Abstract
The allergic airway diseases chronic rhinosinusitis (CRS), allergic fungal rhinosinusitis (AFRS), asthma, allergic bronchopulmonary mycosis/aspergillosis (ABPM/A), and cystic fibrosis (CF) share a common immunological signature marked by T H 2 and TH 17 cell predominant immune responses, the production of IgE antibody, and atypical inflammatory cell infiltrate that includes eosinophils and other innate immune effector cells. Severe forms of these disorders have long been recognized as being related to hypersensitivity reactions to environmental fungi. Increasingly however,environmental fungi are assuming a more primary role in the etiology of these disorders, with airway mycosis, a type of non-invasive airway fungal infection,recognized as an essential driving factor in at least severe subsets of allergic airway diseases. In this review, we consider recent progress made in understanding the immune mechanisms that drive airway mycosis-related diseases, improvements in immune-based diagnostic strategies, and therapeutic approaches that target key immune pathways.
Keywords: Airway mycosis; Asthma; allergic bronchopulmonary mycosis; cystic fibrosis; Chronic Rhinosinusitis; allergic fungal rhinosinusitis; protease; fibrinogen; airway, Hyperresponsiveness
Introduction
The allergic airway diseases chronic rhinosinusitis (CRS), allergic fungal rhinosinusitis (AFRS), asthma, allergic bronchopulmonary mycosis/aspergillosis (ABPM/A), and cystic fibrosis (CF) share a common immunological signature marked by Th2 and Th17 cell predominant immune responses, the production of IgE antibody, and a typical inflammatory cell infiltrate that includes eosinophils. Severe forms of these disorders have long been recognized as being related to hypersensitivity reactions to environmental fungi. Increasingly however, environmental fungi are assuming a more primary role in the etiology of these disorders, with airway mycosis, a type of non-invasive airway fungal infection, recognized as an essential driving factor in at least severe subsets of allergic airway diseases. In this review, we consider recent progress made in understanding the immune mechanisms that drive airway mycosis-related diseases, improvements in immune-based diagnostic strategies, and therapeutic approaches that target key immune pathways.
I. Immune and molecular disease mechanisms
Innate Immunity
The immune and molecular mechanisms that drive development of asthma are initiated by environmental exposure to antigen and infection with fungal pathogens. Deposition of fungal spores or conidia in the airway leads to spore hydration and germination resulting in the release of fungal allergens derived from cellular components, secreted proteases, ribonucleases, and other fungal products.(1-3) Cell wall components such as chitin, β-glucan, galactomannans, and mannoproteins are well known allergens recognized by pathogen recognition receptors (PRRs): Toll-like receptors (TLRs) and C-type lectin-like receptors (CLRs). Stimulation of PRRs activates the epithelium to releases alarmins like interleukin 1β (IL-1β), IL-25, IL-33 and thymic stromal lymphopoietin (TSLP). These alarmins, namely IL-33, induce progenitor innate lymphoid cells (pILC) that develop into group 2 ILC (ILC2) that secrete the type 2 T helper (TH2) cytokine IL-5 and IL-13. Fungal proteases can cleave full length IL-33 to a more potent form, enhancing ST2 signaling (IL-33 receptor) on ILC2 and type 2 cytokine production.(4) ILC2 production of IL-5 enhances recruitment and enrichment of inflammatory eosinophils in the lungs, a hallmark of eosinophilic asthma.(5) The alarmin IL-1β can induce group 3 ILC (ILC3) to produce the TH17 cytokine IL-17A and granulocyte-macrophage colony-stimulating factor (GM-CSF) that enhance macrophage phagocytosis and fungal killing.(6) Additionally, the CLR Dectin-1 on macrophages can recognize fungal β-glucan directly to enhance phagocytic activity.(1)
Fungal-derived proteases also activate the 7-transmembrane G protein-coupled receptor family of protease-activated receptors (PARs) by directly cleaving the ligand domain, freeing the ligand to bind the receptor’s ligand binding domain and induce alarmin secretion from epithelial cells. Pharmacologically inhibiting the PAR-2 receptor or loss of Regulator of G protein Signaling 4 (RGS4) function leads to attenuated disease in murine models and highlights the importance of fungal proteases in the pathogenesis of fungal induced asthma.(7, 8) Degradation of epithelial cell tight junctions by protease during fungal invasion increases permeability and influx of serum proteins such as fibrinogen. Proteases can cleave fibrinogen into cryptokine fibrinogen cleavage products (FCP) that activate macrophages through TLR4 and the macrophage integrin (Mac-1).(3) FCP-mediated TLR4-dependant activation of mast cells further enhances IL-13 production in the lungs, promoting PD-L2+ dendritic cell (DC) activation.(9) Activated DCs and macrophages increase antigen sampling and migration to the draining lymph node as a result of these factors.
Adaptive Immunity
Activated DCs in the lymph node present antigen to naïve T cells, inducing differentiation into TH2 and TH17 effector T cells. Effector T cells then home to the lungs and promote the developing immune response. TH2 T cells in lymph nodes also secrete IL-4 to promote B cell development and antigen specific IgE, another established marker of allergic disease. Serum IgE is absorbed on the plasma membrane of tissue resident mast cells by the high-affinity IgE receptor (FcεR1).
In the lungs, TH2 cells secret large quantities of IL-4, IL-5, and IL-13 to sustain the innate inflammatory response. IL-4 and IL-13 induce goblet cell metaplasia of epithelial cells, which leads to increased mucus production in the lungs and airway obstruction by mucus plugs. TH2 cytokines also stimulate epithelial cells to express chemokines such as CCL11 and CCL26 that direct eosinophil to the lungs. The recruited T cells also promote eosinophil survival through IL-5 and IL-13 ( Geslewits, W.E. 2018 Eosinophil). IL-33 in the lungs induces amphiregulin expression in TH2 cells, which reprograms inflammatory eosinophil to produce the pro-fibrotic osteopontin (Morimoto, Y. 2018 amphiregulin). The TH2 cytokine IL-13, produced by TH2 cells, eosinophil, and mast cells is thought to promote the smooth hyperplasia behind airway hyperreactivity that is characteristic of asthma. The multifaceted immune response that culminates in a dominant TH2 phenotype drives the eosinophilic asthma phenotype characterized by airway hyperreactivity, increase mucus in the airway and fibrosis.
II. Immune Responses in airway mycosis-related allergic airway disease
Sinus mycosis, the non-invasive growth of fungi along the sino-mucosal surface, is associated with distinct clinical syndromes that include CRS with and without nasal polyposis and AFRS. These clinical phenotypes, together with their accompanying immunological presentations, depend to a substantial degree on the host immune status and the specific fungi involved. The spectrum of fungal disease within the sinuses includes invasive fungal sinusitis (IFS, immunocompromised), fungal balls (FBs, immunocompetent) and allergic fungal rhinosinusitis (AFRS, immunocompetent with an atopic background). In the immunocompromised patient (such as a diabetic), class I major histocompatibility complexes have been shown to be downregulated and the function of neutrophils, complement fixation and phagocytes were decreased while angioinvasion was stimulated (10). Further, diabetic ketoacidosis patients have increased iron levels which can lead to an augmented vulnerability of endothelial cells to fungal damage (10). In immunocompetent patients, fungus balls are a common presentation of airway mycosis (11). More commonly affecting females and older patients, FBs incite macrophagic, neutrophilic, plasma cells and lymphocytic activity associated with elevated expression of IL-8, G-CSF, and IgA (11, 12). This robust local immune response contains the inflammation to the one affected sinus cavity and prevents tissue infiltration of the fungi.
An endotype of chronic rhinosinusitis with nasal polyps (CRSwNP), AFRS occurs in immunocompetent patients with an atopic background. AFRS is defined by tissue eosinophilia, mucin layden with fungi, fungal hypersensitivity and specific computed tomography (CT) findings among many other characteristics (13). Current studies support fungi-activated molecular signaling, an exaggerated Type 2 immune response, and defects in the mucosal barrier as main drivers of the fungal induced CRSwNP phenotypes.
Fungal components and protease activity can activate various immune receptors found on respiratory epithelial cells to trigger release of epithelial cell derived cytokines (EDCs) important in orchestrating a Type 2 immune response such as IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) (14). CRSwNP patients have elevated levels of Interleukin-33 (IL-33), the receptor for IL-33 (ST2), Protease Activated Receptor 2 (PAR2), and Toll like Receptor 4 (TLR4) in the sinonasal mucosa in comparison to healthy controls (14). Dietz et al demonstrated that A. fumigatus extract via PAR2 activation could trigger IL-33 release (14). These studies highlight the importance of fungi-mediated molecular signaling in the Type 2 immune response.
Fungal-induced release of EDCs activate both the innate and adaptive Type 2 immune response. Of the innate response, group 2 innate lymphoid cells (ILC2s) express receptors for IL-33, IL-25 and TSLP. In response to IL-33, ILC2s are a major source of IL-13 in CRS (15). In addition, cross talk between ILC2s and Th2 cells is one means by which fungi can activate the adaptive Type 2 immune response. Illustrating the activated adaptive immune response to fungi, peripheral blood mononuclear cells from AFRS patients demonstrate enhanced IL-4 T cell memory when challenged with fungi (16). Finally, whole genome analysis comparing AFRS to CRSwNP identified many differentially upregulated genes in AFRS related to adaptive immunity including TCR and co-stimulatory signaling (13).
AFRS is also characterized by defects in the mucosal barrier and permissive sinus environment for fungal germination. In vitro studies with respiratory epithelial cells show loss of integrity when challenged with Type 2 cytokines IL-4 and -13 (17). While expression of antimicrobial peptides is elevated in sinonasal mucosa from CRSwNP patients, expression of histatin and other AMPs is significantly depressed in AFRS (13), suggesting a possible mechanism to explain the exuberant overgrowth of fungi that in part defines AFRS.
In asthma, Aspergillus fumigatus culture positive asthma has been linked to exaggerated bronchial inflammation. Thirty-three moderate to severe asthmatics with negative sputum cultures were compared to 19 similar asthmatics with positive sputum cultures with numerous inflammatory mediators quantitated from sputum. While expression of all inflammatory biochemical markers was elevated in fungus culture-positive relative to culture negative subjects, soluble tumor necrosis factor receptor 2 (TNF-R2) and CCL5 provided the greatest discrimination between the two groups (18).
III. Diagnosis of airway mycosis-associated diseases
The only subtype of asthma for which the use of systemic antifungal therapy is not controversial is allergic bronchopulmonary aspergillosis or ABPA. While current first line therapy for ABPA exacerbation is still systemic corticosteroids, current IDSA guidelines do recommend the use of triazole antifungals such as itraconazole or voriconazole as adjunct therapy (19). The Rosenberg-Patterson criteria are undoubtedly the criteria most clinicians use to assess for the presence of ABPA (20). However these criteria call for the strict adherence to several metrics that make the diagnosis of ABPA exceedingly difficult and likely leading to significant underestimation of the true prevalence of this condition. Since the Rosenberg-Patterson criteria’s inception in 1977, our understanding of ABPA has brought forth various weaknesses of this criteria. Current efforts to update our current definition of ABPA focuses on widening the criteria for ABPA to allow for easier diagnosis, a higher emphasis on molecular mechanisms that drive this condition and a recognition that ABPA can occur in patients other than those with asthma or cystic fibrosis.
In a comparison between the Rosenberg, International Society for Human and Animal Mycology (ISHAM and Greenberg criteria), Mortazee et al., demonstrated that the Greenberg criteria were more sensitive than the Rosenberg criteria due to the need to satisfy fewer clinical criteria. This study also showed inferior sensitivity of the ISHAM criteria compared to Rosenberg due to the need for a higher total IgE cutoff (21). Saxena, et al., demonstrated marginal improvement of the ISHAM criteria compared to the Rosenberg criteria, but after modifying the ISHAM criteria to lower the total IgE cutoff from 1000 to 500, they demonstrated significant improvement of the ISHAM criteria (22). Asano et al., also demonstrated superior sensitivity of the ISHAM criteria compared to Rosenberg, however this group also created their own criteria that proved more sensitive to either of the former criteria while requiring fewer criteria compared to Rosenberg, a lower IgE cutoff compared to ISHAM, and less emphasis on needing asthma as a precursor to the diagnosis of ABPA (23).
IV. Immune therapies for airway mycosis related disease
Severe forms of airway mycosis-related disease can be particularly difficult to manage clinically. Over the past five years biologics and other novel therapies have been explored for their potential utility in several of these disorders. Cystic fibrosis (CF) is a chronic, relentlessly progressive disorder of primarily the airways that is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that leads to inability to control bacterial and fungal infections of the upper and lower airways. Airway mycosis in CF often manifests as allergic bronchopulmonary mycosis/aspergillosis (ABPM/A) in which fungi, often Aspergillus spp, massively overgrow in the airways, leading to abnormal airway dilatation, impaired ability to clear secretions, and markedly elevated serum IgE levels. Vitamin D supplementation, which has shown promise in alleviating asthma and other allergic airway diseases related to airway mycosis, was explored in CF complicated by ABMP due to Aspergillus spp (24). Seven patients were supplemented with 4000 vitamin D units daily for 24 weeks in a phase I clinical trial. The primary outcomes were change in Aspergillus-specific T cell responses after antigen-specific rechallenge and change in serum IgE levels. The authors found that vitamin D supplementation was safe and significantly reduced both IL-13-specific T cell recall responses after antigen challenge in vitro and serum Aspegillus-specific IgE.
Additional small studies were conducted in CF patients with elevated serum IgE levels to determine if omalizumab, a monoclonal anti-IgE antibody, aided management. Six patients with CF and ABPM and elevated serum IgE levels were treated with omalizumab for 6-18 months in a retrospective case series (25). The authors found that omalizumab was associated with modest reductions in glucocorticoid use, mild symptom improvement, and reductions in serum IgE, although the latter is an inescapable effect of the drug. However, a second multi-national small study failed to find significant benefit (26) and it remains unclear if anti-IgE therapy will be pursued for CF in the future.
More recently, the monoclonal antibody mepolizumab that neutralizes the Th2 cytokine IL-5 has been explored in early clinical studies of multiple airway mycosis-related diseases. Multiple case reports of mepolizumab used in the context of ABPA complicated by eosinophilic CRS (27), concurrent CRS and otitis media (28), or nontuberculous mycobacterial disease (29) all indicate that this agent usefully complements standard anti-inflammatory, bronchodilator, and antifungal therapy to enhance disease management and symptom resolution. Where mepolizumab has not proven effective in ABPA, dupilumab, a monoclonal antibody that blocks a shared component of the IL-4 and IL-13 receptors, IL-4Rα, has shown remarkable efficacy in reducing or abrogating disease features (30).
Mepolizumab has also been evaluated in a small case series of CF patients marked by type 2 inflammation, which manifests as elevated serum IgE and blood eosinophils together with enhanced production of the cytokines IL-4, IL-5, and IL-13. Zhang, et al., reported three corticosteroid-dependent CF patients with a type 2 phenotype and mold allergy who received mepolizumab as add-on therapy. The biologic was well tolerated and resulted in substantial reductions in both glucocortocoid use and serum IgE levels, althought lung function and disease exacerbation rates were not improved (31). Despite such encouraging early reports, randomized prospective controlled studies are needed to confirm these preliminary observations with mepolizumab and dupilumab in CF and ABPA.
A final therapeutic study of note was conducted in mice and involved a unique small molecule antagonist of signal transducer and activator of transcription 6 (STAT6) and STAT5, factors that are both essential for the expression of airway mycosis-dependent asthma. PM-43I, a lead compound that evolved through a series of in vitro and in vivo studies, binds to the Sarc Homology 2 (SH2) domains of STAT5 and STAT6, but not other STAT factors, to prevent their dimerization and ability to transactivate key asthma-related genes. Administered intranasally to mice challenged with the spores of Aspergillus niger to mimic airway mycosis-related asthma, PM-43I both prevented and reversed airway hyperresponsiveness and reduced lung IL-4 production and airway eosinophilia (32). PM-43I is thus the first of a new class of small molecules targeting transcription factors intracellularly that may be suitable for further clinical development against asthma.
V. Concluding remarks
The airway mycosis-related airway diseases are primarily, but not exclusively, allergic in nature, involving predominant Th2, ILC2, and eosinophil-driven airway inflammation that is frequently accompanied by elevated serum IgE levels. Over the past four years, substantial advances have been made in deciphering the immune mechanisms of airway mycosis-related diseases, improving diagnostic approaches for especially ABPA, and immune based therapies. These discoveries portend exciting future research that will further unlock the mechanisms by which molds, but also yeasts, elicit allergic inflammation that will in turn inform future clinicians as to improved diagnostic and therapeutic approaches. Undoubtedly, these additional findings will extend to other diseases related to fungi.
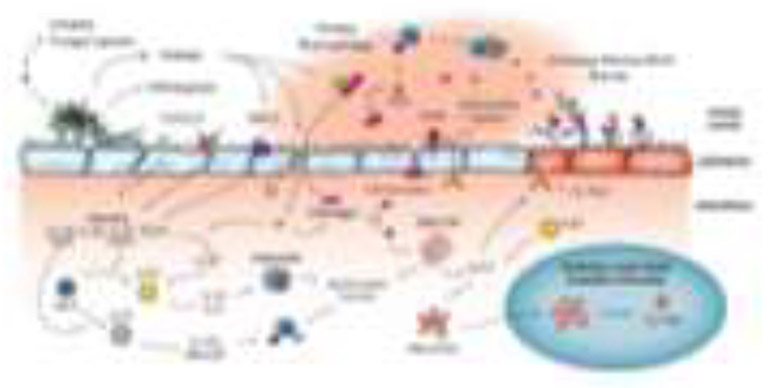
Figure 1.
Recent advances in understanding immune response to airway mycosis. Inhaled fungal spores germinate within the airway and begin releasing immunostimulatory molecules such as chitin, β-glucan, and proteases. Toll-like receptors (TLR) and C-type lectin-like receptors (CLR) recognize antigen on the epithelium to release the alarmins IL-1β, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) that promote adaptive immune responses. Secreted proteases can activate protease activated receptor 2 (PAR-2) on epithelial cells to induce alarmin release, degrade epithelial tight junctions (TJ) to increase permeability and influx of fibrinogen into the airway, and cleave fibrinogen to promote clot formation and the creation of fibrinogen cleavage products (FCP). FCPs are recognized by TLR4 and promote macrophage phagocytic activity, induce mast cell degranulation and IL-13 release, and activate epithelial cells to increase IL-13α1 expression and production of antimicrobial peptides. IL-1β release by epithelial cells promotes progenitor innate lymphoid cells (pILC) development into type 3 ILC (ILC3) to secrete IL-17A and GM-CSF, which enhance macrophage phagocytic and other antifungal activities. ILC2 development from pILC is also induced by alarmins, especially IL-33. Protease-mediate cleavage of full-length IL-33 to a more potent form enhances ILC2 development and cytokine production (IL-13 and IL-5). ILC2 cytokines promote eosinophil recruitment and secretion of antimicrobial peptides, both of which are potently antifungal. ILC2 and FCP-stimulated mast cells released IL-13 that promote goblet cell metaplasia, increase mucus production, and enhanced barrier formation together with fibrinogen. Activated mast cells release IL-13 that activates PD-L2+ dendritic cells (DC) to migrate into regional lymph nodes to again prime adaptive immune responses to both fungi and bystander antigens.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the United States National Institutes of Health or the Veterans Administration Office of Research and Development. Supported by US National Institutes of Health grants R01HL117181, HL140398, R01AI135803, and R41AI124997; and VA Office of Research and Development grant I01BX004828.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griffiths JS, Thompson A, Stott M, Benny A, Lewis NA, Taylor PR, et al. Differential susceptibility of Dectin-1 isoforms to functional inactivation by neutrophil and fungal proteases. FASEB J. 2018;32(6):3385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JJ, He YS, Zhong XJ, Cai ZL, Lyu YS, Zhao ZF, et al. Ribonuclease T2 from Aspergillus fumigatus promotes T helper type 2 responses through M2 polarization of macrophages. Int J Mol Med. 2020;46(2):718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landers CT, Tung HY, Knight JM, Madison MC, Wu Y, Zeng Z, et al. Selective cleavage of fibrinogen by diverse proteinases initiates innate allergic and antifungal immunity through CD11b. J Biol Chem. 2019;294(22):8834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. **.Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol. 2018;19(4):375–85.• This is the first study to demonstrate that allergenic proteases cleave mammalian cytokines such as IL-33 to create more potent isoforms.
- 5.Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Santos N, Wiesner DL, Fites JS, McDermott AJ, Warner T, Wuthrich M, et al. Lung Epithelial Cells Coordinate Innate Lymphocytes and Immunity against Pulmonary Fungal Infection. Cell Host Microbe. 2018;23(4):511–22 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ocasio-Rivera M, Marin-Maldonado F, Trossi-Torres G, Ortiz-Rosado A, Rodriguez-Irizarry V, Rodriguez-Lopez E, et al. Targeting of protease activator receptor-2 (PAR-2) antagonist FSLLRY-NH2 as an asthma adjuvant therapy. Medicine (Baltimore). 2020;99(43):e22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. *.Wong GS, Redes JL, Balenga N, McCullough M, Fuentes N, Gokhale A, et al. RGS4 promotes allergen- and aspirin-associated airway hyperresponsiveness by inhibiting PGE2 biosynthesis. J Allergy Clin Immunol. 2020;146(5):1152–64 e13.• The authors show that regulator of G protein signaling 4 (RGS4) epithelial expression correlates with increasing severity of asthma patients and RGS4−/− mice are resistant to fungal protease-induced airway hyperreactivity and inflammation. This study highlights the importance of epithelial cell prostaglandin E2 and regulation of G-protein-coupled receptors in the development of allergic disease.
- 9.Cho M, Lee JE, Lim H, Shin HW, Khalmuratova R, Choi G, et al. Fibrinogen cleavage products and Toll-like receptor 4 promote the generation of programmed cell death 1 ligand 2-positive dendritic cells in allergic asthma. J Allergy Clin Immunol. 2018;142(2):530–41 e6. [DOI] [PubMed] [Google Scholar]
- 10.Nyunt TPK, Mullol J, and Snidvongs K. Immune response to fungi in diabetic patients with invasive fungal rhinosinusitis. Asian Pac J Allergy Immunol. 2020;38(4):233–8. [DOI] [PubMed] [Google Scholar]
- 11.Jiang RS, Huang WC, and Liang KL. Characteristics of Sinus Fungus Ball: A Unique Form of Rhinosinusitis. Clin. 2018;11:1179550618792254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HJ, Seoh JY, Han KH, Moon KR, and Lee SS. The role of mucosal immunity in fungus ball of the paranasal sinuses. Acta Otolaryngol (Stockh). 2012;132 Suppl 1:S58–62. [DOI] [PubMed] [Google Scholar]
- 13.Tyler MA, Padro Dietz CJ, Russell CB, Citardi MJ, Assassi S, Ying J, et al. Distinguishing Molecular Features of Allergic Fungal Rhinosinusitis. Otolaryngol Head Neck Surg. 2018;159(1):185–93. [DOI] [PubMed] [Google Scholar]
- 14. *.Dietz CJ, Sun H, Yao WC, Citardi MJ, Corry DB, Luong AU. Aspergillus fumigatus induction of IL-33 expression in chronic rhinosinusitis is PAR2-dependent. Laryngoscope. 2019;129(10):2230–2235. doi: 10.1002/lary.28000• The authors had previously established that sinonasal epithelial cells release IL-33 which stimulated with fungal extracts. Using recombinant PAR2 and fungal protease inhibitors, they demonstrate that this induction of IL-33 by fungi is via fungal protease activation of PAR2 signaling.
- 15.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188(4):432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter PC, Lim DJ, Maskatia ZK, Mak G, Tsai CL, Citardi MJ, et al. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol. 2014;134(2):325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, and Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol. 2014;4(5):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghebre MA, Desai D, Singapuri A, Woods J, Rapley L, Cohen S, et al. Sputum Inflammatory Mediators Are Increased in Aspergillus fumigatus Culture-Positive Asthmatics. Allergy Asthma Immunol Res. 2017;9(2):177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, and Harris KE. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86(4):405–14. [DOI] [PubMed] [Google Scholar]
- 21.Mortezaee V, Mahdaviani SA, Pourabdollah M, Hassanzad M, Mirenayat MS, Mehrian P, et al. Diagnosis of allergic bronchopulmonary aspergillosis in patients with persistent allergic asthma using three different diagnostic algorithms. Mycoses. 2021;64(3):272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. **.Saxena P, Choudhary H, Muthu V, Sehgal IS, Dhooria S, Prasad KT, et al. Which Are the Optimal Criteria for the Diagnosis of Allergic Bronchopulmonary Aspergillosis? A Latent Class Analysis. J Allergy Clin Immunol Pract. 2021;9(1):328–35.e1.• This study showed that by lowering the IgE cutoff for the ISHAM criteria, there was a significant improvement in sensitivity compared to the Rosenberg criteria in the diagnosis of ABPA.
- 23. **.Asano K, Hebisawa A, Ishiguro T, Takayanagi N, Nakamura Y, Suzuki J, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2020;10:10.• The authors of this study proposed new diagnostic criteria for ABPA that are superior to both the Rosenberg and ISHAM criteria by lowering the IgE cutoff, reducing the number of clinical criteria and reducing the emphasis on the need for asthma to make a diagnosis of ABPA.
- 24.Nguyen NL, Pilewski JM, Celedon JC, Mandalapu S, Blanchard ML, DeRicco A, et al. Vitamin D supplementation decreases Aspergillus fumigatus specific Th2 responses in CF patients with aspergillus sensitization: a phase one open-label study. Asthma res. 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emiralioglu N, Dogru D, Tugcu GD, Yalcin E, Kiper N, and Ozcelik U. Omalizumab Treatment for Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis. Ann Pharmacother. 2016;50(3):188–93. [DOI] [PubMed] [Google Scholar]
- 26.Ashkenazi M, Sity S, Sarouk I, Bar Aluma BE, Dagan A, Bezalel Y, et al. Omalizumab in allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Asthma Allergy. 2018;11:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto N, Shigekusa T, Matsuo A, Tsubouchi H, Yanagi S, and Nakazato M. Allergic bronchopulmonary aspergillosis complicated by eosinophilic chronic rhinosinusitis successfully treated with mepolizumab. Respirol. 2019;7(7):e00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirota S, Kobayashi Y, Ishiguro T, Nishida T, Kagiyama N, Shimizu Y, et al. Allergic bronchopulmonary aspergillosis successfully treated with mepolizumab: Case report and review of the literature. Respir Med Case Rep. 2019;26:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsubouchi H, Tsuchida S, Yanagi S, Shigekusa T, Miura M, Sakaguchi K, et al. Successful treatment with mepolizumab in a case of allergic bronchopulmonary aspergillosis complicated with nontuberculous mycobacterial infection. Respir Med Case Rep. 2019;28:100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mummler C, Kemmerich B, Behr J, Kneidinger N, and Milger K. Differential response to biologics in a patient with severe asthma and ABPA: a role for dupilumab? Allergy Asthma Clin Immunol. 2020;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Borish L, Smith A, Somerville L, and Albon D. Use of mepolizumab in adult patients with cystic fibrosis and an eosinophilic phenotype: case series. Allergy, Asthma & Clinical Immunology. 2020;16(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. **.Knight JM, Mandal P, Morlacchi P, Mak G, Li E, Madison M, et al. Small molecule targeting of the STAT5/6 Src homology 2 (SH2) domains to inhibit allergic airway disease. J Biol Chem. 2018;293(26):10026–40.• This is the first study to develop a small molecule antagonist of the SH2 domain of immune-related transcription factors that has proven effective preclinically in attenuating allergic airway disease.