a cohort of children enrolled in the Vertical Exposure to Zika Virus and its Consequences for Child Neurodevelopment study. It was conducted at the Fernandes Figueira Institute , Rio de Janeiro, Brazil.
The infants who were prenatally exposed to the Zika virus were born between March 2016 and April 2017 and followed until the age of 22 months, with periodic anthropometric and developmental measurements.
We included children born with evidence of prenatal exposure to the Zika virus, but without congenital Zika syndrome at birth.
The exclusion criteria were the presence of congenital Zika syndrome (3), exposure to other congenital infections, namely syphilis, toxoplasmosis, cytomegalovirus, rubella, and herpes and asphyxia, congenital malformations, genetic syndromes, inborn errors of metabolism and fetal hydrops. We also excluded infants with incomplete anthropometric data at 12 months of age and missing neurodevelopment assessments.
Exposure
We related the timing of the exposure to the virus to the gestational age when the clinical symptoms of the Zika virus infection emerged in the pregnant women. These symptoms were fever with a maculopapular or pruritic rash (10). Exposure to the Zika virus during pregnancy was confirmed by testing the mother’s blood and urine, using polymerase chain reaction (RT-PCR) during the symptomatic acute viremia period.
Anthropometric measurements were performed at birth and at the follow-up visits. Weight was measured with a Filizola digital scale with 5 gram precision (Filizola®, São Paulo, Brazil). Height was measured with an appropriate ruler, with the child in the supine position, and head circumference was measured with an inelastic tape measure. Z-scores were calculated for each measurement using the Intergrowth-21 (11) reference curve at birth and the WHO reference curve (12) at one month of age and older.
All the children included in the study were evaluated by the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) (13). The assessment was performed between 12 and 18 months. Ages were adjusted for prematurity when the children were born preterm. The Bayley-III (13) covers three broad domains of neurodevelopment, which evaluate five independent outcomes. These are cognition, global language, including receptive and expressive language, global motor development and its components and fine and gross motor skills. The assessment was conducted in an enclosed room by trained psychologists. The composite scores for the broad domains range from 40 to 160 points, with a mean of 100 and a standard deviation of 15. This means that 85 would be one standard deviation below the mean and 115 points would be one standard deviation above the mean. A score of below 85 indicates mild delay and below 70 indicates severe delay. Expressive and receptive language scores and fine and gross motor development scores represent the child’s performance in a given sub-score and range from 1-19, with a mean of 10 and a standard deviation of three. For example, a score of seven represents one standard deviation below the mean and a score of 13 represents one standard deviation above the mean (13).
We evaluated both the continuous scores and the scores that indicated normal or delayed development. Values below one standard deviation of the standardised mean in at least one of the domains were considered to indicate delay. Values below two standard deviations were considered to indicate severe delay.
Variables
The variables that were analysed were gestational age in complete weeks, type of delivery, birth weight, height and head circumference with their respective standard deviations (Z-scores).
The socioeconomic evaluation of the families covered a number of factors, including age, any use of illicit drugs, maternal alcohol use or smoking and household income, which was assessed in relation to the Brazilian minimum wage. Maternal education was stratified into partial high school or less, complete high school to partial higher education and complete higher education and more. High school education normally ends when adolescents are 17 years of age. The following factors were also recorded: breastfeeding, months of exclusive breastfeeding and exclusive milk formula use, the number of people living in the same house and whether the father participated in childcare. All these variables were considered confounding factors with regard to development.
Sample size
Non-probabilistic convenience sampling was performed and included all children born asymptomatic who were included in the cohort of the main project.
Statistical analysis
A descriptive analysis was performed. Measures of central tendencies and variability, such as means and standard deviations and medians with interquartile ranges (IQRs), were used for continuous variables. Categorical variables were analysed by calculating proportions and performing association analyses using the chi-square test.
The Student’s t-test was performed to verify the association between confounding factors and the results of the Bayley-III scores in the cognitive, motor and language domains, as well as the receptive and expressive language and fine and gross motor sub-scores. The confounding factors considered were gestational age, type of delivery, maternal education, use of illicit drugs or alcohol or maternal smoking, breastfeeding, the number of people living in the same house and whether the father participated in childcare.
The results of these scores were considered to indicate normal or delayed development, as previously described. We stratified the children by the trimester of exposure during pregnancy. A multiple regression model was constructed to evaluate the influence of exposure to the Zika virus during pregnancy on the developmental domains of the Bayley-III. This considered a number of independent variables. These included maternal education, the trimester when the mother became infected, term and preterm birth, birth weight and the head circumference Z-score at birth and the weight and head circumference Z-score at the follow-up evaluation at about 12 months.
The regression models included the significant variables with a p value of 0.20. Five multiple regression models were constructed, with the following Bayley-III domains as dependent variables: cognition, global motor composite, fine and gross motor sub-tests, global language composite and receptive and expressive language sub-tests.
The database was prepared with EpiInfo software, version 7 (Centers for Disease Control and Prevention. Georgia, USA) and data were analysed with SPSS, version 22.0 (IBM Corp, New York USA). The significance level was p=0.05.
Ethical considerations
This study was approved by the Institution’s Research Ethics Committee (approval number CAAE 526756616000005269) and registered on clinical trials.gov (NCT03255369).
The mothers provided informed consent before being included in the study.
RESULTS
We examined 132 newborn infants, born without congenital Zika syndrome, after their mothers had been exposed to the Zika virus (Figure 1). Of these, 96 met the inclusion and exclusion criteria and were included in the study.
Figure 1-.
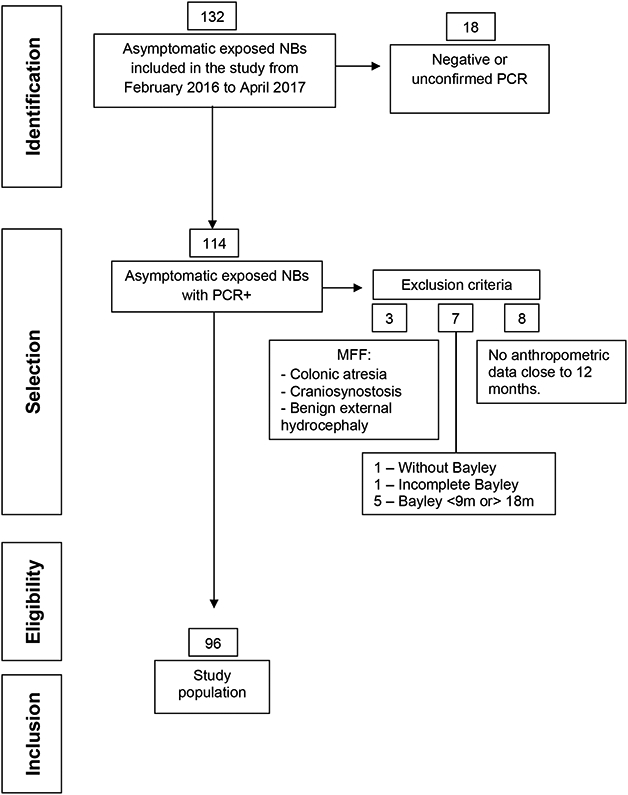
Study Flowchart
Maternal age ranged from 16 to 43 years, with a median of 31 years. (IQR 1). The median number of pregnancies was two (range 1-6) and median parity was two, with 1-4 deliveries. Overall, the mothers had a high level of education: 92.2% had completed high school and 30.0% had completed at least some kind of higher education. The mean monthly household income was equal to the median of 3.2 (IQR 3.3) Very few mothers used illicit drugs or alcohol (2.0%) or admitted tobacco use (4.0%).
Exclusive or combination breastfeeding was widely practiced during the first year of life: 43.5% of the mothers exclusively breastfed until at least four months and only 15.2% used milk formula from birth.
A homogeneous gender distribution was observed in the infant population. Most of them were born at term (87.5%), delivered by Caesarean section and had an adequate weight for their gestational age (Table 1). Admission to the neonatal intensive care unit was 19.8% and the main indication was early respiratory distress.
Table 1.
Perinatal and demographic characteristics of the studied population
| n | other values | |
|---|---|---|
| Male gender, n (%) | 96 | 47 (49) |
| Small for gestational age, n (%) | 96 | 5 (5.2) |
| Caesarean delivery, n (%) | 95 | 73 (76.8) |
| NICU admission, n (%) | 95 | 19 (20) |
| Maternal arterial hypertension, n (%) | 94 | 19 (20.2) |
| Pregnancy-induced diabetes, n (%) | 94 | 5 (5.3) |
| APGAR score at one minute, n, median (IQR) - range | 93 | 9 (1) - (3-10) |
| APGAR score at five minutes n, median (IQR) - range | 93 | 9 (1) - (6-10) |
| Birth weight (grams), n, mean (SD) | 96 | 3,206 (544.4) |
| Gestational age (weeks) n, mean (SD) | 96 | 38.3 (1.8) |
| Head circumference (cm) n, mean (SD) | 96 | 34.5 (1.7) |
NICU, neonatal intensive care unit; IQR, interquartile range; SD, standard deviation.
The median age at anthropometric assessment was 14 months (IQR 3) and ranged from 8-22 months and the mean Z-scores for weight, length and head circumference were within the normal ranges (Table 2).
Table 2.
Mean and standard deviations scores of anthropometric measurements at birth and at approximately 12 months of age.
| Birth | 12 months | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Weight (g) | 0.26 (0.98) | 0.50 (1.09) |
| Height (cm) | 0.25 (1.26) | 0.18 (1.24) |
| Head circumference (cm) | 0.91 (1.16) | 0.81 (1.09) |
SD, standard deviation.
Maternal exposure to the virus occurred predominantly in the second trimester (65.3%), with 20.0% exposed in the first trimester and 14.7% in the third.
The median age at the Bayley-III evaluation was 12 months (IQR 1), ranging from 11 to 19 months. Although the mean motor, language and cognitive scores were within the normal ranges for most of the children, the mean language scores were near the lower limit of normality (Table 3). The receptive language scores were lower than the expressive language scores, with 26/96 (27.0%) scoring less than seven for receptive language and 19/96 (19.7%) scoring less than seven for expressive language.
Table 3.
Mean and standard deviation scores of the different domains in Bayley-III and their subtests at the age of assessment.
| Domains | Mean (SD) |
|---|---|
| Cognition | 106.3 (12.5) |
| Language | 89.5 (13.5) |
| Motor | 98.9 (10.3) |
| Receptive language | 7.6 (2.2) |
| Expressive language | 8.8 (2.7) |
| Fine motor | 9.7 (2.1) |
| Gross motor | 9.8 (2.1) |
SD, standard deviation.
We found that 35.4% of the children had a delay in at least one Bayley-III domain, and 91.2% of the delays were in language. Of the 34 children with any altered scores, 88.2% showed a delay in only one domain, one child exhibited delays in two domains and three children exhibited delays in three domains. There were eight children who scored below 70, indicating severe delay: six in the three language domains, one in the motor domain and one in the cognition domain.
Anthropometric data at birth and at 12 months of age were related to psychometric test results in the language domain and these were considered to indicate normal or delayed development. The only anthropometric measure that showed statistical significance when the groups with delayed and normal language scores were compared was the Z-score for head circumference at birth. The Z-score was significantly lower, when measured with the Student’s t-test (p=0.001), in children with results indicating delay than in those with results indicating no language delay (0.37 ±1.3 versus 1.17 ±1.0). Other anthropometric data were not associated with the Bayley-III results at birth or at approximately 12 months. The multiple regression analysis identified significant associations between the head circumference Z-score at birth with all language domains. The coefficient for the global language score was 4.98 (p=0.002, 95% CI 1.86-8.09).For the receptive score, it was 0.39 (p=0.048, 95% CI 0.003-0.78) and for the expressive score it was 1.07 (p=0.001, 95% CI 0.42-1.72). All the models were adjusted for maternal education, birthweight and preterm birth. When it came to the cognitive and motor domains, the multiple regression analysis only identified a significant association between maternal education and fine motor function, with a coefficient of 1.16 (p=0.007, 95% CI 0.32-2.01).
Scores in the motor and cognition domains were not significantly associated with the trimester of exposure or with birthweight and head circumference at birth. We found a homogeneous distribution of scores that indicated delayed exposure by trimester, with a slight predominance in infections in the first trimester. However, the differences were not significant, according to the chi-square test (p=0.76) (Table 4). Despite the high prevalence of language delay, there was no predominant delay in any of the trimesters.
Table 4 –
Distribution of the proportion of delays by trimester of maternal Zika virus infection, according to the Bayley-III. Any score below 85 in any of the domains indicates delay.
| Trimester of exposure |
Delay in any domain | Language delay | Total | ||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| 1st trimester | 11 (57.9) | 8 (42.1) | 12 (63.2) | 7 (36.8) | 19 (100) |
| 2nd trimester | 42 (67.7) | 20 (32.3) | 44 (70.9) | 18 (29.0) | 62 (100) |
| 3rd trimester | 9 (64.3) | 5 (35.7) | 9 (64.3) | 5 (35.7) | 14 (100) |
| Total | 62 (65.3) | 33 (34.7) | 65 (68.4) | 30 (31.6) | 95 (100) |
One mother was not sure when the Zika virus infection occurred, but her asymptomatic newborn twin was investigated because the other twin was born with microcephaly.
DISCUSSION
The offspring of mothers with Zika infections, confirmed by RT-PCR, had median scores in all Bayley-III domains and these fell within the normal range. Mean scores for language, especially receptive language, were within the expected age range. However, they were close to the lower limit, unlike those in the other evaluated domains. We found a high proportion of children with language delay when we evaluated scores below the normal range in this population. The proportion of children with severe delay, defined by a score of less than 70, in the language domain and its sub-scores, was also analysed. This showed that scores below 70 were not common, indicating that impairment in this function was not severe, at least at this early stage of development. Few studies have addressed the development of children exposed to, and infected by, the Zika virus during the fetal period (4,14-17). One multi-centre study used the General Movement Assessment to evaluate 111 infants exposed to the Zika virus in utero and 333 control infants. It reported the absence of fidgety movements in infants with microcephaly and identified adverse outcomes in newborn infants without microcephaly (5).
Other studies of the same population that our sample originated from analysed the developmental outcomes in a cohort of children, with and without microcephaly, whose mothers were diagnosed with the Zika virus during pregnancy (4,5,8,16). Delays in the motor and cognition domains were found in 16.4% and 9.6% of the assessed children, respectively (16). Another study conducted in Salvador, Brazil, investigated the development of children vertically exposed to the Zika virus without microcephaly at birth. The results showed that 34% of these infants had delays in at least one of the Bayley-III domains, predominantly language delay (31%). In contrast, motor and cognitive delays were observed in 3% and 14% of the children, respectively (7). Another important finding of our study was the association between the head circumference Z-score at birth with global language delay and its components, including receptive and expressive language. A study published by our research group analysed the spectrum of clinical findings in a cohort of 219 children with confirmed intrauterine exposure to the Zika virus. We reported associations between smaller head circumferences at birth with below-average cognitive and language scores (9).
Language development is known to be compromised even when brain insults do not result in global or predominant motor delays (17-19). Cognitive impairments have been reported in children with a history of neonatal encephalopathy, but without alterations in their motor skills (17,18). Although children with neurodevelopmental disorders have a high rate of motor impairment, most of the need for educational support is due to delays in language and reading ability (20). In our study, language delay was most prevalent, especially receptive language delay. This may be because cognitive changes become more evident at older ages, when demands become increasingly complex. Some studies have shown that subtle cognitive abnormalities become more evident when the child reaches school age and that normal neurodevelopment in the initial assessment does not predict future functionality (19,20). One example is language impairment. The child can initially present with minor early language delay, with otherwise normal development, but signs of language impairment develop later in childhood (21). Receptive language is needed, so that children can understand commands and instructions and it is essential for the educational process (22). A delay in language development has been reported in children born very preterm, with the greatest impairment in receptive language (23). A disturbance in expected language development at an early age, within the first 12 months of life, has been reported (24). A review by Conti-Ramsden and Durkin reported that language impairment is a multifactorial dysfunction that can have an impact on the educational process. Affected children can present with early language delay, even when the neuroimaging characteristics are not apparent (22). However, the results of the Bayley-III assessment should be interpreted with caution, as there is increasing discussion in the literature about the whether the Bayley Scales overestimate development. This would, in turn, underestimate the prevalence of developmental delays (25). Thus, the slight alterations in development observed by our study may signify greater damage. They may also predict future cognitive deficits when the need to understand verbal commands becomes more demanding.
In this study, most newborn infants were born at term with an adequate weight for gestational age. Over the past two years, many studies have shown that when mothers contract the Zika virus infection during pregnancy this can lead to spontaneous abortions and stillbirths (26-28). Most of the newborn infants that survived the aggressive effects of this virus during pregnancy in those studies were term infants, like the majority in our study.
We found no predominance of delayed scores in children whose mothers were infected in the first trimester. It has been reported that insults to brain development are more severe when maternal infection occurs early in pregnancy. This suggests that the timing of exposure is one of the most relevant risk factors for the development of congenital Zika syndrome (29). As our cohort only comprised children who were asymptomatic at birth, those who were severely compromised or died in utero as a result of early exposure were not included. Some published studies have evaluated neurological impairment in children exposed to the Zika virus and have shown that such impairment was severe. They noted a high predominance of microcephaly and neurodevelopmental disorders associated with maternal infection during early pregnancy (16,30).
Strengths and limitations
Our study was conducted at a federal reference institution for the congenital Zika virus infection, which allowed us to evaluate a relatively large population. This was a prospective study that included children exposed to the Zika virus in utero, who did not have malformations or other clinical symptoms at birth. All children were born from mothers who showed clinical symptoms and had positive RT-PCR results.
A limitation of this study was that we were unable to obtain Bayley-III assessments or anthropometric measurements for approximately 13% of the children. This was because the mothers missed the appointments These missing data may have caused selection bias and that should be considered when interpreting the results. Another limiting factor was that we did not have a control group that were not exposed to the virus during fetal life and this restricts the generalisability of the results. The finding of early language delays at 12 months of age was a limitation when it came to interpreting the results, but this may have been an indication of future language impairment.
CONCLUSION
This study analysed the 12-month outcomes of children who were prenatally exposed to the Zika virus and asymptomatic at birth. We found that the greatest number of scores that indicated developmental delay were in the language domain, predominantly the receptive language sub-score. This finding may indicate long-term cognitive impairment and suggests that children who are asymptomatic at birth, despite being exposed to the Zika virus during the fetal period, may still be susceptible to adverse health outcomes later in life. This highlights the importance of conducting further studies with long-term follow-up periods, especially with regard to language impairment and learning difficulties. Future studies should also include a control group of children born outside the Zika virus epidemic regions, to assure lack of exposure. This is because it is very difficult to determine the absence of exposure in pregnant women from epidemic areas.
KEY NOTES.
This study used the Bayley Scales of Infant and Toddler Development, Third Edition to measure outcomes in 96 children who were prenatally exposed to the Zika virus and asymptomatic at birth.
We found that 35.4% scored below the normal range in at least one domain and 91.2% of these had language delays.
Delays were not associated with gestational age at exposure but were associated with smaller head circumferences at birth.
Acknowledgments
FUNDING
This study received funding from the Wellcome Trust, the United Kingdom’s Department for International Development (205377/Z/16/Z) and the European Union’s Horizon 2020 research and innovation programme (ZikaPLAN grant agreement no. 734584)
ABBREVIATIONS
- Bayley-III
Bayley Scales of Infant and Toddler Development, Third Edition
- RT-PCR
real time polymerase chain reaction
- WHO
World Health Organization
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/APA.15878
REFERENCES
- 1.Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika Virus and Birth Defects – Reviewing the Evidence for Causality. N Engl J Med. 2016; 19:374(20):1981–1987. [DOI] [PubMed] [Google Scholar]
- 2.CDC. CDC Concludes Zika Causes Microcephaly and Other Birth Defects. Centers for Disease Control and Prevention. 2016. Available from: http://www.cdc.gov/media/releases/2016/s0413-zika-microcephaly.html . [Google Scholar]
- 3.Moore CA, Staples JE, Dobyns WB, Pessoa A, et al. Characterizing the Pattern of Anomalies in Congenital Zika syndrome for Pediatric Clinicians. JAMA Pediatr. 2017; 1:171(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes Moreira ME, Nielsen-Saines K, Brasil P, et al. Neurodevelopment in Infants Exposed to Zika Virus In Utero. N Engl J Med. 2018;13:379(24):2377–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einspieler C, Utsch F, Brasil P, et al. Association of Infants Exposed to Prenatal Zika Virus Infection With Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw Open. 2019; 4;2(1):e187235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol. 2015; 42(1):77–103. [DOI] [PubMed] [Google Scholar]
- 7.Faiçal AV, de Oliveira JC, Oliveira JVV, et al. Neurodevelopmental delay in normocephalic children with in utero exposure to Zika virus. BMJ Paediatr Open. 2019; 5;3(1):e000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peçanha PM, Gomes Junior SC, Pone SM, et al. Neurodevelopment of children exposed intrauterus by Zika virus: A case series. PLoS One. 2020; 28:15(2):e0229434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cranston JS, Tiene SF, Nielsen-Saines K, et al. Association Between Antenatal Exposure to Zika Virus and Anatomical and Neurodevelopmental Abnormalities in Children. JAMA Netw Open. 2020;3(7):e209303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atif M, Azeem M, Sarwar MR, Bashir A. Zika virus disease: a current review of the literature. Infection. 2016;44(6):695–705. [DOI] [PubMed] [Google Scholar]
- 11.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and gender: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 6;384(9946):857–868. [DOI] [PubMed] [Google Scholar]
- 12.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr (Suppl): 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 13.Bayley N Bayley Scales of Infant Mental Development. Third Editon. San Antonio, Texas: The Psychological Corporation; 2006. [Google Scholar]
- 14.Reid S, Rimmer K, Thakur K. Zika Virus and Neurologic Disease. Neurol Clin. 2018;36(4):767–787. [DOI] [PubMed] [Google Scholar]
- 15.Brasil P, Pereira JP Jr, Moreira ME, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;15;375(24):2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen-Saines K, Brasil P, Kerin T, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med. 2019;25(8):1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro KA, Kim H, Mandelli ML, et al. Early changes in brain structure correlate with language outcomes in children with neonatal encephalopathy. Neuroimage Clin. 2017; 10;15:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas A, Shankaran S, McDonald SA, et al. Cognitive Outcomes After Neonatal Encephalopathy. Obstet Gynecol Surv. 2015;70(8):487–488. [Google Scholar]
- 19.Gilkerson J, Richards JA, Warren SF, et al. Language Experience in the Second Year of Life and Language Outcomes in Late Childhood. Pediatrics. 2018;142(4):e20174276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luciana M Cognitive development in children born preterm: implications for theories of brain plasticity following early injury. Dev Psychopathol. 2003;15(4):1017–1047. [DOI] [PubMed] [Google Scholar]
- 21.Conti-Ramsden G, Durkin K. What factors influence language impairment? Considering resilience as well as risk. Folia Phoniatr Logop. 2015;67:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selassie GR, Jennische M, Kyllerman M, et al. Comorbidity in severe developmental language disorders: neuropediatric and psychological considerations. Acta Paediatr. 2005;94(4):471–478. [DOI] [PubMed] [Google Scholar]
- 23.Góes FV, Méio MDBB, Mello RR, et al. Evaluation of neurodevelopment of preterm infants using Bayley III Scale. Rev Bras Saúde Matern Infant. 2015;15(1):47–55. [Google Scholar]
- 24.Gonzalez-Gomez N, O’Brien F, Harris M. The effects of prematurity and socioeconomic deprivation on early speech perception: A story of two different delays. Developmental Science. 2021;24(2):e13020. [DOI] [PubMed] [Google Scholar]
- 25.Anderson PJ, Burnett A. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin Neuropsychol. 2017;31(2):371–381. [DOI] [PubMed] [Google Scholar]
- 26.Wen Z, Song H, Ming GL. How does Zika virus cause microcephaly? Genes Dev. 2017; 31(9):849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds MR, Jones AM, Petersen EE, et al. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(13):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker CL, Little ME, Roby JA, et al. Zika virus and the nonmicrocephalic fetus: why we should still worry. Am J Obstet Gynecol. 2019;220(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chimelli L, Melo ASO, Avvad-Portari E, et al. The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol. 2017;133(6):983–999. [DOI] [PubMed] [Google Scholar]
- 30.Marques VM, Santos CS, Santiago IG, et al. Neurological Complications of Congenital Zika Virus Infection. Pediatr Neurol. 2019;91:3–10. [DOI] [PubMed] [Google Scholar]


