Abstract
Background and aims:
Creatinine-based MDRD and CKD-EPI equations include a race correction factor, which results in higher eGFR in Black patients. We evaluated the impact on our patient population upon adoption of the CKD-EPI equation and the removal of the race correction factor from the equation.
Materials and methods:
Retrospective analysis of blood creatinine results and respective eGFR values calculated by the MDRD or CKD-EPI equation without the race correction factor (CKD-EPINoRace) in a large academic medical system over a 20.5-month period.
Results:
In our population, when changing from MDRD to CKD-EPINoRace, we observed that 3.5% of all patients were reclassified to categorically have worse kidney function. However, we also observed fewer patients overall with eGFR below 60 mL/min/1.73 m2. Around 60 and 20 mL/min/1.73m2, 2.96% and 0.16% of all patients >65 years of age were reclassified, as were 4.29 and 0.03% of all Black patients, respectively. When calculated with CKD-EPINoRace, median eGFR was not meaningfully different between Black and non-Black patients (p = 0.02).
Conclusions:
Changing from MDRD to CKD-EPINoRace could lead to a lower referral rate to nephrology. The distributions of creatinine and eGFR calculated with CKD-EPINoRace were not meaningfully different in Black and non-Black patients.
Keywords: glomerular filtration rate, MDRD, CKD-EPI, chronic kidney disease, race
Introduction
Chronic kidney disease (CKD) is a growing public health concern with an estimated prevalence of 11.5% [1]. Therapeutic decisions rely largely on laboratory assessment of kidney function [2]. While measured glomerular filtration rate (GFR) is considered the most accurate indicator of filtration function, it is expensive and impractical for routine analysis [3–5]. Instead, serum/plasma creatinine is routinely used to estimate glomerular filtration rate. Creatinine concentration by itself is insufficient as an indicator of kidney function, because while creatinine is primarily cleared by glomerular filtration, the amount of creatinine produced in each patient varies by muscle mass and diet. As a result, the same creatinine concentration equates with different levels of kidney function, depending on the person [6]. Based on these principles, the National Kidney Disease Education Program (NKDEP) recommended that clinical laboratories report estimated GFR (eGFR) along with creatinine in adults [7].
There have been many equations developed over time to estimate GFR from blood creatinine, including Cockcroft-Gault, six- and four- parameter Modification of Diet in Renal Disease (MDRD), isotope-dilution mass spectrometry-traceable MDRD, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [1, 8–12]. Currently, the MDRD family of equations are used in >65% of North American clinical laboratories [13]. Despite their widespread use, these equations have limited precision and systematically underestimate GFR at higher values, which may be due to the fact that the MDRD Study equation was developed in a CKD population without the inclusion of healthy volunteers [14]. To overcome the limitations of the MDRD equations, the more accurate CKD-EPI equation was developed in 2009, which is now widely recommended [1, 15, 16].
The MDRD and CKD-EPI equations each include a correction factor to account for patient race (i.e., there is a parameter that increases eGFR when a patient is identified as Black or African American). This parameter resulted from mathematical modeling, which suggested that race improved the correlation between measured and estimated GFR, even when including sex and age in the model. Similar to many other health care systems, our laboratory provided eGFR calculated with and without the race correction factor, leaving providers to choose among the two calculations. This placed our providers in the awkward position of directing clinical management based on a poorly defined variable – race. The choice between using the eGFR calculated with or without the race correction could ultimately affect when a patient is referred to a nephrologist (e.g., eGFR <60 mL/min/1.73m2 in our system), be considered for dialysis, or be made eligible for kidney transplant (e.g., eGFR <20 mL/min/1.73m2 in our system) [16]. Because the race correction factors in the MDRD and CKD-EPI equations lead to a higher estimated GFR, patients who are identified as Black or African American by their provider could be less likely to receive more advanced interventions than non-Black or non-African American patients with the same age, sex, and blood creatinine concentration. Many institutions have reconsidered the appropriateness of using these race correction factors [17, 18].
In our medical system, consideration of removing the race correction factor from the calculation of eGFR was motivated by medical students and faculty insisting that race does not have the scientific rigor that we expect of our diagnostic tools. More specifically: it is not possible to make appropriate medical decisions when employing a race correction in clinical algorithms. This point is made clear when patients are multiracial or do not identify with any race. Currently, a joint task force convened by the American Society of Nephrology and the National Kidney Foundation is evaluating approaches to replace current race-based equations for eGFR [19].
Our institution moved from MDRD to CKD-EPI in calculating eGFR and consciously decided to remove the race correction factor during the process. As part of this transformation, we performed a retrospective analysis of blood creatinine results in our patient population, which we present here with the goal of informing the ongoing nationwide conversation about the use of race in calculating and reporting eGFR.
Materials and Methods
Individual serum and plasma creatinine results from patients evaluated within the University of Washington Medicine System from January 1, 2018 to August 15, 2019 were retrieved from a departmental data warehouse containing order and result data from Sunquest Laboratory (Tucson, AZ, UW Medicine’s laboratory information system). Creatinine was measured using the standardized Jaffe method with calibrators traceable to the NIST Standard Reference Material (SRM 3667) on the Beckman Coulter AU system. For each result, associated metadata included laboratory accession number, age, sex, and race, patient care location, sample collection time, result time, test name, and order name. Demographic information (i.e., age, sex, race, and patient location) and creatinine results were used in the final data analysis. The analysis of de-identified data for aggregation and publication was approved by the Human Subjects Division at the University of Washington (STUDY00012562).
Data exclusion, transformation, analysis, and visualization were conducted using packages and functions (e.g., tidyverse, dplyr, ggplot2, hablar) within the R open source statistical software environment (version 4.0.2, https://www.R-project.org). Estimated GFR was calculated with one of two creatinine-based equations (with serum creatinine values standardized to the IDMS reference method): the 4-variable MDRD or the CKD-EPI equations:
4-variable MDRD equation
where SCr is the creatinine concentration;
CKD-EPI equation
where κ = 0.7 (females) or 0.9 (males), α = −0.329 (females) or −0.411 (males), min(SCr/κ, 1) = the minimum of SCr/κ or 1, max(SCr /κ, 1) = the maximum of SCr/κ or 1. The units of variables are: age in years, serum creatinine (SCr) in mg/dL, and eGFR in mL/min/1.73 m2.
The race correction factor was excluded in some calculations where denoted. In data visualization, we truncated eGFR if it exceeded 150 mL/min/1.73 m2 by the MDRD equation because (i) it represented a small proportion of all tests and (ii) measured GFR exceeding 150 mL/min/1.73 m2 was rarely observed/not included in the CKD-EPI study [1]. Reclassification rates were calculated by dividing the number of patients that changed category when eGFR was calculated by the second equation by the number of patients originally in the category when eGFR was calculated by the first equation.
Statistical Analysis
For multiple group comparison of creatinine and eGFR distributions, Kruskal-Wallis test was performed. In order to identify meaningful differences in distributions, a p-value of 0.01 was considered statistically significant [20].
Results
Demographic and laboratory information of recruited patient population
Information was extracted from the laboratory data warehouse for all UW Medicine outpatients, inpatients, and emergency room patients over a 20.5-month period and totaled 1,059,002 creatinine results. To avoid the bias in distribution caused by patients with multiple creatinine results [mean (SD) creatinine for all results was 1.17 (1.19) mg/dL vs. 0.94 (0.75) mg/dL for only first results], only the first result was used for each patient. Race was self-reported as White, Asian, American Indian or Alaska native, Black or African American, or Native Hawaiian or other Pacific Islander. Sex was self-reported as female or male. Patients with unknown race or sex were excluded. We included all adult patients but excluded geriatric patients older than 105 years due to concerns for date of birth errors in the electronic health record. After exclusions (repeat results, unknown race or sex, age < 18 or >105 years), 241,760 patient results were available for subsequent data analysis (Figure S1).
Patient demographics are summarized in Table 1. The cohort was composed of 51% females and the median (interquartile range) age was 53 (28) years. The proportions of patients aged 18–40, 41–65, and older than 65 y were 31, 45, and 24%, respectively. The percentages of Black patients and patients of other racial minorities were 9 and 22%, respectively. Among these racial minorities, the Asian population was 10% of the full cohort. Outpatients and emergency department patients accounted for 96% of the laboratory values, with only 4% from inpatient settings. The mean creatinine was 0.94 mg/dL (range 0.2–29.07 mg/dL) (Table S1).
Table 1.
Patient demographic information
| Median age (interquartile range), y | 53 (28) |
| Age, N (%) | |
| 18–40 y | 73,742 (31) |
| 41–65 y | 107,961 (45) |
| 66–70 y | 21,593 (9) |
| 71–75 y | 15,696 (6) |
| 76–80 y | 10,198 (4) |
| >80 y | 12,570 (5) |
| Women, N (%) | 122,521 (51) |
| Race, N (%) | |
| White | 167,105 (69) |
| Black or African American | 20,714 (9) |
| Asian | 25,071 (10) |
| American Indian or Alaska Native | 3,970 (2) |
| Native Hawaiian or other Pacific Islander | 2,214 (1) |
| Other | 22,686 (9) |
| Location, N (%) | |
| Outpatient | 170,941 (71) |
| Emergency room | 58,889 (24) |
| Inpatient | 11,930 (5) |
Serum creatinine distributions in Non-Black, Black, and Asian patient populations
We first examined the distributions of serum creatinine in different patient populations. Histograms of creatinine ranging 0–5 mg/dL were plotted (Figure 1). There were no obvious differences between Black and non-Black patients; however, Asian patients appeared to have lower creatinine results on average. There was a statistically significant difference between the medians of each group (0.83, 0.88, and 0.78 mg/dL for non-Black and non-Asian, Black, and Asian populations, respectively; p<0.0001, Kruskal-Wallis; Table S2). The concentrations of creatinine at the 10th, 25th, 50th, 75th, and 90th percentiles of each patient population were compared (Table S2) and the greatest difference of creatinine concentration between groups was 0.18 mg/dL (i.e., the difference between 1.35 mg/dL in Black and 1.17 mg/dL in Asian) at the 90th percentile.
Figure 1. Serum creatinine distributions.
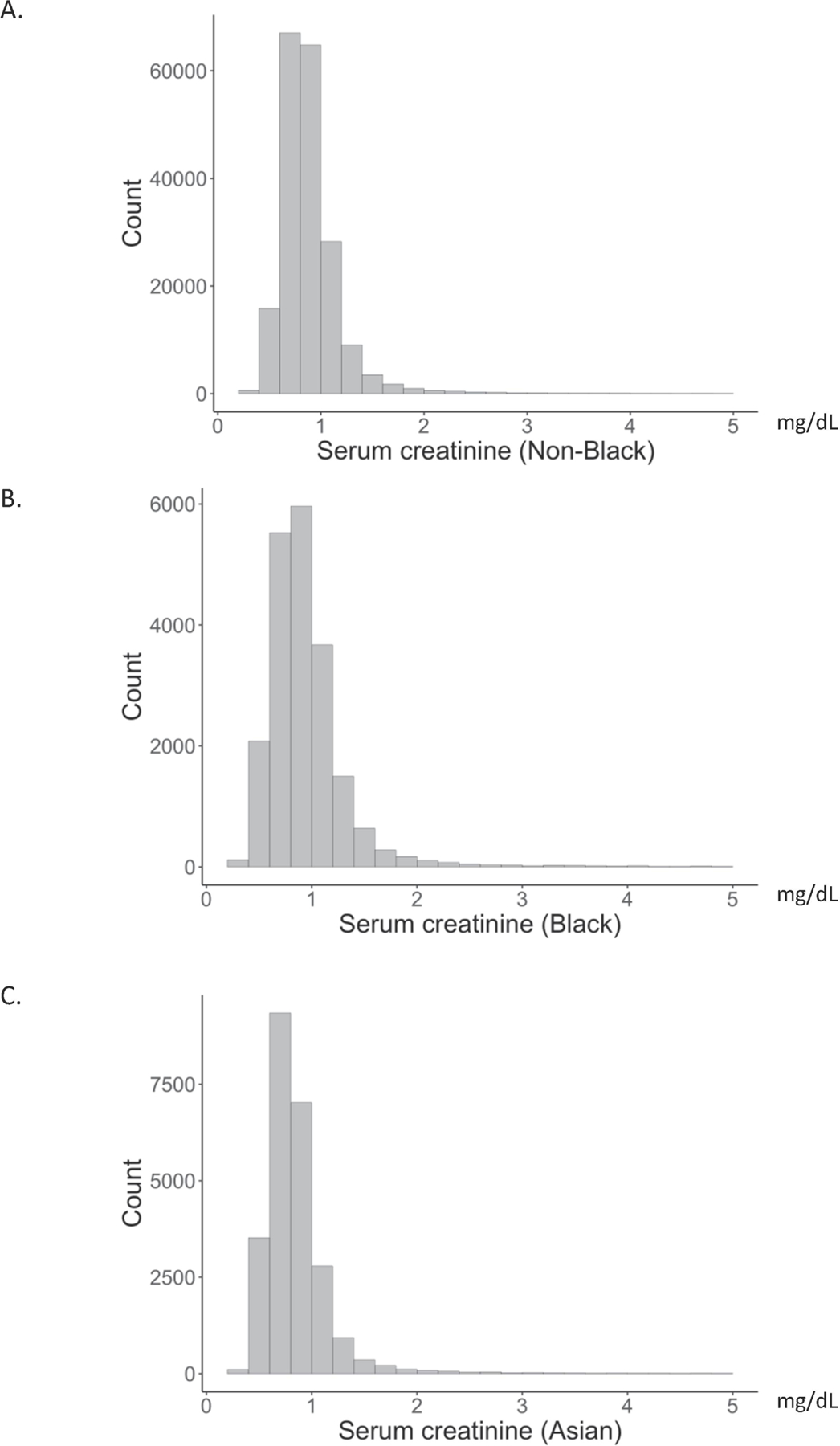
Histograms of serum creatinine in self-described non-Black (A), Black (B), and Asian (C) patients. The x-axis represents serum creatinine, which was truncated at 5 mg/dL, and y-axis represents the number of patients in each range.
Comparing MDRD and CKD-EPI equations with the race correction factors
To evaluate the performance of the MDRD and CKD-EPI equations in our local patient population, we performed method comparisons (Figure S2). As expected from previous studies, the two equations demonstrated similar performance when eGFRMDRD < 60 mL/min/1.73m2, with eGFRCKD-EPI = 1.062 × eGFRMDRD −1.623. Also as expected, for eGFRMDRD in the range of 60 to 105 mL/min/1.73m2, the CKD-EPI equation led to slightly higher values, with eGFRCKD-EPI = 1.064 × eGFRMDRD + 1.326 [1]. For eGFRMDRD >105 mL/min/1.73m2, a high proportion (59%) of eGFRCKD-EPI values were below eGFRMDRD.
Changes in the number of patients in different eGFR ranges
To assess the downstream effects on the medical system of switching from the MDRD to the CKD-EPI equation without the race correction factor (CKD-EPINoRace) (i.e., potential changes in referral or consideration for kidney replacement therapies), the population distribution was evaluated across the full eGFR range (Table 2). When eGFR was calculated by CKD-EPINoRace, the number of patients with eGFR in the ranges of 60–89 and 45–59 mL/min/1.73m2 were 9% and 2% lower, respectively, compared to when eGFR was calculated by the MDRD equation. No changes in the number of patients were observed for the ranges below 45 mL/min/1.73m2.
Table 2.
Number and proportion of patients within different eGFR ranges based on eGFR calculated by four different equations
| eGFR (ml/min/1.73m2) | with race factor N (%) |
without race factor N (%) |
||
|---|---|---|---|---|
| MDRD | CKD-EPI | MDRD | CKD-EPI | |
| ≥ 90 | 108,503 (45) | 136,348 (56) | 103,507 (43) | 132,957 (55) |
| 60–89 | 102,494 (42) | 78,447 (32) | 105,904 (44) | 80,940 (33) |
| 45–59 | 18,251 (8) | 14,734 (6) | 19,335 (8) | 15,298 (6) |
| 30–44 | 7,234 (3) | 6,879 (3) | 7,611 (3) | 7,119 (3) |
| 15–29 | 2,973 (1) | 2,989 (1) | 3,049 (1) | 3,043 (1) |
| <15 | 2,305 (1) | 2,363 (1) | 2,354 (1) | 2,403 (1) |
Reclassification of eGFR category when moving from MDRD to CKD-EPINoRace
To explore the reclassification associated with the change from MDRD to CKD-EPINoRace, we calculated the reclassification rates across the full cohort (Table 3) and in specific patient populations, including Black patients (Table S3), Asian patients (Table S4), and senior patients older than 65 years (Table S5). In the full cohort, the reclassification rate was highest (29.60%) for patients in the 60–89 range for the MDRD equation, who were reclassified into the ≥ 90 mL/min/1.73m2 category with the CKD-EPINoRace. The reclassification rate was similarly high for the patients who moved from 45–59 to 60–89 mL/min/1.73m2 (23.49%, Table 3). In contrast, the highest reclassification rate in the Black population (38.08%) was for patients that were reclassified from 45–59 to 30–44 mL/min/1.73m2. For the older patient population (> 65 years of age), the highest reclassification rate was highest for the patients that moved from ≥ 90 to 60–89 mL/min/1.73m2 (34.83%, Tables S3 & S4).
Table 3.
Reclassification of eGFR category upon adoption of the CKD-EPI equation without the race correction factor (CKD-EPINoRace) in the full cohort.a
| MDRD (mL/min/1.73m2), N | CKD-EPINoRace (mL/min/1.73m2), N (%) | |||||
|---|---|---|---|---|---|---|
| ≥ 90 (N=132,957) | 60–89 (N=80,940) | 45–59 (N=15,298) | 30–44 (N=7,119) | 15–29 (N=3,043) | < 15 (N=2,403) | |
| ≥ 90 (N=108,503) | 5,887 (5.43) | |||||
| 60–89 (N=102,494) | 30,341 (29.60) | 1,387 (1.35) | ||||
| 45–59 (N=18,251) | 4,287 (23.49) | 857 (4.70) | ||||
| 30–44 (N=7,234) | 804 (11.11) | 307 (4.24) | ||||
| 15–29 (N=2,973) | 139 (4.68) | 129 (4.34) | ||||
| < 15 (N=2,305) | 31 (1.34) | |||||
The reclassification rate was calculated by dividing the number of patients who were classified into a different category when eGFR was calculated with the CKD-EPINoRace equation compared to the MDRD equation by the total number of patients in the original category when calculated by the MDRD equation.
To understand the characteristics of the patients with categorical change of eGFR, we focused on two cut-offs, 60 and 20 mL/min/1.73m2, which are criteria often used in our clinical practice to determine referral to nephrology or eligibility for transplant listing, respectively. Notably, most of the patients reclassified to have worse kidney function were older than 65 y (852/1387 patients, 61%; 1.42% of all patients >65 y in the full cohort; Table 4). The proportion of patients who self-identified as Black (888/1387 patients, 64%; 4.29% of all Black patients) was higher than the proportion of patients self-identified as Black in the whole cohort (9%, Table 4).
Table 4.
Characteristics of the patient population who were reclassified upon adoption of the CKD-EPINoRace equation.a
| eGFR MDRD | ≥ 60 | < 60 | ≥ 20 | < 20 |
| eGFR CKD-EPI.NoRace | < 60 | ≥ 60 | < 20 | ≥ 20 |
| No. of patients, N (% of the entire cohort) | 1,387 (0.6) | 4,287 (1.8) | 133 (0.06) | 50 (0.02) |
| Male, N (% of all males) | 891 (0.75) | 1,588 (1.33) | 93 (0.08) | 14 (0.01) |
| Age > 65 y, N (% of patients >65 y) | 852 (1.42) | 927 (1.54) | 94 (0.16) | 1 (0.0) |
| Black race, N (% of Black patients) | 888 (4.29) | 0 (0.0) | 62 (0.03) | 0 (0.0) |
eGFR Units: mL/min/1.73 m2.
Comparison of eGFR distributions for Black and non-Black patients
The distribution of eGFR for non-Black patients shifted to higher values with CKD-EPI (CKD-EPINoRace is the same as CKD-EPI for non-Black patients, Figure 2A, B, D & S4B), as expected. Upon eliminating the race correction factor from the CKD-EPI equation, the distribution of eGFR for Black patients shifted lower (Figure 2A, B, C & S4B). Interestingly, the distributions of eGFR for CKD-EPINoRace were not meaningfully different for Black and non-Black patients (Figure 2B & S4B), with median eGFR of 92 for both groups (p = 0.02, Kruskal-Wallis). The mean change in eGFR when replacing the standard MDRD equation with CKD-EPINoRace was −15 and 3 mL/min/1.73 m2 for Black and non-Black patients, respectively (Figure S3B & S3C). The removal of the race correction factor had similar effects on the distribution of eGFR for males and females (Figure S5).
Figure 2. eGFR distributions in self-described Black and non-Black patients.
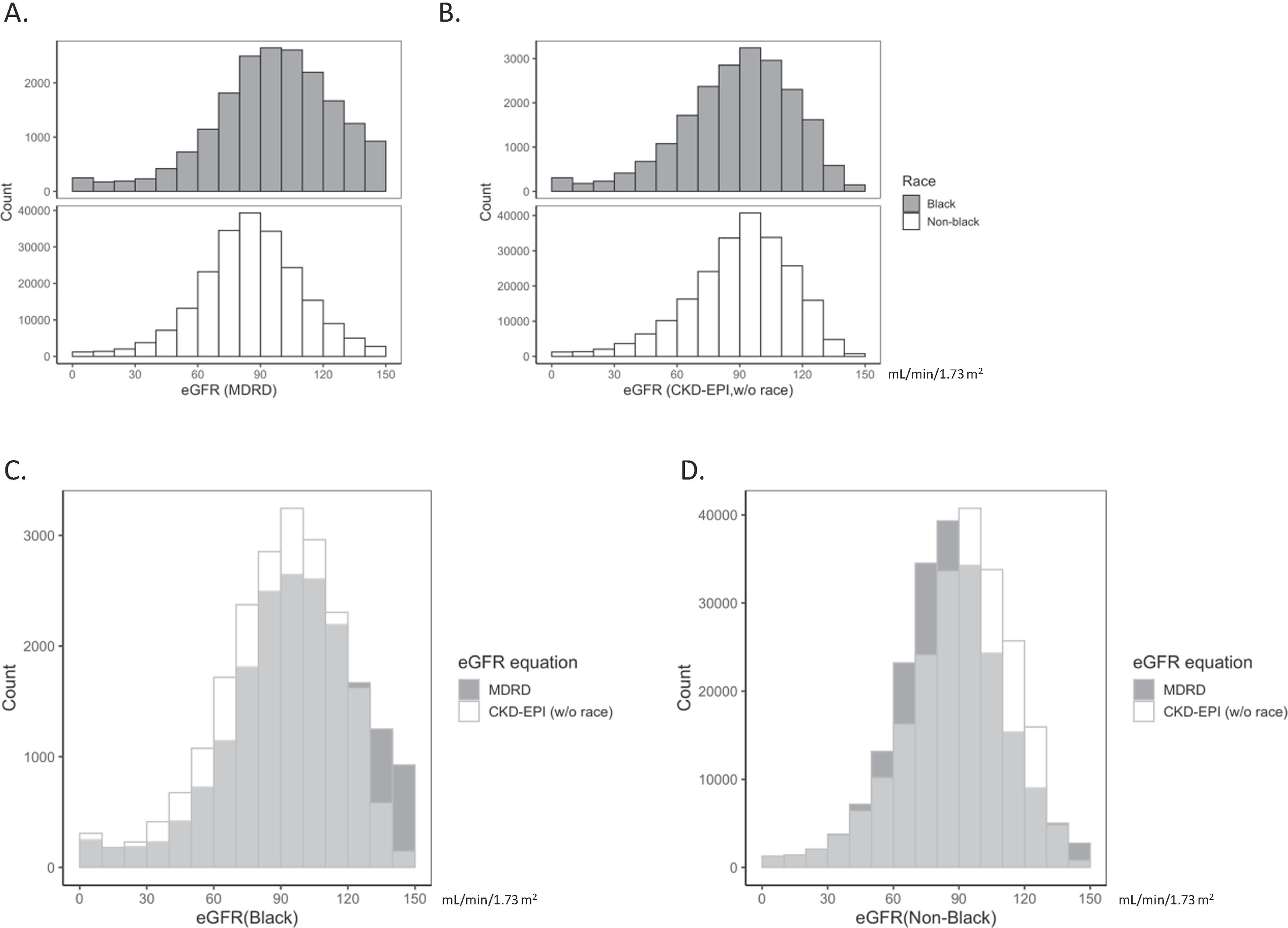
(A, B) Histograms of eGFR for self-described Black (dark gray bars) and non-Black (white bars) patient results are illustrated with the x-axis segmented by 10 mL/min/1.73 m2. eGFR was calculated with the MDRD equation (A) or CKD-EPI equation without the race correction factor (B). (C, D) Overlay of eGFR distributions for the MDRD (dark gray bars) and CKD-EPI without the race correction factor (white bars) in the Black (C) and non-Black (D) population.
Discussion
Our health care system recently adopted the CKD-EPI equation to improve the accuracy of the calculation of eGFR from plasma and serum creatinine concentrations. In addition, we removed the race correction factor from the equation, which reduced the eGFR for our self-identified or provider-identified Black patients by ~16%. We used retrospective data analysis to evaluate how these two changes would affect the patients in our large medical system as a whole and found that the distribution of eGFR across self-identified ethnicities was more uniform when using our new approach, which has the potential to improve equity in health care. Compared to the cohort used for external validation in the CKD-EPI study [1], our medical system population (N = 241,760 patients) included more patients older than 65 years of age (24 vs. 14%) and more racial minorities (31 vs. 14%), from diverse clinical settings, including outpatient, inpatient, and emergency department, with a lower mean creatinine concentration (0.94 vs. 1.52 mg/dL), and a wider range of eGFR. As a result, the observations from this study are widely generalizable.
As expected, adoption of the CKD-EPI equation led to higher eGFR results for many patients, a change that has previously been associated with fewer cardiovascular events and provides better risk stratification in this patient group [21]. There is also a group of patients for whom the CKD-EPI equation resulted in a lower eGFR, who were predominately young adults with creatinine within or below the reference range and eGFRMDRD was above 105 mL/min/1.73 m2. The fact that some of these results shifted from above to below 90 mL/min/1.73 m2, with the lowest being 78 mL/min/1.73 m2, suggests to report eGFR values above 60 mL/min/1.73 m2 as >60 mL/min/1.73 m2 rather than as an actual number, even with the CKD-EPI equation.
In laboratory medicine, assay results are put in context by using reference ranges, which are often developed based on a distribution of observed values from a healthy population but can also be assigned using clinical cut-offs developed by scientific evidence and expert opinion. For the diagnosis, management, and prognosis of CKD, clinical guidelines rely on eGFR. It is important to note that the guidelines are blinded to the color of a person’s skin, their race, and their ethnicity. Indeed, the guidelines are applied universally across all adults. As previously mentioned, both the MDRD and CKD-EPI equations include a race correction factor, which leads to 21.2% and 15.9% higher eGFR, respectively, and can impact health care for patients. These adjustments were included to improve the correlation between measured and estimated GFR in the original cohorts studied and have the potential benefit of allowing the same creatinine derived eGFR cut-offs to be used in Black patients as well as non-Black patients as previous studies have suggested that serum creatinine is higher in Black participants [22, 23]. In our patient population, however, there was no obvious difference in serum creatinine between Black and non-Black patients. This observation, together with the higher prevalence of CKD in the Black population and the fact that higher proportions of Black patients have advanced CKD and are at risk for kidney failure [2, 24], raises questions about the appropriateness of including a race correction factor in the calculation of eGFR.
The use of the race correction factor is even more questionable for the following reasons: (i) the initial cohorts were biased with self-identified Black participants making up less than a third of the cohort [1, 9]; (ii) it is unclear if comorbidities and other clinical features were well-matched between self-identified races in the initial cohorts; (iii) recent studies have shown that the race correction factor does not improve accuracy in South Africans, mixed ancestry and sub-Saharan Africans, and African Brazilians [25–28]; and (iv) there are no differences in measured GFR between races in a community-based study that included 47% Black and 53% non-Black individuals [29]. These arguments strongly support using the calculation of eGFR without a race correction factor, so that clinical cut-offs are relevant across all people.
While race carries no biological certainty and lacks the scientific rigor needed for biomedical research and diagnostic equations, understanding how it causes many of the health inequities we see is a concept that can and should be embraced. By demonstrating nearly identical distributions of eGFR in multiple self-described racial groups after removing the race correction factor from the CKD-EPI equation, our data provide support for a more equitable use of serum and plasma creatinine concentrations in the care of our patients. In fact, the race correction factor is not used in eGFR in many multicultural countries with individuals from many racial backgrounds [30]. A recent review discussed different race-free approaches to refit serum creatinine-based eGFR [31]. Evaluation of these alternatives using the CKD-EPI development data showed decreased accuracy and increased bias relative to race-based eGFRcreatinine and race-free eGFRcystatinC. However, these conclusions on accuracy and bias were still based on the old CKD-EPI development data.
In a recent study by Ahmed and colleagues, a total of 33.4% (743/2225) of African-American patients in a CKD registry were reclassified to have a more severe CKD stage when removing the race correction factor from the CKD-EPI study [24]. Of these reclassified patients, 3.1% would be reassigned from eGFR > 20 ml/min/1.73 m2 to eGFR ≤ 20 ml/min/1.73 m2 and none would have missed appropriate medical care and treatment. Our study demonstrated that 0.22% of Black patients would be reclassified from an eGFR above 20 ml/min/1.73 m2 to below (44/20272, proportion of reclassified patients with eGFRMDRD > 20 ml/min/1.73 m2), which was more than 10 times lower. This can be explained by the healthier population in our study.
It might be concerning that a change from an MDRD equation with a race correction factor to a CKD-EPI without a race correction might have a significant impact on the number of patients being referred to and cared for by nephrology. However, the overall number of subjects with an eGFR < 60 mL/min/1.73m2 decreased by 2% which may actually lead to fewer referrals overall, particularly in light of the small number of patients reclassified to have worse kidney function in our patient population. Another potential result of removing the race correction factor is a change in the safety and effectiveness of pharmacotherapy and proper approaches to dosing. After the change, lower doses of certain medications will be administered to some Black patients due to the lower eGFR that is calculated when the race correction factor is excluded, which may prevent overdose and renal toxicity, but could also lead to less effective therapy.
Our study has limitations. We have no clinical follow-up for our patients to understand how adopting the CKD-EPI equation without a race correction factor will affect outcomes, which may be particularly interesting for patients with eGFR > 60 mL/min/1.73 m2 [16]. We have also not considered urine albumin concentrations, but have instead relied solely on serum and plasma creatinine concentrations, which are affected by muscle mass, diet, and chronic illness [32]. In addition, although equations based on serum and plasma cystatin C concentrations exist, widespread application of cystatin C will take time due to assay cost, lack of standardization, and the need for thorough evaluation of clinical performance [12, 25, 33, 34]. Lastly, we didn’t remove patients on dialysis or pregnant women, although they only account for a small fraction of our patient population. However, as a strength, our study included more AsianAmericans (10%) than some of the prior studies in the US. Although the Asian patient population appeared to have lower creatinine and higher eGFR on average with both MDRD and CKD-EPI equations, further studies are warranted for this specific population.
In conclusion, we provided data of implementation of a CKD-EPI equation without the race correction factor at a large academic medical system to inform the ongoing nationwide conversation with the goal to provide more equitable care, to improve risk assessment, and to enhance public health advances.
Supplementary Material
Our institution moved from MDRD to CKD-EPI in calculating eGFR and consciously decided to remove the race correction factor during the process.
We provided the reclassification data upon the adoption of the new calculation of eGFR in our patient population.
The distributions of creatinine and eGFR calculated with CKD-EPI without the race correction factor were not meaningfully different in Black and non-Black patients.
Acknowledgements
We would like to acknowledge our UW Medical Students who initially brought up this issue with the faculty and the faculty who have supported the efforts to make this clinical change.
Abbreviations:
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- NKDEP
National Kidney Disease Education Program
- eGFR
estimated glomerular filtration rate
- MDRD
Modification of Diet in Renal Disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- SCr
serum creatinine
- CKD-EPINoRace
CKD-EPI equation without the race correction factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Junyan Shi, Department of Laboratory Medicine and Pathology, University of Washington School of Medicine
Edwin G. Lindo, Department of Family Medicine, Department of Bioethics & Humanities, Office of Healthcare Equity, University of Washington School of Medicine
Geoffrey S. Baird, Department of Laboratory Medicine and Pathology, University of Washington School of Medicine
Bessie Young, Kidney Research Institute, Division of Nephrology, Department of Medicine, University of Washington School of Medicine
Michael Ryan, Division of Nephrology, Department of Medicine, University of Washington School of Medicine
J. Ashley Jefferson, Division of Nephrology, Department of Medicine, University of Washington School of Medicine
Rajnish Mehrotra, Kidney Research Institute, Division of Nephrology, Department of Medicine, University of Washington School of Medicine
Patrick C. Mathias, Department of Laboratory Medicine and Pathology, Department of Biomedical Informatics and Medical Education, University of Washington School of Medicine
Andrew N. Hoofnagle, Department of Laboratory Medicine and Pathology, Kidney Research Institute, Division of Metabolism, Endocrinology, and Nutrition, Department of Medicine, University of Washington School of Medicine.
References
- [1].Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, C.-E.C.K.D.E. Collaboration, A new equation to estimate glomerular filtration rate, Ann Intern Med 150(9) (2009) 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G, Clinical practice guidelines for chronic kidney disease in adults: Part I. Definition, disease stages, evaluation, treatment, and risk factors, Am Fam Physician 70(5) (2004) 869–76. [PubMed] [Google Scholar]
- [3].Israelit AH, Long DL, White MG, Hull AR, Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate, Kidney Int 4(5) (1973) 346–9. [DOI] [PubMed] [Google Scholar]
- [4].Perrone RD, Steinman TI, Beck GJ, Skibinski CI, Royal HD, Lawlor M, Hunsicker LG, Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study, Am J Kidney Dis 16(3) (1990) 224–35. [DOI] [PubMed] [Google Scholar]
- [5].Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM, Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group, J Am Soc Nephrol 4(5) (1993) 1159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Estimate Glomerular Filtration Rate (GFR). (Accessed November 24 2020).
- [7].Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, N.K.D.E.P.L.W. Group, Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program, Clin Chem 52(1) (2006) 5–18. [DOI] [PubMed] [Google Scholar]
- [8].Cockcroft DW, Gault MH, Prediction of creatinine clearance from serum creatinine, Nephron 16(1) (1976) 31–41. [DOI] [PubMed] [Google Scholar]
- [9].Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group, Ann Intern Med 130(6) (1999) 461–70. [DOI] [PubMed] [Google Scholar]
- [10].Levey A, Greene T, Kusek J, Beck G, A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J. Am. Soc. Nephrol. 11 (2000) 155A. [Google Scholar]
- [11].Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, C.K.D.E. Collaboration, Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values, Clin Chem 53(4) (2007) 766–72. [DOI] [PubMed] [Google Scholar]
- [12].Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, C.-E. Investigators, Estimating glomerular filtration rate from serum creatinine and cystatin C, N Engl J Med 367(1) (2012) 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller G, Vassalotti JA, Kidney Biomarkers: the Kidney Profile Order, Urine Albumin-Creatinine Ratio (uACR), and Estimated Glomerular Filtration Rate (eGFR), College of American Pathologists (2020).
- [14].Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, Rahman M, Deysher AE, Zhang YL, Schmid CH, Levey AS, Evaluation of the modification of diet in renal disease study equation in a large diverse population, J Am Soc Nephrol 18(10) (2007) 2749–57. [DOI] [PubMed] [Google Scholar]
- [15].Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI, KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD, Am J Kidney Dis 63(5) (2014) 713–35. [DOI] [PubMed] [Google Scholar]
- [16].KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management ofChronic Kidney Disease, Kidney Int Suppl 3(1) (2013) 1–160. [DOI] [PubMed] [Google Scholar]
- [17].Eneanya ND, Yang W, Reese PP, Reconsidering the Consequences of Using Race to Estimate Kidney Function, JAMA 322(2) (2019) 113–114. [DOI] [PubMed] [Google Scholar]
- [18].Vyas DA, Eisenstein LG, Jones DS, Hidden in Plain Sight - Reconsidering the Use of Race Correction in Clinical Algorithms, N Engl J Med 383(9) (2020) 874–882. [DOI] [PubMed] [Google Scholar]
- [19].Delgado C, Baweja M, Burrows NR, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, St Peter WL, Warfield C, Powe NR, Reassessing the Inclusion of Race in Diagnosing Kidney Diseases: An Interim Report from the NKF-ASN Task Force, J Am Soc Nephrol (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thiese MS, Ronna B, Ott U, P value interpretations and considerations, J Thorac Dis 8(9) (2016) E928–E931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD, Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation, Am Heart J 162(3) (2011) 548–54. [DOI] [PubMed] [Google Scholar]
- [22].Lim E, Miyamura J, Chen JJ, Racial/Ethnic-Specific Reference Intervals for Common Laboratory Tests: A Comparison among Asians, Blacks, Hispanics, and White, Hawaii J Med Public Health 74(9) (2015) 302–10. [PMC free article] [PubMed] [Google Scholar]
- [23].Rappoport N, Paik H, Oskotsky B, Tor R, Ziv E, Zaitlen N, Butte AJ, Comparing Ethnicity-Specific Reference Intervals for Clinical Laboratory Tests from EHR Data, The Journal of Applied Laboratory Medicine 3(3) (2019) 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ahmed S, Nutt CT, Eneanya ND, Reese PP, Sivashanker K, Morse M, Sequist T, Mendu ML, Examining the Potential Impact of Race Multiplier Utilization in Estimated Glomerular Filtration Rate Calculation on African-American Care Outcomes, J Gen Intern Med (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bukabau JB, Yayo E, Gnionsahé A, Monnet D, Pottel H, Cavalier E, Nkodila A, Makulo JRR, Mokoli VM, Lepira FB, Nseka NM, Krzesinski JM, Sumaili EK, Delanaye P, Performance of creatinine- or cystatin C-based equations to estimate glomerular filtration rate in sub-Saharan African populations, Kidney Int 95(5) (2019) 1181–1189. [DOI] [PubMed] [Google Scholar]
- [26].Holness JL, Bezuidenhout K, Davids MR, Warwick JM, Validation of equations to estimate glomerular filtration rate in South Africans of mixed ancestry, S Afr Med J 110(3) (2020) 229–234. [DOI] [PubMed] [Google Scholar]
- [27].Rocha AD, Garcia S, Santos AB, Eduardo JCC, Mesquita CT, Lugon JR, Strogoff-de-Matos JP, No Race-Ethnicity Adjustment in CKD-EPI Equations Is Required for Estimating Glomerular Filtration Rate in the Brazilian Population, Int J Nephrol 2020 (2020) 2141038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zanocco JA, Nishida SK, Passos MT, Pereira AR, Silva MS, Pereira AB, Kirsztajn GM, Race adjustment for estimating glomerular filtration rate is not always necessary, Nephron Extra 2(1) (2012) 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Inker LA, Shafi T, Okparavero A, Tighiouart H, Eckfeldt JH, Katz R, Johnson WC, Dermond N, Tariq Z, Benayache I, Post WS, Coresh J, Levey AS, Shlipak MG, Effects of Race and Sex on Measured GFR: The Multi-Ethnic Study of Atherosclerosis, Am J Kidney Dis 68(5) (2016) 743–751. [DOI] [PubMed] [Google Scholar]
- [30].El-Khoury JM, Ovalle AA, Cervinski MA, Levey AS, Jones G, Eneanya ND, Lieske JC, Jha V, Moderators:, Experts:, Is It Time to Move On? Reexamining Race in Glomerular Filtration Rate Equations, Clin Chem 67(4) (2021) 585–591. [DOI] [PubMed] [Google Scholar]
- [31].Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK, In Search of a Better Equation - Performance and Equity in Estimates of Kidney Function, N Engl J Med (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stevens LA, Coresh J, Greene T, Levey AS, Assessing kidney function--measured and estimated glomerular filtration rate, N Engl J Med 354(23) (2006) 2473–83. [DOI] [PubMed] [Google Scholar]
- [33].Peralta CA, Katz R, Sarnak MJ, Ix J, Fried LF, De Boer I, Palmas W, Siscovick D, Levey AS, Shlipak MG, Cystatin C identifies chronic kidney disease patients at higher risk for complications, J Am Soc Nephrol 22(1) (2011) 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang YL, Greene T, Levey AS, Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD, Am J Kidney Dis 51(3) (2008) 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


