Abstract
Curcumin, a pure compound extracted from the flowering plant, turmeric (Curcuma longa. Zingiberaceae), is a common dietary ingredient found in curry powder. It has been studied extensively for its anti-inflammatory, antioxidant, antimicrobial and anti-tumour activities. Evidence is accumulating demonstrating its potential in chemoprevention and as an anti-tumour agent for the treatment of cancer. Despite demonstrated safety and tolerability, the clinical application of curcumin is frustrated by its poor solubility, metabolic instability and low oral bioavailability. Consequently researchers have tried novel techniques of formulation and delivery as well as synthesis of analogues with enhanced properties to overcome these barriers. This review presents the synthetic analogues of curcumin that have proven their anticancer potential from different studies. It also highlights studies that combined these analogues with approved chemotherapies and delivered them via novel techniques. Currently, there are no reports of clinical studies on any of the synthetic congeners of curcumin and this presents an opportunity for future research. This review presents the synthetic analogues of curcumin and makes a compelling argument for their potential application in the management of cancerous disease.
Keywords: Chemoprevention, Curcumin, Synthetic analogues, Molecular Targets, Apoptosis
Graphical Abstract
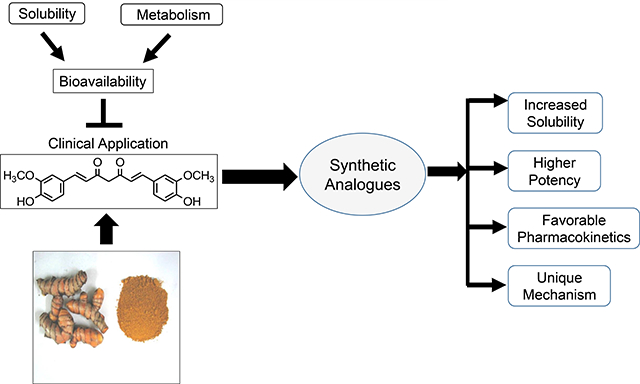
Low bioavailability resulting from poor aqueous solubility and metabolic instability impedes the clinical application of curcumin, a diketone compound isolated from curry powder. Synthetic analogues with better aqueous solubility, higher potency, favorable pharmacokinetic properties, some with unique mechanisms of action might be clinically applicable.
1. INTRODUCTION
Cancer is a debilitating and complex disease engaging scientists around the globe working tirelessly to find possible cures. Billions of dollars have been spent every year for cancer research and treatment globally with questionable returns on investment. In the United States for the year 2010 alone, more than $124.6 billion has been spent to treat over 13.7 million survivors with 1.5 million newly diagnosed cancer patients in 2010, a decade later the battle is far from won (Park et al., 2013). None-the-less, giant strides have been made in the management of cancerous disease, from surgery in the 19th century to the introduction of chemotherapy in the 1940’s. Most recently, advancements have been made employing molecular-targeted and immune-targeted therapy along with modern delivery systems. However, the work is far from done. According to the World Health Organization (WHO), 1 in every 6 deaths is due to cancer and 70% of these deaths occur in low- and middle-income countries where early detection and effective chemotherapy and/or modern therapies is expensive and hard to come by (Organization, 2018). With an increasing human life expectancy and increasingly aging population, the incidence of cancer and related costs is expected to rise (Park et al., 2013). Cancer development is a rather insidious multi-step process that often starts from exposure to a carcinogen, which leads to hyperplasia, dysplasia, carcinoma in situ and ultimately invasive cancer (Haddad and Shin, 2008). Notably while it is reported that 30–40% of cancers could be prevented by maintaining healthy lifestyle practices such as consumption of healthy diet, regular physical activity and maintaining optimum body weight (Glade, 1999, Amin et al., 2009) the incidence of this disease continues to rise. Given issues with toxicity, efficacy and the cost of care of many of the current therapies, it is imperative that we look towards alternative therapies for cancer prevention and treatment such as cost-effective and efficient natural remedies. An ideal agent should be safe for human use, effective at low doses, economical and readily available. One such agent that has proven effective for both cancer treatment and prevention is curcumin, a natural product from the common plant turmeric (Curcuma longa, Zingiberaceae). Several studies have corroborated the findings of Kuttan and co-workers who first tested the clinical efficacy of 1% curcumin ointment on skin cancer lesions (Kuttan et al., 1987). Multiple reports show that curcumin inhibits growth by arresting the cells in the G1/S and G2/M phase of the cell cycle and/or inducing apoptosis of cancerous cells in various body tissues like B and T cells, colon, epidermis, prostate, breast, and head and neck squamous cell carcinoma (Martínez-Castillo et al., 2018, Tamvakopoulos et al., 2007). Moreover, curcumin is safe and tolerable at doses up to 12g/day. However, the realisation of curcumin as an effective cancer treatment is limited by its very low oral bioavailability (less than 1%) due to metabolic instability (Cheng et al., 2001). This has led to the discovery of numerous curcumin analogues (synthetic and semi-synthetic) with higher potency and enhanced bioavailability, which could overcome the limitations of the clinical efficacy of curcumin (Kudo et al., 2011a, Jordan et al., 2018). We acknowledge that there is an exhaustive list of analogues that have been synthesized/semi-synthesized from structure-activity relationship studies over the years (Agrawal and Mishra, 2010, Rodrigues et al., 2019, Vyas et al., 2013), this review focuses on synthetic analogues that have demonstrated anti-tumour potential with higher potency in multiple studies (Fig. 1, Table 1). These analogues have diverse molecular mechanistic pathways (Fig. 2) and have proven prospects for the prevention and management of cancerous disease. Here we provide a perspective from cell biology and molecular pharmacology (summarized in Table 2).
Figure 1:
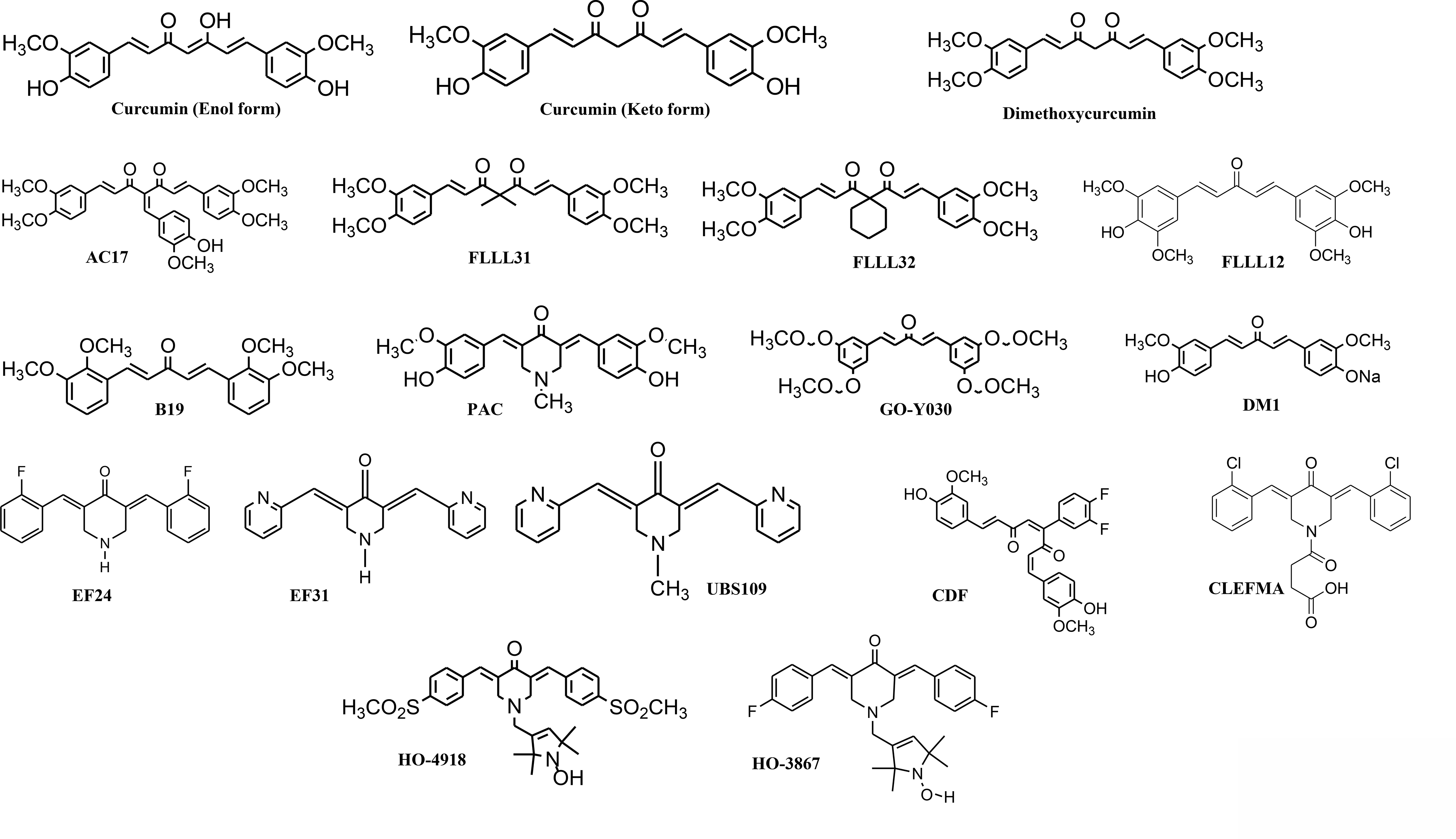
The chemical structures of synthetic analogues of curcumin. B19, GO-Y030, FLLL12 and DM1 form a group of monocarbonyl analogues of curcumin, while EF series have a common piperidone core. The newer generation members of the FLLL series (FLLL31 and 32) functionalize the methylene group of curcumin.
Table 1:
Classification of analogues based on structural modifications
| Structural Modification | Analogues |
|---|---|
| Dimethoxy Substitution | AC17, Dimethoxycurcumin, FLLL31, and FLLL32 |
| Mono-ketone (Pentadieneone bridge) | B19, CDF, DM1, FLLL12, and GO-Y030 |
| Piperidine-4-one core | EF24, EF31, CLEFMA, HO-4918, HO-3867, PAC, and UBS109 |
| Fluorination | CDF, EF24 |
Figure 2:
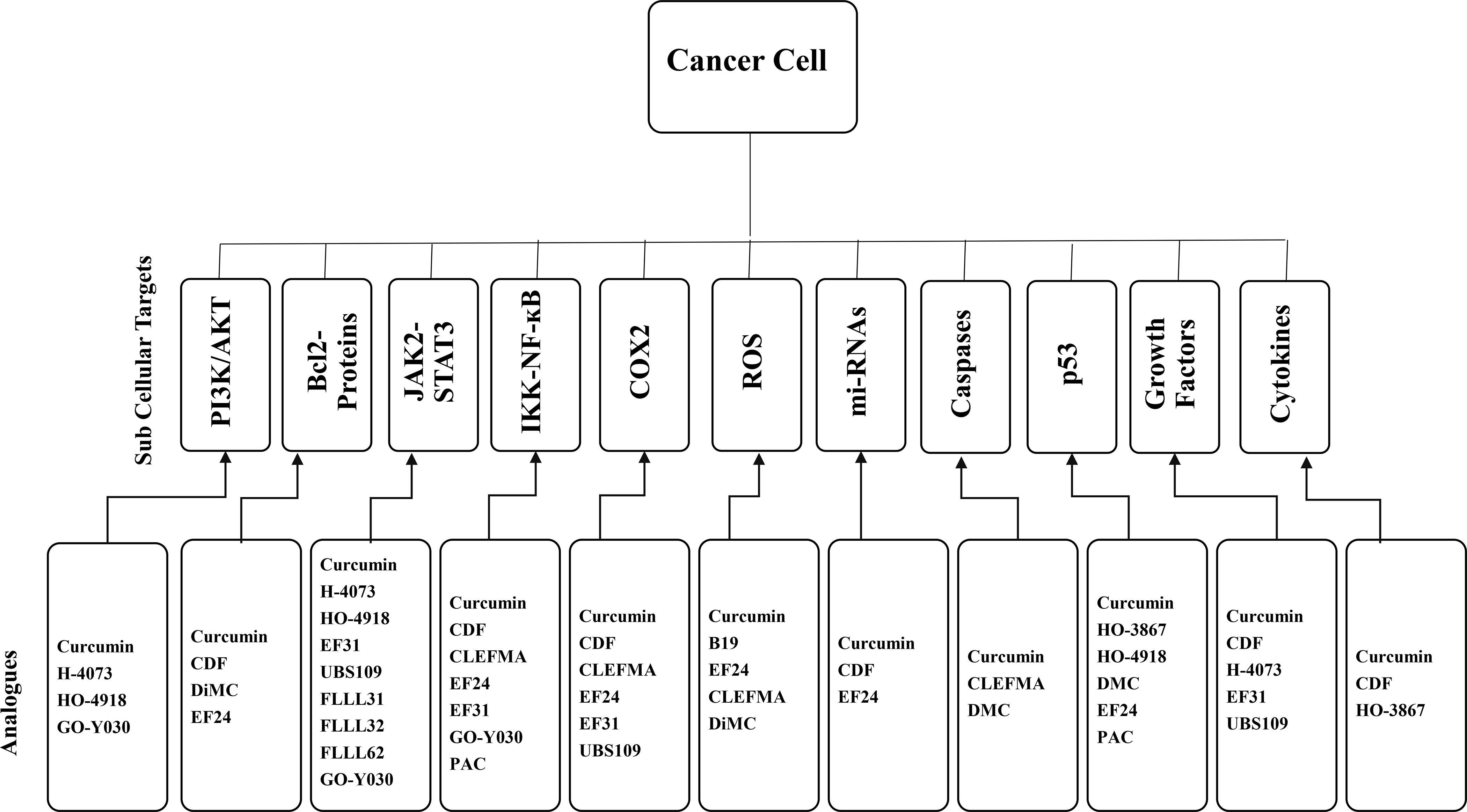
A schematic representation of the molecular mechanism/ sub-cellular targets of curcumin and its synthetic analogues.
Table 2:
Synthetic analogues of curcumin and their anti-tumour mechanism of action
| Analogue | Cancer/Cell Type | Assay type | Molecular Targets and Mechanism | Study Conclusion | References |
|---|---|---|---|---|---|
| AC17 | Non-Small Cell Lung Cancer (A549) | In vitro and in vivo (mice) | 19S deubiquitinase inhibition. | 4-arylidene curcumin analogues have great potential as anticancer agents. | (Zhou et al., 2013) |
| B19 | Non-Small Cell Lung Cancer (H460) | In vitro and in vivo | ER stress mediated apoptosis. | B19’s induces ER stress-CHOP mediated antitumor effect. | (Wang et al., 2011) |
| Ovarian Carcinoma (A2780, CP70) | In vitro | ER stress activation, ROS generation. | B19 induces apoptosis of ovarian cancer cells through oxidative stress and ER stress. | (Zhang et al., 2012) | |
| Ovarian Carcinoma (HO8910) | In vitro | UPR and ER stress response | B19 induces ER stress-mediated apoptosis which is increased by inhibition of autophagy. | (Qu et al., 2013) | |
| Human umbilical vein endothelial cells | In vitro and Ex vivo (Rat and Chicken) | Inhibition of VEGF, ERK, p38MAPK and AKT | B19 exhibits potent anti-angiogenic effect in vivo and ex vivo | (Sun et al., 2014) | |
| Gastric Cancer (SGC-7901, BGC-823 and KATO III) | In vitro and In vivo (Mice xenografts) | TrxR1 inhibition, ROS-mediated ER-stress activation and mitochondrial dysfunction | B19 induces cell cycle arrest and apoptosis by direct interaction with TrxR1. | (Chen et al., 2016) | |
| CDF | Colon Cancer (HCT116, HT29) | In vitro | Cancer stem cell markers including CD44, CD166; ABCG2, EGFR, IGF-1R, and NF-κB, β-catenin, COX-2, c-Myc and Bcl-xL | CDF overcomes chemo-resistance by eliminating CSCs and inducing apoptosis. | (Dandawate et al., 2012) |
| Colon Cancer (miR-21 over-expressing colon cancer HCT116, SW620) | In vivo (SCID mouse xenograft) | Restoration of PTEN-AKT pathway, inhibition of miR21 | CDF holds potential for application in chemo-resistant colorectal cancers. | (Roy et al., 2013) | |
| Prostate Cancer | In vitro (PC-3, LNCaP) | Inhibition of VEGF, IL-6, Nanog, Oct4, EZHZ and miR-21 | Anti-tumour activity of CDF is mediated by tumour hypoxic pathways. | (Bao et al., 2012) | |
| Pancreatic Cancer (AsPC-1, MiaPaCa-2, BxPC-3, Colo-357, Panc-1) | In vitro and in vivo (mouse xenograft) | Inhibition of EZH2, EGFR, Notch-1, CD44, miR-221; upregulation of PTEN, p27, let-7a,b,c,d, miR-26a, miR-101, miR-146a, and miR-200b,c; activation of p73,c-Myc, Bax, PUMA | CDF decreased tumour growth and aggressiveness, can combine with conventional chemotherapies and can help to design personalized care for human pancreatic cancer. | (Bao et al., 2012, Sarkar et al., 2010) | |
| CLEFMA | Lung adenocarcinoma (A549, H441, CCL151) | In vitro and in vivo (mouse xenograft) | Inhibition of NF-κB transcriptional activity and target genes expression | CLEFMA inhibits growth of lung cancer xenografts by suppressing NF-κB-regulated anti-inflammatory and anti-metastatic effects. | (Yadav et al., 2013) |
| Human osteosarcoma (U2OS, HOS) | In vitro | Activation of proapoptotic effectors caspase 3, 8 and 9; Phosphorylation of JNK and p38 | CLEFMA activates both the extrinsic and intrinsic apoptosis. | (Yang et al., 2019) | |
| HO-3867 | Ovarian cancer (A2780) | In vitro and in vivo (rat) | G2/M cell cycle arrest by modulating p53, p21, p27, cdk2 and cyclin; apoptosis by caspase-8 and caspase-3 activation | Cellular absorption of HO-3867 is substantially greater than curcumin | (Rath et al., 2013) |
| H-4073 | Head and Neck Cancer (UM-SCC-74A, UM-SCC-74B, UM-SCC-1, UM-SCC-38, UM-SCC-47, CAL27 | In vitro and in vivo (SCID mouse xenografts) | Inhibition of JAK-STAT3, FAK, Akt and VEGF | H-4073 overcomes chemotherapy resistance in head and neck cancers. | (Kumar et al., 2014) |
| HO-4918 | Colon Cancer (HCT116) | In vitro | p53, AKT, pAKT and STAT3 | HO-4918 inhibits the proliferation of HCT-116 cells. | (Prabhat et al., 2019) |
| Dimethoxycurcumin (DiMC) | Breast Cancer (MCF7) | In vitro | p53, p21, CDK4, and cyclin-D1, Bax and Bcl-2 | Dimc induced cell death in MCF7 cells through S-phase arrest and apoptosis. | (Kunwar et al., 2012) |
| Hepatocellular carcinoma (HepG2, C3A) | In vitro | Increased CDKN1A, GADD45A, PARP1 gene expression | DiMC induced genotoxicity and apoptosis. | (Zanetti et al., 2019) | |
| Leukemia (CEM, BV-173, Kasumi-1) | In vitro | Epigenetic changes | DiMC was not cytotoxic to leukemia cells and induced G2/M cell cycle arrest. | (Hassan et al., 2015) | |
| Lung (A549) | In vitro | Increase in ROS, decreased in GSH by targeting thioredoxin system | DiMC induced apoptosis and mitosis. | (Jayakumar et al., 2016) | |
| Renal Carcinoma (Caki) | In vitro | ROS generation | Enhanced potency and stability | (Lee et al., 2010) | |
| DM-1 | Melanoma (SKMEL-19, SKMEL-28, SKMEL-29 and A375) | In Vitro. | ADK, ATP6V0B, PEMT, TOP1, ZFP36, ZFP36L1, ZFP36L2 | The anticancer effect is cell line-dependent. | (Oliveira et al., 2017) |
| EF24 | Breast (MBA-MD231), prostate (PC-3, DU145), cholangiocarcinoma (HuCCT-1, TFK-1, HuH28) lung (A549, H157, H460, Calu-1, H358), melanoma (B16), hepatocellular Carcinoma (Hep3B, HepG2) oral squamous cell carcinoma (Cal27) | In vitro and in vivo (mouse xenografts) | ↓HIF1-α and NF-κB; ROS generation and GSH depletion; ↑PTEN | EF24 is a potent curcumin analogue that induced G2/M cell cycle arrest and apoptosis. | (Kasinski et al., 2008, Thomas et al., 2008, Liang et al., 2011, Selvendiran et al., 2007, Tan et al., 2010, Yang et al., 2013) |
| EF31 | Head and Neck Cancer (Tu212) | In vivo (mouse xenograft) | Inhibition of NF-κB p65 phosphorylation | Long term EF31 treatment decreased angiogenesis and metastasis | (Zhu et al., 2012) |
| EF31 & UBS 109 | Pancreatic Cancer (Mia PaCa-2, PANC-1); Colorectal (HCT116, HT29) | In vitro and in vivo | ↓HSP90, HIF-1α and NF-κB, p-STAT3 | EF31 and UBS109 are potent curcumin analogues with potential antiangiogenic activities. | (Rajitha et al., 2016, Nagaraju et al., 2015, Rajitha et al., 2017) |
| FLLL11 & FLLL12 | Pancreatic (PANC-1, BXPC-3, MIA-PACA-2, ASPC-1, and HPAC), Breast (MDA-MB-231, MCF-7, MDA-MB-468, SK-BR-3, BT-474, MDA-MB-453) and Prostate (PC-3 and DU145) Cancer | In vitro | STAT3 and AKT | FLLL11 and FLLL12 are potent anticancer curcumin analogues. | (Friedman et al., 2009, Lin et al., 2009) |
| FLLL12 | Lung Cancer | In vitro | DR-5 | FLLL12 is a potent curcumin analogue that induced extrinsic apoptosis. | (Haque et al., 2015) |
| Head and Neck (Tu212, Tu686, Tu177) | In vitro and in vivo | Inhibits expression of p-EGFR, EGFR, p-AKT, AKT, Bcl-2 and Bid and increased the expression of Bim | FLLL12 is more potent, has good pharmacokinetic properties and induced apoptosis. | (Anisuzzaman et al., 2016) | |
| FLLL31 and FLLL32 | Pancreatic (PANC-1, BXPC-3, HPAC, SW1990) and Breast (MDA-MB-231, SK-BR-3, MDA-MB-468, MDA-MB-453) Cancer | In vitro and in vivo (chicken embryo and mouse xenografts) | Inhibition of STAT3 activation | FLLL32 meets basic requirements for potential chemopreventive and therapeutic agent. | (Lin et al., 2010) |
| FLLL32 and FLLL62 | Melanoma (ACHN RCC, Caki RCC, A375, Hs294T) and Renal (SK-RC-45, SK-RC-54) Carcinoma | In vitro | Jak2-STAT3 pathway inhibition | FLLL32 and FLLL62 are potent curcumin analogues that might serve as lead compounds for STAT3 inhibition. | (Bill et al., 2012) |
| GO-Y030 | Thyroid, pancreatic and cholangiocarcinoma (8505c, HuCCT-1, SH-10-TC SW620, MCF7, PC3 and PK-1) | In vitro | Direct inhibition of IKKβ kinase and suppression of nuclear translocation of NF-ΚB p65 | GO-Y030 is a much stronger inducer of apoptosis with enhanced potency than curcumin | (Sato et al., 2011) |
| GO-Y030 and GO-Y078 | Multiple Myeloma (RPMI8226, U266, OPM2, KMS12-BM) | In vitro | Inhibition of NF-κB, PI3K/AKT, JAK/STAT3, and IRF4 pathways | Both are potential therapeutic candidates for multiple myeloma treatment. | (Kudo et al., 2011) |
| PAC | Breast Cancer (MDA-MB231, MCF-7, T-47D, MDA-MB-468, BT-20, BT-549) | In vitro and in vivo (mouse xenografts) | Inhibition of NKT/NF-κB and WNT/b-catenin signalling, ↓ERα and EMT | PAC showed anti-carcinogenic efficacy by inducing apoptosis against breast cancer cell lines and has better bioavailability. | (Al-Hujaily et al., 2011, Al-Howail et al., 2016) |
2. SYNTHETIC CURCUMINS IN CANCER MANAGEMENT
2.1. EF Series (EF24, EF31 and UBS109)
EF24 was the first member of this series developed through the combined efforts of scientists from the National Cancer Institute and Emory University with the goal to enhance potency and to overcome poor absorption characteristics of curcumin by modifying its structure. After testing over 100 analogues, 10 shortlisted compounds were screened using the National Cancer Institute, NCI-60 cancer cell line panel. EF24 was identified as one of the analogues with great promise (Adams et al., 2004, Kasinski et al., 2008). Subsequently, EF31 and UBS109 with better physicochemical and pharmacologic characteristics were synthesized (Nagaraju et al., 2013).
2.1.1. EF24- Diphenyl difluoroketone/3,5-bis(2-flurobenzylidene) piperidin-4-one
EF24 is a fluorinated curcumin analogue (Thomas et al., 2008) that has been investigated against several cancer types (Table 2) and exhibits approximately 10-fold higher potency than curcumin (Sarkar et al., 2010). Like curcumin, the exact mechanism of action of EF24 is not well defined but it seems to suppress cancer cell proliferation and angiogenesis by altering regulation of genes in a variety of pathways (Table 2). Adam and colleagues reported that through redox-dependent mechanisms, EF24 induced cell cycle arrest and apoptosis in human breast and prostate cancer cells (Adams et al., 2005). Subsequently, Thomas et al. reported that EF24 has a different mechanism than its parent compound. While curcumin acts via inhibition of Hypoxia-inducible factor 1-alpha (HIF-1α) gene transcription, EF24 inhibits HIF-1α post-transcriptionally in both breast and prostate cancer cells (Thomas et al., 2008). Kasinski et al. demonstrated that EF24 was about 10 times more effective than curcumin in suppressing lung cancer cells (A549) in vitro by inhibiting IKK-beta kinase in the NF-κB signalling pathway (Kasinski et al., 2008).
EF24 at a very low dose (0.4 uM) synergizes with p38 inhibitors and suppresses lung cancer cells by blocking the p38 signalling pathway (Thomas et al., 2010). EF24 also suppressed growth of human liver cancer cells in vitro and in mice xenograft models by blocking NF-ҡB signalling pathways involving the expression of Bcl-2, COX-2, Cyclin B1, inhibiting p-ERK, p-AKT and VEGF, and activating p53 and p21 (Liang et al., 2011b, Liu et al., 2012). EF24 is also effective against oral squamous cell carcinomas by inhibiting phosphorylated MEK/ERK (Lin et al., 2017). Against cisplatin resistant human ovarian cancer cells, EF24 induced G2/M arrest and apoptosis by increasing phosphate and tensin homolog (PTEN) expression and regulating downstream signalling pathways (Selvendiran et al., 2007). In another study, EF24 exhibited an 8 to 16 fold higher potency than the parent compound against platinum sensitive and resistant ovarian cancer cells (Tan et al., 2010). EF24 also showed potential anticancer activity against both prostate cancer and melanoma models by selectively targeting the NF-κB pathway, along with enhanced expression of tumour suppressor miRNAs and reduced expression of oncogenic miRNAs including miR-21 (Yang et al., 2013). Inhibition of miR-21 was further confirmed in human prostate cancer cells. Although EF24 unlike curcumin, selectively inhibits the NF-ҡB pathway without affecting the signal transducer and activator of transcription (STAT) pathways (Yang et al., 2013, Yin et al., 2016), a recent study found that EF24 does inhibit phosphorylation of STAT3. This same study also found that EF24-induced apoptosis is mediated by reactive oxygen species (ROS)-dependent oxidative stress (Bisht et al., 2019). In summary, EF24 is a potent curcumin analogue, that like curcumin, affects multiple pathways including NF-ҡB, HIF-1α, p53, STAT3, miRNAs and ROS (He et al., 2018).
2.1.2. EF31 (3,5-Bis(2-pyridylmethylene)-4-piperidone)
EF31 is another fluorinated analogue from the National Cancer Institute and Emory University group and like the other analogues strongly inhibits the NF-ҡB pathway. A study directly comparing EF24 and EF31 suggests that EF31 is more potent than EF24 with respect to inhibition of IKK-beta phosphorylation, NF-ҡB nuclear translocation and DNA binding, and has greater cytotoxicity than EF24 in human ovarian, breast and pancreatic cancer cell lines (Olivera et al., 2012). Another study using colorectal cancer models demonstrates that EF31 induces G0/G1 cell cycle arrest and inhibits in vivo tumour growth in xenograft models by inhibiting NF-ҡB activity. In addition, EF31 via downregulation of E2F-1 and its target gene thymidylate synthase, might overcome 5-FU resistance (Rajitha et al., 2016). Zhu et al. studied the pharmacokinetic (PK) profile and anticancer activity of EF31 in mice using head and neck cancer models. The results show that EF31 is slightly more soluble (<10 and 13 μg/ml) and more potent (IC50: 7μM vs. 8μM) than EF24 and inhibits tumour growth in the xenograft model (Zhu et al., 2012). When administered peritoneally in mice, the peak concentration was reached within 30 mins with a mean residence time of at least 6 h. While prolonged treatment with EF31 led to a decrease in p-IKK-alpha and p-IKK-beta, there were increased levels of p-IҡBα. This indicates that there may be other kinases responsible for the phosphorylation of IҡBα (Zhu et al., 2012). The pleiotropic nature of EF31 was tested against 50 kinases at 5μM dose, confirming that EF31, like curcumin, is pleiotropic in nature. EF31 blocked all the kinases studied to varying degrees (Brown et al., 2013). Nagaraju et al. examined the effects of EF31 on DNA methylation in MiaPaCa-2 and PANC-1 cells and confirmed that EF31 treatment lowered DNA methylation by disrupting interaction of DNA methyltransferase (DNMT)-1 and the chaperone protein heat shock protein 90 (HSP90), which led to increased expression of SPARC, p16 and E-cadherin (Nagaraju et al., 2013). Furthermore, the antiangiogenic potential of EF31 has been studied in pancreatic and colorectal cancer cell lines using human umbilical vein endothelial cells (HUVEC) tube formation, egg chorioallantoic membrane (CAM) assays and matrigel plug assays. EF31 inhibits angiogenesis by inhibiting HSP90, NF-ҡB, HIF1-α and VEGF, which play crucial roles in angiogenesis leading to cancer metastasis (Nagaraju et al., 2015, Rajitha et al., 2017). Taken together, EF31 is a novel mono-carbonyl analogue with enhanced physico-chemical characteristics and proven anti-tumour potential. More studies are required to identify any comparative advantage in the pharmacokinetic profile of EF31 against curcumin and other analogues.
2.1.3. UBS109
UBS109 is another fluorinated curcumin analogue structurally similar to EF24 and EF31, but with higher solubility. Pharmacokinetic studies in mice with a single oral dose revealed that peak plasma concentrations were reached at 0.5 h post-dose with average plasma concentrations of 131 and 248 ng/ml for oral doses of 50 and 150 mg/kg, respectively with terminal elimination half-lives (T½) for these doses averaging 3.7 and 4.5 h, respectively (Zhu et al., 2014). UBS109 also decreased the levels of phosphorylated IKK-beta and phosphorylated p65 both in vitro and in vivo and inhibited the growth of head and neck squamous cell carcinoma xenograft tumours in mice (Zhu et al., 2014). UBS109 has been assessed for its antiangiogenic effects on cancer cells and bone marrow cells. Yamaguchi and colleagues reported that UBS109 stimulates osteoblast mineralization and inhibits lipopolysachharide-induced osteoclastogenesis (Yamaguchi et al., 2012). Subsequently, this finding was corroborated by in vivo mice models transfected with metastatic breast cancer cells. UBS109 prevented bone loss in the mice when compared with the control group (Yamaguchi et al., 2014). In pancreatic cancer models, UBS109 impaired angiogenesis as assessed by in vitro egg CAM assays and HUVEC tube assembly or in vivo vascularization by and repressing HIF-1α, HSP90, COX-2 and VEGF (Nagaraju et al., 2015). In a colorectal cancer model, UBS109 decreased angiogenesis by inhibiting NF-κB activity, HIF-1α, COX-2, STAT-3, and VEGF (Rajitha et al., 2017). Inhibition of NF-ҡB by UBS109 is also associated with inhibition of DNMT-1 expression and DNA methylation (Nagaraju et al., 2013).
2.2. FLLL Series (FLLL11, FLLL12, FLLL31, FLLL32 and FLLL62)
Dr. Fuchs laboratory at Ohio State University initially synthesized 24 curcumin analogues that fall under two major chemical groups- a heptadiendione series (13 analogues) and a pentadienone series (11 analogues) (Fuchs et al., 2009). Structure activity relationship studies revealed that many of these analogues are more potent than curcumin against prostate and breast cancer cell lines, particularly the pentadienone analogues (Fuchs et al., 2009). Later, members of this groups were named FLLL and newer members were introduced. FLLL11 is also available naturally from Curcuma domestica and Curcuma longa. FLLL12 has an extra methoxy group at the 5-position of the aromatic rings (Friedman et al., 2009). FLLL31, FLLL32 and FLLL62 are newer members derived by functionalizing the methylenic position of curcumin (Lin et al., 2010b).
2.2.1. FLLL12
FLLL12 is a promising monoketone analogue with additional methoxy groups at position 5 of both aromatic rings which may account for its superiority over FLLL11 and curcumin as an anticancer agent (Friedman et al., 2009, Ohori et al., 2006). FLLL12 has been screened for its activity against various cancer types such as head and neck, lung, breast, pancreatic and colorectal cancers. Initial screening suggests that FLLL12 is approximately 5–10 times more potent than the parent compounds against prostate (PC-3 and LNCaP) and breast cancer cell lines (MCF-7 and MDA-MB-231) (Fuchs et al., 2009). FLLL12 was also tested against 5 pancreatic cancer cell lines indicating that this analogue is 3–21-fold more potent in inducing apoptosis (Friedman et al., 2009). Further mechanistic studies revealed inhibition of p-AKT and p-STAT3 by FLLL12. Lin et al. also tested the efficacy of FLLL12 against eight different breast and prostate cancer cell lines and found that FLLL12 is approximately 10–50 times more potent and inhibited p-AKT, p-STAT3, STAT3 DNA binding and STAT3 transcriptional activity (Lin et al., 2009). Our laboratory determined the IC50 of FLLL12 against eight well-known lung cancer cell lines. FLLL12 is 5–10 fold more potent in inhibiting cell growth and inducing apoptosis. Mechanistic studies demonstrated activation of the death receptor-5 (DR5)-dependent extrinsic apoptotic pathway (Haque et al., 2015). Our laboratory also conducted a comparative pharmacokinetic study of FLLL12 and curcumin. After oral administration of a dose of 200 mg/kg, peak mouse plasma concentrations were reached at 0.25 and 0.5 h post-dose (Tmax) with average concentrations (Cmax) of 55.65 and 241.5 ng/ml, for curcumin and FLLL12, respectively (Anisuzzaman et al., 2016). This pharmacokinetic advantage could be critical to the clinical application of the analogue over curcumin if it scales through preclinical and clinical evaluations. Finally, we reported that FLLL12 is about 10–24 times more potent against a panel of 9 head and neck cancer cell lines (Anisuzzaman et al., 2016). Interestingly, normal human oral keratinocytes were not affected, suggesting that the compound is non-toxic to non-cancerous cells. Our laboratory also showed that FLLL12 not only inhibited p-AKT but also AKT1 mRNA (Anisuzzaman et al., 2016). More systemic studies are warranted to proceed for further clinical development.
2.2.2. FLLL31 and FLLL32
FLLL31 and FLLL32 are diketone analogues of curcumin specifically designed to bind selectively to Janus Kinase 2 (JAK2) and the STAT3 SH2 domain, which serves crucial roles in STAT3 dimerization and signal transduction (Lin et al., 2010b). These analogues were synthesized by replacing the two hydrogen atoms on the central carbon of curcumin with geminal dimethyl substituents (FLLL31) or a spiro-cyclohexyl ring (FLLL32). Moreover, these analogues contain 3,4-dimethoxy substituents to mimic those of dimethoxycurcumin. As expected FLLL31 and FLLL32 effectively inhibit STAT3 phosphorylation, DNA binding, and transactivation, leading to the induction of apoptosis, inhibition of soft agar colony formation and cell invasion in pancreatic and breast cancer cell lines (Lin et al., 2010b). Moreover, administration of FLLL32 inhibits tumour growth and vascularity in chicken embryo xenografts as well as substantially reducing tumour volumes in mouse xenografts (Lin et al., 2010b). FLLL31 has also shown its potential application in glioblastomas, which often have constitutively active STAT3 (Chiba et al., 2012). In colorectal cancer cell (CRC) lines carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) plays an active role in tumour progression. FLLL31 was shown to reduce the expression of CEACAM5 by inhibiting IL-6-induced STAT3 phosphorylation (Holmer et al., 2015). In addition, due to the link between interleukins and STAT3 activation FLLL31 has also been studied for its potential as an anti-inflammatory agent. However, more studies are required for its potential application in inflammatory conditions like asthma, chronic obstructive pulmonary disease, multiple sclerosis, rheumatoid arthritis and cancer (Yuan et al., 2015, Yuan et al., 2014).
Studies have shown that FLLL32, as a potent inhibitor of STAT3, ablates the cancer promoting effects of this transcription factor. Against human and canine osteosarcoma cells lines, FLLL32 strongly inhibited STAT3 DNA binding, leading to proteasome mediated degradation of STAT3 and consequent ablation of downstream effectors VEGF, MMP2 and survivin expression (Fossey et al., 2011). Yan and colleagues in in vivo studies determined the effects of FLLL32 on human osteosarcoma cells supported these findings (Yan et al., 2015). This study also suggests that STAT3 plays a major role in propagation of osteosarcoma cells and suggests that inclusion of FLLL32 in combination therapies would be a novel strategy to target cancer initiating cells in chemo/radio resistant carcinomas (Yan et al., 2015). Lin et al. reported that FLLL32 selectively inhibits STAT3 and downstream proteins survivin, Bcl-xL and Notch-1, -3, and -4 in colon cancer-initiating cells and inhibited cell viability and tumorsphere formation as well as induced cleavage of caspase-3 (Lin et al., 2011a). These findings were corroborated by their subsequent work in ALDH+ and CD44+/CD24+ stem cell-like pancreatic cancer cells (Lin et al., 2016). Other studies have explored the ability of FLLL32 to overcome chemo/radio resistance of cancer cells. FLLL32 significantly increased the sensitivity of HNSCC cell lines to cisplatin, thereby achieving a similar tumour cell killing with a four-fold lower dose of cisplatin when combined with FLLL32 (Abuzeid et al., 2011). A recent study exploring the innovative delivery of FLLL32 via liposome technology observed that such a strategy increased the sensitivity of pancreatic cancer cells to radiation by inhibiting cancer stem cells (CSCs) through STAT3. More so, they observed a synergistic effect when FLLL32 was combined with gemcitabine and radiation therapy in vivo and in vitro (Wu et al., 2016).
2.3. GO-Y series
In a bid to identify compounds with growth suppressive effects against the colon cancer cell line DLD-1, Ohori et al. screened over 2000 species of compounds from a synthetic organic compound library and discovered GO-Y035, a curcumin analogue that showed remarkable growth suppressive potential. Subsequently, over 50 similar analogues were developed with reference to the structure of GO-Y035. Notable among them was GO-Y030 and GO-Y078 (Murakami et al., 2017, Ohori et al., 2006).
2.3.1. GO-Y030, G0Y031
GO-Y030 is a 1,5-diaryl-3-oxo-1,4-pentadiene (DOP) analogue, and like most other analogues of curcumin, it was synthesized to overcome the low bioavailability of curcumin while retaining the anticancer activities. While GO-Y030 has a 30–50 fold higher potential to suppress growth of cancer cells than curcumin it retains the similar molecular mechanisms of the parent compound (Ohori et al., 2006, Sato et al., 2011b, Shibata et al., 2009). Some of these mechanisms include; cell cycle arrest, downregulation of NF-κB and Wnt signal transactivation as well as activation of Caspase 3 (Shibata et al., 2009). Some studies have shown GO-Y030 to have a stronger inhibitory effect on the Wnt signalling pathway and NF-κB activation than curcumin in thyroid, pancreatic and cholangiocarcinoma (Sato et al., 2011b, Shibata et al., 2009). Shibata et al. reported a 39% reduction of tumour incidence in an Apc580D/+ mice model of intestinal tumorigenesis, thus demonstrating the chemopreventive effects of GO-Y030 (Shibata et al., 2009). Against colon cancer stem cells (ALDH+/CD133+), GO-Y030 was more potent than curcumin in inhibiting STAT3 phosphorylation and expression of STAT3 downstream targets as well as inhibition of cell viability, tumorsphere formation, in vivo tumour growth and induction of apoptosis (Lin et al., 2011c). In an in vivo transgenic mouse model, Uehara et al. demonstrated that GO-Y031 reduced tumour incidence and tumour size of gastric carcinoma by 54.4% and 51.6% respectively by significantly suppressing the levels of β catenin and STAT3 (Uehara et al., 2015).
In multidrug resistant breast cancer cell lines, GO-Y030 directly interacts with the ABCG2 transporter (encoding the breast cancer resistance protein, BCRP) at the substrate binding site thereby inhibiting efflux of its substrates and increasing sensitivity to the anticancer drug, SN-38 (Murakami et al., 2017). GO-Y030 also more strongly inhibited the growth of colorectal cancer cell lines and was found to be more potent than curcumin in the induction of apoptosis (Cen et al., 2009). Against thyroid, pancreatic and cholangiocarcinoma cells, GO-Y030 exhibited about 10-fold higher activity in terms of cell growth inhibition and induction of apoptosis, and directly inhibited IKKβ kinase activity and suppression of nuclear translocation of the NF-κB p65 subunit (Sato et al., 2011a). Taken together, GO-Y030 is more potent than curcumin for multi-target anticancer activity with a good safety profile. Moreover, GO-Y030 is a promising candidate against chemo-resistance of different human cancers.
2.4. 4-arylidene curcumin analogues: AC17
In an attempt to synthesize more potent curcumin analogues different from the mono and diketone analogues, Qiu et al. synthesized a series of 4-arylidene analogues of curcumin and evaluated their anti-tumour and NF-κB inhibitory effects against multiple lung cancer cell lines (Qiu et al., 2010). In cell viability assays, many of these analogues are 10–60 times more potent than curcumin, with GI50 in sub-micromolar range (0.23–0.93μM) and were found to induce apoptosis. These analogues also strongly inhibited TNFα-induced nuclear translocation of NF-κB with IC50 values in the low micromolar range (1.0~4.9 μM). The IC50 value for curcumin is 11.6 μM in this same assay. AC17 (IC50 1.0 μM) is a member of this group showing a more than 10-fold greater potency in blocking the nuclear localization of NF-κB than that of curcumin.
Interestingly, AC17 had direct inhibitory effects on IҡB kinase (IKK), which was not observed with curcumin. Molecular modelling suggests that AC17 could potentially bind to the ATP pocket of IKKβ (Qiu et al., 2010). A further study by Zhou and colleagues identified the deubiquitinase inhibition of 19S regulatory particles (19S RP) by AC17. This is a different mechanism of proteasome inhibition through inactivation of deubiquitinase 19S-RP without inhibiting 20S core particle (CP) mediated proteolysis (Zhou et al., 2013a). This led to accumulation of ubiquitinated proteins resulting in NF-ҡB inhibition and reactivation of proapoptotic protein p53. The in vitro findings were confirmed in vivo in a murine xenograft model of human lung cancer A549. Treatment with AC17 suppresses tumour growth in a manner associated with proteasome inhibition, NF-κB blockage, and p53 reactivation (Zhou et al., 2013a). This contrasts with curcumin’s inhibitory activity on proteasome via DYRK2 inhibition (Banerjee et al., 2018). Since many members of this series possess similar anticancer potency like AC17, further systematic studies are required to elucidate conclusively the molecular mechanisms, in vivo efficacy, and PK properties of these promising compounds.
2.5. B19 [(1E,4E)-1,5-bis(2,3-dimethoxyphenyl) penta-1,4-dien-3-one]
B19 is an analogue of curcumin identified as one of the active molecules among a series of monocarbonyl analogues of curcumin synthesized by Liang and colleagues that induces endoplasmic reticulum (ER) stress (Liang et al., 2009). Although some of these compounds have better stability, more favourable PK parameters and higher antitumour potency against multiple cancer cell lines, only B19 modulated C/EBP-homologous protein (CHOP) expression suggesting that B19 induced apoptosis via ER stress (Wang et al., 2011). Subsequently, Zhang et al. investigated the mechanism of apoptosis induction by B19 in ovarian cancer cells. Their results show that B19 is more potent at growth inhibition and apoptosis induction than curcumin against cisplatin resistant ovarian cancer cells. Moreover, while both curcumin and B19 induced ROS production, only B19 led to ER stress (Zhang et al., 2012). Further mechanistic studies revealed that B19 activates autophagy as evidenced by the significant accumulation of light chain protein 3 (LC3) in cells treated with B19 (Qu et al., 2013). In this case, autophagy serves as a protective mechanism against apoptosis since inhibition of autophagy by 3-methyladenine enhanced apoptosis induced by B19. Later, Li and co-workers explored the anti-angiogenic potential of B19 in human umbilical vein endothelial cells (HUVEC) in vitro and ex vivo via rat aorta ring and chicken chorioallantoic membrane assays (Sun et al., 2014). Their results suggest that B19 inhibited growth, migration and tube formation of HUVEC cells in vitro while preventing micro-vessel outgrowth ex vivo. Molecular docking studies identified thioredoxin reductase (TrxR) as a primary target for B19 (Chen et al., 2016). Furthermore, B19 directly inhibits TrxR1 enzyme activity in gastric cancer cells leading to elevated oxidative stress, ROS-mediated activation of ER Stress and mitochondrial dysfunction, subsequently resulting in cell cycle arrest and apoptosis (Chen et al., 2016). This corroborates earlier conclusions that B19 induces apoptotic cell death by ROS mediated ER stress and mitochondrial dysfunction. Taken together, these studies suggest that B19 is more potent than curcumin as an anti-tumour agent and provides new insights into TrxR1 involvement in ROS production and ER stress. Further studies are warranted to explore the PK properties and in vivo anti-tumour efficacies of this promising curcumin analogue.
2.6. CDF (Difluorinated Curcumin)
Difluorinated-curcumin (CDF) is a diketone curcumin analogue that has been studied extensively against various malignancies like colon, pancreatic and ovarian cancer (Momtazi and Sahebkar, 2016). More importantly, it is one of the few congeners whose in vivo pharmacokinetic properties have been compared with curcumin. In comparative pharmacokinetic studies in mice, CDF was 2.7 times more bioavailable than curcumin after intragastric intubation of a 250 mg/kg single dose (1.22 vs 0.44μg/ml*h) (Padhye et al., 2009a). Furthermore, oral administration of CDF produced a 2.7 fold increase in systemic drug level than curcumin (AUClast, 1.22 vs. 0.44 μg/ml*h) and its distribution skewed in favour of the pancreas (Cmax 44-fold higher than serum). Higher water solubility of CDF (8.4 fold higher than curcumin) might be responsible for the enhanced bioavailability of this analogue. CDF has shown more stability in vivo compared to curcumin, because of its slower absorption, onset of action and longer plasma residence time (Padhye et al., 2009a). Although CDF has a higher bioavailability and aqueous solubility than curcumin, concern exists that its solubility is still too low for good oral absorption (Momtazi and Sahebkar, 2016). Formulations of CDF were developed to further improve the water solubility and bioavailability. CDF-β-cyclodextrin inclusion complex (1:2) (CDFCD) lowered the IC50 value by half when tested against multiple cancer cell lines including pancreatic (BxPC-3), breast (MDA-MB-231) and prostate (PC3) cancer cells. The IC-50 values of CDF against BxPC-3, MDA-MB-231 and PC3 cells were 350 nM, 325 nM and 260 nM whereas those of CDFCD 1:2 conjugate were 125 nM, 150 nM and 120 nM respectively (Dandawate et al., 2012). Such formulations also increased CDF concentrations in serum (110 ng/ml vs. 6 ng/ml at 2h) and pancreas (410 ng/g vs. ~300 ng/g). Interestingly, another CDF formulation, styrene-maleic acid copolymer-CDF nano-micelles only slightly improved efficacy against pancreatic cancer cell lines (Kesharwani et al., 2015a).
Mechanistically, CDF is pleiotropic and just like curcumin acts via multiple mechanisms inhibiting different pathways, genes, and receptors. CDF in combination with 5-FU and oxaliplatin, significantly increased the sensitivity of chemo-resistant HCT-116 and HT-29 cells by eliminating CSCs, downregulation of the membrane transporter ABCG2 and attenuation of EGFR, IGF-1R, and NF-κB signaling (Kanwar et al., 2011). CDF also inactivates the AKT pathway by restoring the expression of PTEN by downregulating the expression of miR-21 in colon cancer cells (Roy et al., 2013). Bao et al. found that treatment of prostate cancer cells with CDF inhibited the production of VEGF and IL-6 along with reduced expression of Nanog, Oct4, EZH2 mRNAs, as well as miR-21 and decreased cell migration under hypoxic conditions suggesting that the anti-tumour effect of CDF is in part mediated through deregulation of tumour hypoxic pathways (Bao et al., 2012a). The same group also reported that CDF decreased pancreatic tumour growth and aggressiveness by targeting an EZH2-miRNA regulatory circuit for epigenetically controlled gene expression (Bao et al., 2012b). CDF decreased pancreatic cancer cell survival, clonogenicity, formation of pancreatospheres, invasive cell migration. Additionally, CDF also decreased CSC function and the expression of EZH2 and increased expression of a panel of tumour-suppressive miRNAs. CDF inhibited tumour growth in an orthotopic xenograft model of human pancreatic cancer in a manner associated with reduced expression of EZH2, Notch-1, CD44, EpCAM, and Nanog and increased expression of let-7, miR-26a, and miR-101 (Bao et al., 2012b). In some ovarian and breast cancer cell lines, the folic acid directed combination of paclitaxel and CDF yielded 10% and 20% greater apoptosis respectively when compared to drug combinations without folic acid (Gawde et al., 2018). In summary, CDF is effective against many cancers and acts by modulating multiple intracellular targets; PI3-K/Akt, HIF-1α, EGFR, VEGF, NF-κB, PTEN, EZH2, EpCAM, CD44 and microRNAs (miR-210 and mir- 21, mir-34, mir-101 and miR-146a).
2.7. 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] CLEFMA
Lagisetty and co-workers synthesized a series of 3,5-bis(2-fluorobenzylidene)-4-piperidone analogues of curcumin and conducted structure activity relationship (SAR) studies against the lung adenocarcinoma cell line H441 (Lagisetty et al., 2010). Based on an initial screening of the results, the group identified 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] (CLEFMA) as a potent curcumin analogue carrying a N-maleic acid functional group, and 2-chloro substitution on the aromatic rings which exhibited anti-proliferative activity against H441 (lung adenocarcinoma), PC-3 (prostate cancer), MiaPaCa-2 and PANC-1(both pancreatic cancer) (Lagisetty et al., 2010). Despite having potent activity CLEFMA did not induce apoptosis, rather it killed cancer cells by inducing autophagic death (Lagisetty et al., 2010). Subsequently, the group extended their studies using gene expression profiling and mechanism of action studies to show that generation of ROS, depletion of the intracellular GSH/GSSG ratio and failure of nuclear factor erythroid 2-related factor 2 (Nrf2) -mediated oxidative stress response mediates the antiproliferative effect of CLEFMA (Sahoo et al., 2012). Interestingly, ROS generation was not observed in normal lung fibroblasts which were also resistant to CLEFMA-induced cell death. Although previously the group reported autophagic cell death without caspase 3 activation, later studies demonstrated that CLEFMA induces apoptosis in lung cancer cell lines, which was evident by the accumulation of cleaved PARP, Caspase-3 and Caspase-9 in a dose-dependent manner and inhibiting the growth of xenografted lung cancer cells in mice (Yadav et al., 2013). Mechanistic study reveals that CLEFMA inhibits the NF-ҡB transcriptional pathway by reducing nuclear localization of phospo-p65 and levels of NF-ҡB1 transcripts. CLEFMA treatment in mice reduced the expression of proinflammatory COX-2 and circulating levels of pro-inflammatory cytokines IL-6 and TNF-α commonly observed in many cancers. Moreover, CLEFMA reduces the potential for angiogenesis and migration by reducing levels of CD31 (Yadav et al., 2013). Recently, new evidence has shown that CLEFMA induced apoptosis is not limited to the intrinsic pathway, but also the extrinsic pathway as CLEFMA has been shown to increase the levels of active caspase 8 in osteosarcoma cells, a marker of extrinsic apoptosis (Yang et al., 2019a). Finally, a formulation was developed by encapsulating CLEFMA by hydroxypropyl-β-cyclodextrin (Agashe et al., 2011b). In vitro studies showed that encapsulation of CLEFMA retained the antiproliferative potency of free CLEFMA, while maintaining its non-toxic nature in normal lung fibroblasts. IV administration of CLEFMA liposomes significantly reduced (94% reduction) tumour volume in nude rats bearing xenograft H441 tumours.
2.8. The diarylidenylpiperidones series
The diarylidenylpiperidones (DAPs) form a novel class of curcumin analogues with the inclusion of a piperidone ring within the beta-diketone backbone structure of curcumin and the additional fluorination of the phenyl groups (Dayton et al., 2010, Dayton et al., 2011). Like most other analogues of curcumin, the DAPs have superior bio-absorption and bioavailability when compared with curcumin. In vitro studies in ovarian cancer cells demonstrated that DAP-F(p)-NOH) was taken up in greater proportion and lasted longer within the cells compared to curcumin (Rath et al., 2013). Another DAP, HO-3867 was taken up in cells within 15 minutes of exposure and in concentrations up to 100 fold higher than curcumin (Dayton et al., 2010, Kalai et al., 2011). More so, in vivo models have shown that DAPs can produce measurable paramagnetic signals in vital organ tissue after intraperitoneal and oral administration (Dayton et al., 2010, Rath et al., 2013). The increased biological stability and bioavailability in tissues accounts for DAPs superior anticancer activity when compared to curcumin. Rath et al. also reported the selective induction of ROS by DAP-F(P)-NOH in cancer cells (Rath et al., 2013). This selective toxicity against cancerous cells has been attributed to the N-hydroxypyrolline function in this molecule, which is suggested to protect non-cancerous cells (Kalai et al., 2011). Similar effects have been observed in other DAPs (HO-4918 and HO-4200) with the N-hydroxypyrolline function. Electron paramagnetic resonance spectroscopy measurements of these DAPs showed metabolic conversion of the N-hydroxylamine to nitroxide with pronounced superoxide radical-scavenging activity in the noncancerous cells compared to cancer cells (Prabhat et al., 2019, Selvendiran et al., 2010a).
Regarding molecular targets, studies show that DAPs target the STAT3 pathway to a greater extent than other pro-oncogenic pathways (Selvendiran et al., 2010a, Selvendiran et al., 2010b). However, a study on the ovarian cancer cells, A2780 reported that HO-3867 induced G2/M cell cycle arrest by modulating cell cycle regulatory proteins p53, p21, p27, cdk2 and promoted apoptosis by caspase 8 and caspase 3 activation (Selvendiran et al., 2010b). Selvendiran et al. also demonstrated that HO-3867 at 10μM upregulated both phosphorylated and total PTEN expression in vascular smooth muscle cells (Selvendiran et al., 2009). Another DAP, H-4073 has been very effective at inhibiting tumour cell proliferation, colony formation and cell migration in HNSCC. The remarkable outcomes of H-4073 could be linked to inhibition of some important pathways such as STAT, FAK, AKT and VEGF (Kumar et al., 2014). Additionally, the synergistic effects of DAPs with other anti-tumour agents has also been reported. H-4073 has been reported to synergistically enhance the anti-tumour efficacy of cisplatin in overcoming resistance in head and neck cancer cells both in vitro and in vivo (Kumar et al., 2014).
2.9. DM-1
DM-1 (sodium 4-[5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2-methoxyphenolate) is a water soluble sodium salt of 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien-3-one, a diarylpentadienone derivative of curcumin (Faiao-Flores et al., 2012). The parent compound, 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien-3-one, exhibited potent anti-tumour activity against 9 human cancer cell lines (IC50: 0.09–2.3 μM) and was well tolerated (LD50–3.68g/kg) (Quincoces Suarez et al., 2010). The anti-tumour efficacy of DM-1 was tested on B16F10 melanoma-bearing mice either alone or in combination with Dacarbazine (DTIC) administered intraperitoneally (Faiao-Flores et al., 2015). The results showed that DM-1 reduced melanoma tumour burden without any detectable toxicological changes in major organs. Moreover, mice were recovered from anemia arising from melanoma and immunomodulation when treated with DM-1+DTIC. Moreover, DM-1 alone or in combination with DTIC induced apoptosis via extrinsic and intrinsic pathways (activation of caspase-3, -8 and -9). Synthetic lethal genetic screening in Saccharomyces cerevisiae suggests that the molecular mechanism of DM-1 was cell line specific and mediated via multiple targets (Oliveira et al., 2017). The TOP-1 gene was important for naïve cell lines treated with BRAF inhibitors whereas modulation of the adenosine kinase gene was more important for cell lines resistant to BRAF inhibition (Oliveira et al., 2017). Melanomas are known to invade and metastasize rapidly due to overexpression of metalloproteinases. Recently, it was demonstrated that DM-1 treatment inhibited migration of both naive and BRAF- resistant SKMEL-28 melanoma cell lines by suppressing the expression of metalloproteinases (de Souza et al., 2020). In conclusion, DM-1 is a potent curcumin analogue with in vitro and in vivo activity against multiple cancer types although most studies were conducted against melanomas. Further exploration of its in vivo activity against other cancer types as well as pharmacokinetic studies are warranted.
2.10. Dimethoxycurcumin
Dimethoxycurcumin (DiMC) is a synthetic analogue of curcumin in which the two phenolic-OH groups are replaced with methoxy groups. This small structural modification allows superior anti-cancer activity against multiple cancer types as well as better metabolic stability (Table 2) (Teymouri et al., 2018). Kunwar et al. have investigated the potential of DiMC (5–50 μM) against MCF7 breast cancer cells and found that it inhibited the cancer cells by a mechanism linked to generation of ROS, reductions in glutathione level, and induction of DNA damage and mitochondrial dysfunction leading to induction of S-phase cell cycle arrest and apoptosis (Kunwar et al., 2012). Another study comparing the anticancer activity and metabolism of curcumin and DiMC confirmed that even at low concentrations of 5–10 ug/ml DiMC was more potent at inhibiting growth and inducing apoptosis in human colon carcinoma HCT116 cells. In addition, DiMC was found to be more stable in HCT116 cells and mouse models which could contribute to its increased efficacy (Tamvakopoulos et al., 2007). These findings have been supported by another study that showed significantly improved anticancer efficacy of DiMC over curcumin in human renal carcinoma Caki cells (Lee et al., 2010). Moreover, it was observed that the efficacy for generation of ROS and induction of apoptosis was in the order DiMC > Curcumin > bis-demethoxycurcumin, suggesting that the methoxy group might have a significant role in the activity and/or stability of curcumin and its congeners (Lee et al., 2010). Several studies demonstrate that DiMC kills cells by a mechanism known as paraptosis which is associated with proteosomal inhibition leading to mitochondrial dysfunction, ER- stress, and ROS production, especially mitochondrial superoxide (Yoon et al., 2014, Adeyeni et al., 2016). Against the hepatocellular carcinoma cell line HepG2/C3A, DiMC exhibited its anticancer activity by inducing mitotic arrest through monopolar spindle formation and irreversible DNA damage. This was due to oxidative stress leading to the activation of BAK1 and caspase 7 via the intrinsic apoptotic pathway (Zanetti et al., 2019). In a study with various leukemia cells such as CEM, BV-173 and Kasumi-1, DiMC was found to induce epigenetic changes which were not seen with treatment with curcumin (Hassan et al., 2015). Multiple studies implicate DiMC-induced apoptosis to ROS generation (Teymouri et al., 2018). Furthermore, DiMC induces ROS-associated DNA damage in the radio-resistant A549 lung cancer cells in combination with radiotherapy (Jayakumar et al., 2016). Taken together, DiMC is a metabolically more stable curcumin analogue with better cytoprotective activity in normal tissues as well as more potent anti-cancer activity acting mainly by generation of ROS.
3. Combination Strategies with Curcumin Analogues
It is well accepted that most cancers are heterogenous with complex pathogenetic pathways involving several enzymes and receptors. Normal cells must acquire a series of essential hallmark abilities to become cancerous. This makes cancer management difficult with a single agent, and in most cases resistance to a single agent ensues. In fact, few cancer patients are treated with single drug, rather typical treatment involves multiple drugs. The main purpose in using a combinatorial approach is to either increase sensitivity or overcome resistance through additive or synergistic effects of multiple agents with different molecular targets and/or mechanisms of action. Previous work has shown that curcumin and its congeners are pleiotropic and would be good candidates for combination therapies (Hurtado et al., 2018). For example, FLLL32 suppresses cancer stem cells by inhibiting STAT3 and is able to reverse resistance to chemo and radiation therapies (Abuzeid et al., 2011, Wu et al., 2016). Similarly, EF24 demonstrated synergistic growth inhibitory effects with approved chemo drugs for both medullary thyroid cancer and pancreatic cancer (Bertazza et al., 2018, Bisht et al., 2016). DM1 and CDF also enhanced the efficacy of paclitaxel in breast cancer (Faiao-Flores et al., 2015), cervical cancer and ovarian cancers (Gawde et al., 2018). Table 3 gives a brief overview of such studies and their findings.
Table 3:
Combination strategies of curcumin analogues with conventional chemotherapy
| Combination | Purpose | Results/ Study Conclusion | Reference |
|---|---|---|---|
| CDF + Paclitaxel | Evaluation for synergistic effects | CDF and paclitaxel revealed synergistic potential portending promising clinical translation potential. | (Gawde et al., 2018) |
| DM-1 + Dacarbazine | Overcome chemo-resistance | DM-1 showed in vivo antitumor activity alone or in combination with chemotherapeutic DTIC in B16F10 melanoma-bearing mice. | (Faião-Flores et al., 2015) |
| DM-1 + Paclitaxel | Adjuvant chemotherapy | Combination increased the occurrence of apoptosis up to 35% more than in the group treated with paclitaxel alone. | (Faião-Flores et al., 2011) |
| EF24+ Carbozantinib | Evaluation for synergistic effects | EF24 and XL184 act synergistically irrespective of RET mutation status | (Bertazza et al., 2018) |
| EF24 + Gemcitabine | Evaluate the efficacy of combination | Enhanced tumour growth inhibition was observed from combination compared to individual agents | (Bisht et al., 2016) |
| FLLL32 + Cisplatin | To increase sensitivity | FLLL32 monotherapy induces a potent anti-tumour effect and sensitizes cancer cells to cisplatin. | (Abuzeid et al., 2011) |
| FLLL32 + Gemcitabine/Radiation | To enhance efficacy and overcoming resistance | Reversal of radio-resistance induced by pancreatic CSCs | (Wu et al., 2016) |
| HO-3867 + Doxorubicin | Cardio-protection | Mice treated with both HO-3867 and DOX showed marked improvement in cardiac functional parameters over mice treated with DOX alone | (Dayton et al., 2011) |
4. Molecular Mechanisms-Curcumin Vs Analogues
Curcumin context-dependently (cells or malignancies) exerts its anticancer effects via multiple molecular mechanisms which include inhibition of survival signals, activation of proapoptotic signals, generation of ROS and through anti-inflammatory actions (Park et al., 2013). Similarly, curcumin analogues mediate their anti-cancer effects by various mechanisms dependent on the cancer cell type (Table 2, Fig. 2). A summary of the molecular mechanisms of curcumin analogues are illustrated below.
4.1. Survival Signals- Nuclear Factor-ҡB
NF-ҡB is considered a master oncogenic transcription factor for carcinogenesis. NF-ҡB activation leads to the expression of genes that are critical for the survival of cancer cells. Moreover, NF-κB targets genes that are crucial for other necessary traits of tumours including angiogenesis, resisting cell death, invasion and metastasis etc. (Gupta et al., 2010). Inactive NF-ҡB associates with IκBα in the cytoplasm, upon cleavage of this association by IKK-beta kinase, NF-ҡB moves to the nucleus to promote the transcription of genes responsible for cell survival. In many cancers, NF-ҡB is constitutively activated. Like natural curcumin, the inhibition of the NF-κB pathway remains one of the major molecular mechanisms of many of curcumin analogues including CDF in colon cancer stem-like cells (Kanwar et al., 2011), CLEFMA in lung adenocarcinoma (Yadav et al., 2013), EF24 in lung, breast, ovarian, and cervical cancer cells (Kasinski et al., 2008, Liang et al., 2011a, Yang et al., 2013), EF31/ UBS109 in colorectal, HNSCC, and pancreatic cancer cells (Nagaraju et al., 2015, Rajitha et al., 2016, Zhu et al., 2012), GO-Y030/ GO-Y078 in multiple myeloma, thyroid, and pancreatic cancers (Kudo et al., 2011b, Sato et al., 2011b) and PAC in ER negative breast cancer cells (Al-Hujaily et al., 2011).
4.2. Janus Kinase-Signal Transducer and Activator of Transcription 3 Pathway
STAT3 is also an oncogenic transcription factor constitutively activated in many cancers and is involved in the transcription of genes that are important for tumorigenesis as well as for proliferation, survival, differentiation, and apoptosis. Multiple upstream kinases, including the JAKs, EGFR and Src family of kinases, are responsible for aberrant activation of STAT3 which results in its phosphorylation and nuclear translocation (Yang et al., 2019b). Some curcumin analogues inhibit the JAK-STAT pathways, specifically the FLLL series of analogues share a common mechanism of inhibiting this pathway. For example, FLLL11 and FLLL12 inhibit phosphorylation of STAT3 in breast and prostate cancer cells (Lin et al., 2009), FLLL31 and FLLL32 in pancreatic and breast cancer cells (Lin et al., 2010a), and FLLL32 and FLLL62 in melanoma and renal carcinoma (Bill et al., 2012). Moreover, DAP (H-4073) inhibited JAK-STAT3 pathway in cisplatin resistant HNSCC cell lines (Kumar et al., 2014). EF24 also inhibits phosphorylation of STAT3 in cholangiocellular carcinomas (Bisht et al., 2019). GO-Y030 inhibits STAT3 phosphorylation and expression of STAT3 target genes in colon cancer stem cells (Lin et al., 2011c) and suppresses the expression of STAT3 in a in vivo transgenic mouse model (Uehara et al., 2015).
4.3. Apoptotic Signals -Intrinsic and Extrinsic
Induction of apoptosis is important for successfully eliminating cancer cells from the body. There are two basic apoptotic pathways; the intrinsic pathway mediated mainly by the Bcl-2 proteins and the extrinsic pathway mediated by activation of death receptors and caspase 8. Like curcumin, many curcumin analogues also induce apoptosis through both intrinsic and extrinsic pathways (Table 2). For example, FLLL12 induced apoptosis of lung cancer cells and SCCHN by activating the DR5 pathway and modulating multiple Bcl-2 proteins, respectively (Haque et al., 2015, Anisuzzaman et al., 2016). CDF induced apoptosis of colon cancer stem-like cells by inhibiting NF-κB and Bcl-xL and activating proapoptotic Bax (Kanwar et al., 2011), CLEFMA induces apoptosis of osteosarcoma cells by activating the extrinsic and intrinsic apoptotic processes through JNK1/2 and p38 pathways (Yang et al., 2019a). DiMC induced apoptosis of breast cancer (MCF-7) cells by significantly increasing the Bax/Bcl-2 ratio in favour of apoptosis (Kunwar et al., 2012) and in human renal carcinoma by cytochrome c release and caspase 3 activation (Lee et al., 2010). DiMC also activated intrinsic apoptosis pathways in human hepatocellular carcinomas (Zanetti et al., 2019). EF24 induced apoptosis of oral squamous cell carcinoma cells by increasing levels of activated caspase 3 and 9 (Lin et al., 2017).
4.4. Trophic Signals- Growth factors and Cytokines
Receptor tyrosine kinases (RTKs) activate and regulate many major biological pathways involved in cell proliferation, differentiation, metabolism, and survival. Many cancers have mutations in genes that encode RTKs, leading to the overexpression of growth factor receptors (Hsu and Hung, 2016). Like curcumin, tropic factors including growth factors and cytokines are targets of many synthetic analogues. CDF in colon cancer cells attenuated EGFR and Insulin-like growth factor 1 (IGF-1) receptor signalling, while in prostate cancer cells it inhibited the production of VEGF and IL-6 (Bao et al., 2012a, Kanwar et al., 2011). Similarly, DAP (H-4073) blocked VEGF production in HNSCC to prevent angiogenesis (Kumar et al., 2014). More so, by inhibiting NF-ҡB, EF31 and UBS109 decreased transcription and expression of VEGF in colorectal cancer cells (Rajitha et al., 2017).
4.5. Oxidative Stress
Oxidative stress occurs when there is a prolonged disparity in the production and clearance of oxidative metabolites especially reactive oxygen species (ROS), which leads to damage of cell structure and function (Reuter et al., 2010). Cancer cells generate high concentrations of ROS as a result of high proliferation and metabolism often exceeding ROS thresholds that would be toxic (Sosa et al., 2013). Curcumin analogues like B19 further induce ROS generation that aids apoptosis in ovarian, lung and gastric carcinoma cells (Chen et al., 2016, Qu et al., 2013, Zhang et al., 2012). DiMC also exhibited pro-oxidant apoptotic effects through ROS generation in renal, breast and lung cancer cells (Jayakumar et al., 2016, Kunwar et al., 2012, Lee et al., 2010). EF24-induced apoptosis is mediated by ROS-dependent oxidative stress (Bisht et al., 2019). Furthermore, the antiproliferative effect of CLEFMA is associated with cancer cell specific generation of ROS, depletion of intracellular GSH/GSSG ratio and failure of Nrf2-mediated oxidative stress response (Sahoo et al., 2012). DAF-(P)-NOH also selectively induced ROS in cancer cells (Rath et al., 2013).Taken together, the effects of curcumin analogues on ROS is skewed in favour of anticancer activity.
4.6. Tumour Microenvironment
Inflammation is another known hallmark of cancerous cells that aids the growth and survival of tumours, promotes angiogenesis, metastasis, and ablates immune response in the tumour microenvironment (TME) (Mantovani et al., 2008, Diakos et al., 2014). Cyclooxygenase-2 (COX-2) is an enzyme frequently expressed in the TME which promotes inflammation, angiogenesis, and metastasis (Hashemi Goradel et al., 2019). Notably, synthetic analogues of curcumin have demonstrated anti-inflammatory effects via inactivation of COX-2. For instance, CDF caused COX-2 inactivation in chemo-resistant colon cancer cells (Kanwar et al., 2011). UBS109 impaired angiogenesis by repressing HIF-1α, Hsp90, COX-2 and VEGF in pancreatic cancer model (Nagaraju et al., 2015) and by inhibiting NF-kB activity, HIF-1α, COX-2, STAT-3, and VEGF in colorectal cancer model (Rajitha et al., 2017). CLEFMA reduced the expression of COX-2, and CD31 (angiogenesis marker) and the circulatory level of IL-6 and TNF-α in mice (Yadav et al., 2013).
4.7. Cancer Stem Cells and miRNA Expression
Cancer stem cells (CSCs) form a group of continuously replicating cells with little possibility for senescence. The ability to circumvent senescence may be responsible for tumour initiation and failure of current chemotherapy. New studies have identified CSCs as a potential target for refractory cancers. Consequently, curcumin and its analogues have been demonstrated to target CSCs as well. Some of which include CDF, which deregulated CSCs and suppressed their markers (Nanog, Oct4 and EZH2) via its effects on the HIF-α pathway (Bao et al., 2012a, Park et al., 2013). Also, Lin and colleagues identified STAT 3 inhibition by FLLL32 as an effective target for CSCs in pancreatic and colon cancer (Lin et al., 2011b, Lin et al., 2016). Additionally, curcumin and its analogues have epigenetic effects on the expression of tumour suppressor and/or oncogene miRNAs. CDF induces re-expression of miR-146a in pancreatic cancer (Ali et al., 2014) and EF24 inhibits miR21 expression in human prostate cancer (Yang et al., 2013).
4.8. Proteasome Inhibition
Tumour cells by virtue of their abnormal and uncontrolled growth are known to stockpile large amounts of defective and overexpressed proteins. This could be toxic to cells, which makes tumours dependent on proteasome activity as an essential pathway for degradation of cytosolic and nuclear proteins (Grigoreva et al., 2015, Livneh et al., 2016). This implies that inhibition of proteasome 26S activity could be another promising anti-tumour mechanism of curcumin for proteasome dependent cancers. Like curcumin, some analogues possess inhibitory effects on proteasome activity. For instance, CDF has shown significant inhibition of 26S proteasome activity in HCT116 colon cancer cells, which was comparable to that of curcumin (Padhye et al., 2009b). Likewise, AC17 inhibits 26S proteasome by inhibiting deubiquitinase activity of the 19S Regulatory Particle (RP) (Zhou et al., 2013b).
5. Conclusion and future directions
Anticancer agents are considered clinically useful when they induce apoptosis at doses that are not toxic to normal cells. Without doubt, it is evident from different studies that the synthetic analogues of curcumin have shown great potential as anticancer agents when compared with curcumin. This may be attributed to the enhanced specificity of some analogues in certain cancer types or some molecular targets over the lesser specificity of curcumin. More importantly, some analogues like EF24, FLLL12 and CDF have physico-chemical properties, including better solubility and bioavailability, that overcome the limitations of curcumin (Padhye et al., 2009a, Reid et al., 2014). Unfortunately, none of these potential analogues has yet moved into clinical trials. This is likely due to the lack of systematic efforts focusing on a potential candidate to bring it to the clinic. One reason might be lack of effective, long-term collaboration between medicinal chemists and cancer pharmacologists. Medicinal chemists, cancer pharmacologists and clinicians should work together with the long-term goals of developing these analogues for clinical use.
Analogues like CDF, EF24, EF31, FLLL12, GO-Y030 and UBS109 have shown the greatest promise. CDF, DiMC and PAC have shown enhanced metabolic stability (Al-Howail et al., 2016, Padhye et al., 2009a, Teymouri et al., 2018). However, pharmacokinetic properties, key parameters for clinical development, of many promising analogues are unknown. Focus should be given to PK studies since bioavailability of curcumin is the major drawback. Some analogues have also shown promise for combination therapies to increase efficacy or to overcome resistance to approved chemotherapy drugs (Bertazza et al., 2018, Bisht et al., 2019, Faiao-Flores et al., 2015, Faiao-Flores et al., 2012, Reid et al., 2014, Wu et al., 2016). Focus should also be given in this area to make potential analogues clinically applicable.
Although many analogues have similar molecular mechanisms as curcumin, some analogues have unique mechanisms that have not been associated with curcumin. For instance, B19 and not curcumin, directly inhibits TrxR1 enzyme, which leads to ROS mediated ER stress (Chen et al., 2016), AC17 blocks proteasome by inhibiting the deubiquitinase activity of 19S RP (Zhou et al., 2013a). More studies are required to evaluate specific benefits of one pathway of inhibition over the other in proteasome dependent cancers. Targeted drug delivery through formulation is gaining increasing attention to reduce drug-associated toxicities, improve bioavailability and increase efficacy. Studies have conjugated homing moieties to curcumin analogues to direct their delivery and accumulation at specific sites. These conjugates were either attached directly to the analogues or to their carriers (dendrimers or nanoparticles). Some of the homing moieties tested include hyaluronic acid (HA) targeted nanomicelles, which selectively killed triple negative breast cancer cells. Also, HA dendrimers increased the IC50 value of CDF 1.71 fold when compared with non-targeted formulations (Kesharwani et al., 2015b, Wang et al., 2018). EF24 has been conjugated to fVIIa targeted tissue factor, which is aberrantly expressed on tumour vascular endothelial cells. This conjugate significantly reduced tumour size of human breast cancer xenografts in athymic mice when compared with unconjugated EF24 (Shoji et al., 2008). Folic acid conjugated EF24 offered greater anticancer activity and accumulation in folate expressing tumour cells (Alsaab et al., 2017, Luong et al., 2016). CDF, CLEFMA, EF24 and FLLL32 have been encapsulated in liposomes for enhanced delivery at the tumour sites (Agashe et al., 2011a, Agashe et al., 2011b, Basak et al., 2015, Bisht et al., 2016, Wu et al., 2016). Table 4 summarizes these studies. Future studies should be designed to explore other cost-effective delivery technologies for curcumin analogues.
Table 4:
Novel delivery approaches using synthetic curcumins
| Analogue | Delivery Approach | Resultant Effect | Reference |
|---|---|---|---|
| CDF | CD44 targeted Poly(amidoamine) (PAMAM) dendrimer nanocarrier and hyaluronic acid (HA) as a targeting ligand (HA-PAMAM-CDF) | Dose-dependent cytotoxicity and higher cellular uptake | (Kesharwani et al., 2015b) |
| Non-covalent association of CDF with styrene-maleic acid copolymer (SMA-CDF) nano-micelles | Excellent aqueous solubility, stability, favourable hemocompatibility and sustained drug release characteristics. | (Kesharwani et al., 2015a) | |
| Folate receptor-targeted polymeric micelles | Enhanced aqueous solubility. Highly potent cytotoxicity | (Alsaab et al., 2017) | |
| CDF encapsulated in hyaluronic Acid styrene maleic anhydride (HA-SMA-TPGS) system for targeting TNBC | Higher cell killing compared with non-targeted nanomicelles and free CDF. HA favoured uptake by CD44+ cells by receptor mediated endocytosis | (Wang et al., 2018) | |
| CLEFMA | CLEFMA in hydroxypropyl-β-cyclodextrin liposomes | Retained antiproliferative potency without additional toxicity to normal lung fibroblasts | (Agashe et al., 2011b) |
| EF24 | EF24 chemically conjugated to fVIIa for targeted delivery | Conjugate inhibited VEGF-induced angiogenesis in in vivo rabbit model. Also induced apoptosis and tumour size reduction in human breast cancer xenografts | (Shoji et al., 2008) |
| EF24 in hydroxypropyl-β-cyclodextrin (HPβCD) liposomes | Enhanced aqueous solubility and superior antiproliferative activity | (Agashe et al., 2011a) | |
| FLLL32 | Liposome encapsulated FLLL32 delivery via enhanced permeability and retention | Lip-FLLL32 effectively accumulated at tumour xenografts, dose-dependent inhibition of tumour cell growth | (Wu et al., 2016) |
Footnotes
Conflict of Interest: The authors declare no potential conflict of Interest.
Financial support: Marshall University School of Pharmacy, WV-INBRE (P20GM103434)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- ABUZEID WM, DAVIS S, TANG AL, SAUNDERS L, BRENNER JC, LIN J, FUCHS JR, LIGHT E, BRADFORD CR, PRINCE ME & CAREY TE 2011. Sensitization of head and neck cancer to cisplatin through the use of a novel curcumin analog. Arch Otolaryngol Head Neck Surg, 137, 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADAMS BK, CAI J, ARMSTRONG J, HEROLD M, LU YJ, SUN A, SNYDER JP, LIOTTA DC, JONES DP & SHOJI M 2005. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs, 16, 263–75. [DOI] [PubMed] [Google Scholar]
- ADAMS BK, FERSTL EM, DAVIS MC, HEROLD M, KURTKAYA S, CAMALIER RF, HOLLINGSHEAD MG, KAUR G, SAUSVILLE EA, RICKLES FR, SNYDER JP, LIOTTA DC & SHOJI M 2004. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem, 12, 3871–83. [DOI] [PubMed] [Google Scholar]
- ADEYENI TA, KHATWANI N, SAN K & EZEKIEL UR 2016. BMI1 is downregulated by the natural compound curcumin, but not by bisdemethoxycurcumin and dimethoxycurcumin. Physiol Rep, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGASHE H, LAGISETTY P, SAHOO K, BOURNE D, GRADY B & AWASTHI V 2011a. Liposome-encapsulated EF24-HPβCD inclusion complex: a preformulation study and biodistribution in a rat model. J Nanopart Res, 13, 2609–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGASHE H, SAHOO K, LAGISETTY P & AWASTHI V 2011b. Cyclodextrin-mediated entrapment of curcuminoid 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] or CLEFMA in liposomes for treatment of xenograft lung tumor in rats. Colloids Surf B Biointerfaces, 84, 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGRAWAL DK & MISHRA PK 2010. Curcumin and its analogues: potential anticancer agents. Med Res Rev, 30, 818–60. [DOI] [PubMed] [Google Scholar]
- AL-HOWAIL HA, HAKAMI HA, AL-OTAIBI B, AL-MAZROU A, DAGHESTANI MH, AL-JAMMAZ I, AL-KHALAF HH & ABOUSSEKHRA A 2016. PAC down-regulates estrogen receptor alpha and suppresses epithelial-to-mesenchymal transition in breast cancer cells. BMC Cancer, 16, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-HUJAILY EM, MOHAMED AG, AL-SHARIF I, YOUSSEF KM, MANOGARAN PS, AL-OTAIBI B, AL-HAZA’A A, AL-JAMMAZ I, AL-HUSSEIN K & ABOUSSEKHRA A 2011. PAC, a novel curcumin analogue, has anti-breast cancer properties with higher efficiency on ER-negative cells. Breast Cancer Res Treat, 128, 97–107. [DOI] [PubMed] [Google Scholar]
- ALI S, AHMAD A, ABOUKAMEEL A, AHMED A, BAO B, BANERJEE S, PHILIP PA & SARKAR FH 2014. Deregulation of miR-146a expression in a mouse model of pancreatic cancer affecting EGFR signaling. Cancer Lett, 351, 134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALSAAB H, ALZHRANI RM, KESHARWANI P, SAU S, BODDU SH & IYER AK 2017. Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma. Pharmaceutics, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMIN AR, KUCUK O, KHURI FR & SHIN DM 2009. Perspectives for cancer prevention with natural compounds. J Clin Oncol, 27, 2712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANISUZZAMAN AS, HAQUE A, RAHMAN MA, WANG D, FUCHS JR, HURWITZ S, LIU Y, SICA G, KHURI FR, CHEN ZG, SHIN DM & AMIN AR 2016. Preclinical In Vitro, In Vivo, and Pharmacokinetic Evaluations of FLLL12 for the Prevention and Treatment of Head and Neck Cancers. Cancer Prev Res (Phila), 9, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANERJEE S, JI C, MAYFIELD JE, GOEL A, XIAO J, DIXON JE & GUO X 2018. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc Natl Acad Sci U S A, 115, 8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAO B, AHMAD A, KONG D, ALI S, AZMI AS, LI Y, BANERJEE S, PADHYE S & SARKAR FH 2012a. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One, 7, e43726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAO B, ALI S, BANERJEE S, WANG Z, LOGNA F, AZMI AS, KONG D, AHMAD A, LI Y, PADHYE S & SARKAR FH 2012b. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res, 72, 335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASAK SK, ZINABADI A, WU AW, VENKATESAN N, DUARTE VM, KANG JJ, DALGARD CL, SRIVASTAVA M, SARKAR FH, WANG MB & SRIVATSAN ES 2015. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget, 6, 18504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTAZZA L, SENSI F, CAVEDON E, WATUTANTRIGE-FERNANDO S, CENSI S, MANSO J, VIANELLO F, CASAL IDE E, IACOBONE M, PEZZANI R, MIAN C & BAROLLO S 2018. EF24 (a Curcumin Analog) and ZSTK474 Emphasize the Effect of Cabozantinib in Medullary Thyroid Cancer. Endocrinology, 159, 2348–2360. [DOI] [PubMed] [Google Scholar]
- BILL MA, NICHOLAS C, MACE TA, ETTER JP, LI C, SCHWARTZ EB, FUCHS JR, YOUNG GS, LIN L, LIN J, HE L, PHELPS M, LI PK & LESINSKI GB 2012. Structurally modified curcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS One, 7, e40724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHT S, NOLTING J, WENZEL J, BROSSART P & FELDMANN G 2019. EF24 Suppresses Cholangiocellular Carcinoma Progression, Inhibits STAT3 Phosphorylation, and Induces Apoptosis via ROS-Mediated Oxidative Stress. J Oncol, 2019, 8701824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHT S, SCHLESINGER M, RUPP A, SCHUBERT R, NOLTING J, WENZEL J, HOLDENRIEDER S, BROSSART P, BENDAS G & FELDMANN G 2016. A liposomal formulation of the synthetic curcumin analog EF24 (Lipo-EF24) inhibits pancreatic cancer progression: towards future combination therapies. Journal of nanobiotechnology, 14, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A, SHI Q, MOORE TW, YOON Y, PRUSSIA A, MADDOX C, LIOTTA DC, SHIM H & SNYDER JP 2013. Monocarbonyl curcumin analogues: heterocyclic pleiotropic kinase inhibitors that mediate anticancer properties. J Med Chem, 56, 3456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEN L, HUTZEN B, BALL S, DEANGELIS S, CHEN CL, FUCHS JR, LI C, LI PK & LIN J 2009. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer, 9, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN W, ZOU P, ZHAO Z, WENG Q, CHEN X, YING S, YE Q, WANG Z, JI J & LIANG G 2016. Selective killing of gastric cancer cells by a small molecule via targeting TrxR1 and ROS-mediated ER stress activation. Oncotarget, 7, 16593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG AL, HSU CH, LIN JK, HSU MM, HO YF, SHEN TS, KO JY, LIN JT, LIN BR, MING-SHIANG W, YU HS, JEE SH, CHEN GS, CHEN TM, CHEN CA, LAI MK, PU YS, PAN MH, WANG YJ, TSAI CC & HSIEH CY 2001. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res, 21, 2895–900. [PubMed] [Google Scholar]
- CHIBA T, MACK L, DELIS N, BRILL B & GRONER B 2012. Stat3 inhibition in neural lineage cells. Horm Mol Biol Clin Investig, 10, 255–63. [DOI] [PubMed] [Google Scholar]
- DANDAWATE PR, VYAS A, AHMAD A, BANERJEE S, DESHPANDE J, SWAMY KV, JAMADAR A, DUMHE-KLAIRE AC, PADHYE S & SARKAR FH 2012. Inclusion complex of novel curcumin analogue CDF and beta-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res, 29, 1775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAYTON A, SELVENDIRAN K, KUPPUSAMY ML, RIVERA BK, MEDURU S, KÁLAI T, HIDEG K & KUPPUSAMY P 2010. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol Ther, 10, 1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAYTON A, SELVENDIRAN K, MEDURU S, KHAN M, KUPPUSAMY ML, NAIDU S, KÁLAI T, HIDEG K & KUPPUSAMY P 2011. Amelioration of doxorubicin-induced cardiotoxicity by an anticancer-antioxidant dual-function compound, HO-3867. J Pharmacol Exp Ther, 339, 350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE SOUZA N, DE OLIVEIRA É A, FAIÃO-FLORES F, PIMENTA LA, QUINCOCES JAP, SAMPAIO SC & MARIA-ENGLER SS 2020. Metalloproteinases Suppression Driven by the Curcumin Analog DM-1 Modulates Invasion in BRAF-Resistant Melanomas. Anticancer Agents Med Chem. [DOI] [PubMed] [Google Scholar]
- DIAKOS CI, CHARLES KA, MCMILLAN DC & CLARKE SJ 2014. Cancer-related inflammation and treatment effectiveness. Lancet Oncol, 15, e493–503. [DOI] [PubMed] [Google Scholar]
- FAIAO-FLORES F, QUINCOCES SUAREZ JA, FRUET AC, MARIA-ENGLER SS, PARDI PC & MARIA DA 2015. Curcumin analog DM-1 in monotherapy or combinatory treatment with dacarbazine as a strategy to inhibit in vivo melanoma progression. PLoS One, 10, e0118702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAIAO-FLORES F, SUAREZ JA, PARDI PC & MARIA DA 2012. DM-1, sodium 4-[5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2-methoxy-phenolate: a curcumin analog with a synergic effect in combination with paclitaxel in breast cancer treatment. Tumour Biol, 33, 775–85. [DOI] [PubMed] [Google Scholar]
- FOSSEY SL, BEAR MD, LIN J, LI C, SCHWARTZ EB, LI PK, FUCHS JR, FENGER J, KISSEBERTH WC & LONDON CA 2011. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer, 11, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN L, LIN L, BALL S, BEKAII-SAAB T, FUCHS J, LI PK, LI C & LIN J 2009. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anticancer Drugs, 20, 444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUCHS JR, PANDIT B, BHASIN D, ETTER JP, REGAN N, ABDELHAMID D, LI C, LIN J & LI PK 2009. Structure-activity relationship studies of curcumin analogues. Bioorg Med Chem Lett, 19, 2065–9. [DOI] [PubMed] [Google Scholar]
- GAWDE KA, SAU S, TATIPARTI K, KASHAW SK, MEHRMOHAMMADI M, AZMI AS & IYER AK 2018. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloids Surf B Biointerfaces, 167, 8–19. [DOI] [PubMed] [Google Scholar]
- GLADE MJ 1999. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition, 15, 523–6. [DOI] [PubMed] [Google Scholar]
- GRIGOREVA TA, TRIBULOVICH VG, GARABADZHIU AV, MELINO G & BARLEV NA 2015. The 26S proteasome is a multifaceted target for anti-cancer therapies. Oncotarget, 6, 24733–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUPTA SC, KIM JH, PRASAD S & AGGARWAL BB 2010. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev, 29, 405–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADDAD RI & SHIN DM 2008. Recent advances in head and neck cancer. New England Journal of Medicine, 359, 1143–1154. [DOI] [PubMed] [Google Scholar]
- HAQUE A, RAHMAN MA, FUCHS JR, CHEN ZG, KHURI FR, SHIN DM & AMIN AR 2015. FLLL12 induces apoptosis in lung cancer cells through a p53/p73-independent but death receptor 5-dependent pathway. Cancer Lett, 363, 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHEMI GORADEL N, NAJAFI M, SALEHI E, FARHOOD B & MORTEZAEE K 2019. Cyclooxygenase-2 in cancer: A review. J Cell Physiol, 234, 5683–5699. [DOI] [PubMed] [Google Scholar]
- HASSAN HE, CARLSON S, ABDALLAH I, BUTTOLPH T, GLASS KC & FANDY TE 2015. Curcumin and dimethoxycurcumin induced epigenetic changes in leukemia cells. Pharm Res, 32, 863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE Y, LI W, HU G, SUN H & KONG Q 2018. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front Oncol, 8, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMER R, WÄTZIG GH, TIWARI S, ROSE-JOHN S & KALTHOFF H 2015. Interleukin-6 trans-signaling increases the expression of carcinoembryonic antigen-related cell adhesion molecules 5 and 6 in colorectal cancer cells. BMC Cancer, 15, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU JL & HUNG MC 2016. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev, 35, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURTADO M, SANKPAL UT, RANJAN A, MARAM R, VISHWANATHA JK, NAGARAJU GP, EL-RAYES BF & BASHA R 2018. Investigational agents to enhance the efficacy of chemotherapy or radiation in pancreatic cancer. Crit Rev Oncol Hematol, 126, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAYAKUMAR S, PATWARDHAN RS, PAL D, SHARMA D & SANDUR SK 2016. Dimethoxycurcumin, a metabolically stable analogue of curcumin enhances the radiosensitivity of cancer cells: Possible involvement of ROS and thioredoxin reductase. Biochem Biophys Res Commun, 478, 446–454. [DOI] [PubMed] [Google Scholar]
- JORDAN BC, KUMAR B, THILAGAVATHI R, YADHAV A, KUMAR P & SELVAM C 2018. Synthesis, evaluation of cytotoxic properties of promising curcumin analogues and investigation of possible molecular mechanisms. Chem Biol Drug Des, 91, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALAI T, KUPPUSAMY ML, BALOG M, SELVENDIRAN K, RIVERA BK, KUPPUSAMY P & HIDEG K 2011. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J Med Chem, 54, 5414–21. [DOI] [PubMed] [Google Scholar]
- KANWAR SS, YU Y, NAUTIYAL J, PATEL BB, PADHYE S, SARKAR FH & MAJUMDAR AP 2011. Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res, 28, 827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASINSKI AL, DU Y, THOMAS SL, ZHAO J, SUN SY, KHURI FR, WANG CY, SHOJI M, SUN A, SNYDER JP, LIOTTA D & FU H 2008. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol, 74, 654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESHARWANI P, BANERJEE S, PADHYE S, SARKAR FH & IYER AK 2015a. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf B Biointerfaces, 132, 138–45. [DOI] [PubMed] [Google Scholar]
- KESHARWANI P, XIE L, BANERJEE S, MAO G, PADHYE S, SARKAR FH & IYER AK 2015b. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf B Biointerfaces, 136, 413–23. [DOI] [PubMed] [Google Scholar]
- KUDO C, YAMAKOSHI H, SATO A, NANJO H, OHORI H, ISHIOKA C, IWABUCHI Y & SHIBATA H 2011a. Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC Pharmacol, 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUDO C, YAMAKOSHI H, SATO A, OHORI H, ISHIOKA C, IWABUCHI Y & SHIBATA H 2011b. Novel curcumin analogs, GO-Y030 and GO-Y078, are multi-targeted agents with enhanced abilities for multiple myeloma. Anticancer Res, 31, 3719–26. [PubMed] [Google Scholar]
- KUMAR B, YADAV A, HIDEG K, KUPPUSAMY P, TEKNOS TN & KUMAR P 2014. A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS One, 9, e93208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNWAR A, JAYAKUMAR S, SRIVASTAVA AK & PRIYADARSINI KI 2012. Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch Toxicol, 86, 603–14. [DOI] [PubMed] [Google Scholar]
- KUTTAN R, SUDHEERAN PC & JOSPH CD 1987. Turmeric and curcumin as topical agents in cancer therapy. Tumori, 73, 29–31. [DOI] [PubMed] [Google Scholar]
- LAGISETTY P, VILEKAR P, SAHOO K, ANANT S & AWASTHI V 2010. CLEFMA-an anti-proliferative curcuminoid from structure-activity relationship studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg Med Chem, 18, 6109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE JW, HONG HM, KWON DD, PAE HO & JEONG HJ 2010. Dimethoxycurcumin, a structural analogue of curcumin, induces apoptosis in human renal carcinoma caki cells through the production of reactive oxygen species, the release of cytochrome C, and the activation of caspase-3. Korean J Urol, 51, 870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG G, SHAO L, WANG Y, ZHAO C, CHU Y, XIAO J, ZHAO Y, LI X & YANG S 2009. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem, 17, 2623–31. [DOI] [PubMed] [Google Scholar]
- LIANG Y, YIN D, HOU L, ZHENG T, WANG J, MENG X, LU Z, SONG X, PAN S & JIANG H 2011a. Diphenyl difluoroketone: a potent chemotherapy candidate for human hepatocellular carcinoma. PloS one, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG Y, YIN D, HOU L, ZHENG T, WANG J, MENG X, LU Z, SONG X, PAN S, JIANG H & LIU L 2011b. Diphenyl difluoroketone: a potent chemotherapy candidate for human hepatocellular carcinoma. PLoS One, 6, e23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN C, TU C, MA Y, YE P, SHAO X, YANG Z & FANG Y 2017. Curcumin analog EF24 induces apoptosis and downregulates the mitogen activated protein kinase/extracellular signal-regulated signaling pathway in oral squamous cell carcinoma. Mol Med Rep, 16, 4927–4933. [DOI] [PubMed] [Google Scholar]
- LIN L, FUCHS J, LI C, OLSON V, BEKAII-SAAB T & LIN J 2011a. STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH(+)/CD133(+) stem cell-like human colon cancer cells. Biochem Biophys Res Commun, 416, 246–51. [DOI] [PubMed] [Google Scholar]
- LI L., FUCH J, LI C, OLSON V, BEKAII-SAAB T & LIN J 2011b. STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH+/CD133+ stem cell-like human colon cancer cells. Biochem Biophys Res Commun, 416, 246–51. [DOI] [PubMed] [Google Scholar]
- LIN L, HUTZEN B, BALL S, FOUST E, SOBO M, DEANGELIS S, PANDIT B, FRIEDMAN L, LI C, LI PK, FUCHS J & LIN J 2009. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci, 100, 1719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L, HUTZEN B, ZUO M, BALL S, DEANGELIS S, FOUST E, PANDIT B, IHNAT MA, SHENOY SS, KULP S, LI P-K, LI C, FUCHS J & LIN J 2010a. Novel STAT3 Phosphorylation Inhibitors Exhibit Potent Growth-Suppressive Activity in Pancreatic and Breast Cancer Cells. Cancer Research, 70, 2445–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L, HUTZEN B, ZUO M, BALL S, DEANGELIS S, FOUST E, PANDIT B, IHNAT MA, SHENOY SS, KULP S, LI PK, LI C, FUCHS J & LIN J 2010b. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res, 70, 2445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L, JOU D, WANG Y, MA H, LIU T, FUCHS J, LI PK, LÜ J, LI C & LIN J 2016. STAT3 as a potential therapeutic target in ALDH+ and CD44+/CD24+ stem cell-like pancreatic cancer cells. Int J Oncol, 49, 2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L, LIU Y, LI H, LI PK, FUCHS J, SHIBATA H, IWABUCHI Y & LIN J 2011c. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer, 105, 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU H, LIANG Y, WANG L, TIAN L, SONG R, HAN T, PAN S & LIU L 2012. In vivo and in vitro suppression of hepatocellular carcinoma by EF24, a curcumin analog. PLoS One, 7, e48075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVNEH I, COHEN-KAPLAN V, COHEN-ROSENZWEIG C, AVNI N & CIECHANOVER A 2016. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Research, 26, 869–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUONG D, KESHARWANI P, KILLINGER BA, MOSZCZYNSKA A, SARKAR FH, PADHYE S, RISHI AK & IYER AK 2016. Solubility enhancement and targeted delivery of a potent anticancer flavonoid analogue to cancer cells using ligand decorated dendrimer nano-architectures. J Colloid Interface Sci, 484, 33–43. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A, ALLAVENA P, SICA A & BALKWILL F 2008. Cancer-related inflammation. Nature, 454, 436–44. [DOI] [PubMed] [Google Scholar]
- MARTÍNEZ-CASTILLO M, VILLEGAS-SEPÚLVEDA N, MERAZ-RIOS MA, HERNÁNDEZ-ZAVALA A, BERUMEN J, COLEMAN MA, OROZCO L & CORDOVA EJ 2018. Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells. Oncol Lett, 15, 6777–6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOMTAZI AA & SAHEBKAR A 2016. Difluorinated Curcumin: A Promising Curcumin Analogue with Improved Anti-Tumor Activity and Pharmacokinetic Profile. Curr Pharm Des, 22, 4386–97. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M, OHNUMA S, FUKUDA M, CHUFAN EE, KUDOH K, KANEHARA K, SUGISAWA N, ISHIDA M, NAITOH T, SHIBATA H, IWABUCHI Y, AMBUDKAR SV & UNNO M 2017. Synthetic Analogs of Curcumin Modulate the Function of Multidrug Resistance-Linked ATP-Binding Cassette Transporter ABCG2. Drug Metab Dispos, 45, 1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGARAJU GP, ZHU S, KO JE, ASHRITHA N, KANDIMALLA R, SNYDER JP, SHOJI M & EL-RAYES BF 2015. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett, 357, 557–65. [DOI] [PubMed] [Google Scholar]
- NAGARAJU GP, ZHU S, WEN J, FARRIS AB, ADSAY VN, DIAZ R, SNYDER JP, MAMORU S & EL-RAYES BF 2013. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett, 341, 195–203. [DOI] [PubMed] [Google Scholar]
- OHORI H, YAMAKOSHI H, TOMIZAWA M, SHIBUYA M, KAKUDO Y, TAKAHASHI A, TAKAHASHI S, KATO S, SUZUKI T, ISHIOKA C, IWABUCHI Y & SHIBATA H 2006. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther, 5, 2563–71. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA EA, LIMA DS, CARDOZO LE, SOUZA GF, DE SOUZA N, ALVES-FERNANDES DK, FAIAO-FLORES F, QUINCOCES JAP, BARROS SBM, NAKAYA HI, MONTEIRO G & MARIA-ENGLER SS 2017. Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells. Pharmacol Res, 125, 178–187. [DOI] [PubMed] [Google Scholar]
- OLIVERA A, MOORE TW, HU F, BROWN AP, SUN A, LIOTTA DC, SNYDER JP, YOON Y, SHIM H, MARCUS AI, MILLER AH & PACE TW 2012. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int Immunopharmacol, 12, 368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGANIZATION, W. H. 2018. Cancer [Online]. WHO. Available: https://www.who.int/health-topics/cancer#tab=tab_1 [Accessed 5-5-2021 2021]. [Google Scholar]
- PADHYE S, BANERJEE S, CHAVAN D, PANDYE S, SWAMY KV, ALI S, LI J, DOU QP & SARKAR FH 2009a. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res, 26, 2438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADHYE S, YANG H, JAMADAR A, CUI QC, CHAVAN D, DOMINIAK K, MCKINNEY J, BANERJEE S, DOU QP & SARKAR FH 2009b. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm Res, 26, 1874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK W, AMIN AR, CHEN ZG & SHIN DM 2013. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila), 6, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRABHAT AM, KUPPUSAMY ML, BOGNÁR B, KÁLAI T, HIDEG K & KUPPUSAMY P 2019. Antiproliferative Effect of a Novel 4,4’-Disulfonyldiarylidenyl Piperidone in Human Colon Cancer Cells. Cell Biochem Biophys, 77, 61–67. [DOI] [PubMed] [Google Scholar]
- QIU X, DU Y, LOU B, ZUO Y, SHAO W, HUO Y, HUANG J, YU Y, ZHOU B, DU J, FU H & BU X 2010. Synthesis and identification of new 4-arylidene curcumin analogues as potential anticancer agents targeting nuclear factor-kappaB signaling pathway. J Med Chem, 53, 8260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QU W, XIAO J, ZHANG H, CHEN Q, WANG Z, SHI H, GONG L, CHEN J, LIU Y, CAO R & LV J 2013. B19, a novel monocarbonyl analogue of curcumin, induces human ovarian cancer cell apoptosis via activation of endoplasmic reticulum stress and the autophagy signaling pathway. Int J Biol Sci, 9, 766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUINCOCES SUAREZ JA, RANDO DG, SANTOS RP, GONCALVES CP, FERREIRA E, DE CARVALHO JE, KOHN L, MARIA DA, FAIAO-FLORES F, MICHALIK D, MARCUCCI MC & VOGEL C 2010. New antitumoral agents I: In vitro anticancer activity and in vivo acute toxicity of synthetic 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien-3-one and derivatives. Bioorg Med Chem, 18, 6275–81. [DOI] [PubMed] [Google Scholar]
- RAJITHA B, BELALCAZAR A, NAGARAJU GP, SHAIB WL, SNYDER JP, SHOJI M, PATTNAIK S, ALAM A & EL-RAYES BF 2016. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett, 373, 227–33. [DOI] [PubMed] [Google Scholar]
- RAJITHA B, NAGARAJU GP, SHAIB WL, ALESE OB, SNYDER JP, SHOJI M, PATTNAIK S, ALAM A & EL-RAYES BF 2017. Novel synthetic curcumin analogs as potent antiangiogenic agents in colorectal cancer. Mol Carcinog, 56, 288–299. [DOI] [PubMed] [Google Scholar]
- RATH KS, MCCANN GA, COHN DE, RIVERA BK, KUPPUSAMY P & SELVENDIRAN K 2013. Safe and targeted anticancer therapy for ovarian cancer using a novel class of curcumin analogs. J Ovarian Res, 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID JM, BUHROW SA, GILBERT JA, JIA L, SHOJI M, SNYDER JP & AMES MM 2014. Mouse pharmacokinetics and metabolism of the curcumin analog, 4-piperidinone,3,5-bis[(2-fluorophenyl)methylene]-acetate(3E,5E) (EF-24; NSC 716993). Cancer Chemother Pharmacol, 73, 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUTER S, GUPTA SC, CHATURVEDI MM & AGGARWAL BB 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med, 49, 1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUES FC, ANIL KUMAR NV & THAKUR G 2019. Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. Eur J Med Chem, 177, 76–104. [DOI] [PubMed] [Google Scholar]
- ROY S, YU Y, PADHYE SB, SARKAR FH & MAJUMDAR AP 2013. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS One, 8, e68543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHOO K, DOZMOROV MG, ANANT S & AWASTHI V 2012. The curcuminoid CLEFMA selectively induces cell death in H441 lung adenocarcinoma cells via oxidative stress. Invest New Drugs, 30, 558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARKAR FH, LI Y, WANG Z & PADHYE S 2010. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des, 16, 1801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO A, KUDO C, YAMAKOSHI H, UEHARA Y, OHORI H, ISHIOKA C, IWABUCHI Y & SHIBATA H 2011a. Curcumin analog GO-Y030 is a novel inhibitor of IKKbeta that suppresses NF-kappaB signaling and induces apoptosis. Cancer Sci, 102, 1045–51. [DOI] [PubMed] [Google Scholar]
- SATO A, KUDO C, YAMAKOSHI H, UEHARA Y, OHORI H, ISHIOKA C, IWABUCHI Y & SHIBATA H 2011b. Curcumin analog GO-Y030 is a novel inhibitor of IKKβ that suppresses NF-κB signaling and induces apoptosis. Cancer Sci, 102, 1045–51. [DOI] [PubMed] [Google Scholar]
- SELVENDIRAN K, AHMED S, DAYTON A, KUPPUSAMY ML, TAZI M, BRATASZ A, TONG L, RIVERA BK, KÁLAI T, HIDEG K & KUPPUSAMY P 2010a. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: differential cytotoxicity in healthy and cancer cells. Free Radic Biol Med, 48, 1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELVENDIRAN K, KUPPUSAMY ML, BRATASZ A, TONG L, RIVERA BK, RINK C, SEN CK, KALAI T, HIDEG K & KUPPUSAMY P 2009. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through up-regulation of PTEN expression. J Pharmacol Exp Ther, 329, 959–66. [DOI] [PubMed] [Google Scholar]
- SELVENDIRAN K, TONG L, BRATASZ A, KUPPUSAMY ML, AHMED S, RAVI Y, TRIGG NJ, RIVERA BK, KÁLAI T, HIDEG K & KUPPUSAMY P 2010b. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Mol Cancer Ther, 9, 1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELVENDIRAN K, TONG L, VISHWANATH S, BRATASZ A, TRIGG NJ, KUTALA VK, HIDEG K & KUPPUSAMY P 2007. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem, 282, 28609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA H, YAMAKOSHI H, SATO A, OHORI H, KAKUDO Y, KUDO C, TAKAHASHI Y, WATANABE M, TAKANO H, ISHIOKA C, NODA T & IWABUCHI Y 2009. Newly synthesized curcumin analog has improved potential to prevent colorectal carcinogenesis in vivo. Cancer Sci, 100, 956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOJI M, SUN A, KISIEL W, LU YJ, SHIM H, MCCAREY BE, NICHOLS C, PARKER ET, POHL J, MOSLEY CA, ALIZADEH AR, LIOTTA DC & SNYDER JP 2008. Targeting tissue factor-expressing tumor angiogenesis and tumors with EF24 conjugated to factor VIIa. J Drug Target, 16, 185–97. [DOI] [PubMed] [Google Scholar]
- SOSA V, MOLINÉ T, SOMOZA R, PACIUCCI R, KONDOH H & ME LL 2013. Oxidative stress and cancer: an overview. Ageing Res Rev, 12, 376–90. [DOI] [PubMed] [Google Scholar]
- SUN L, LIU J, LIN SS, SHI WT, ZHU J, LIANG G & YUAN ST 2014. Potent anti-angiogenic activity of B19--a mono-carbonyl analogue of curcumin. Chin J Nat Med, 12, 8–14. [DOI] [PubMed] [Google Scholar]
- TAMVAKOPOULOS C, DIMAS K, SOFIANOS ZD, HATZIANTONIOU S, HAN Z, LIU Z-L, WYCHE JH & PANTAZIS P 2007. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clinical Cancer Research, 13, 1269–1277. [DOI] [PubMed] [Google Scholar]
- TAN X, SIDELL N, MANCINI A, HUANG RP, SHENMING W, HOROWITZ IR, LIOTTA DC, TAYLOR RN & WIESER F 2010. Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells. Reprod Sci, 17, 931–40. [DOI] [PubMed] [Google Scholar]
- TEYMOURI M, BARATI N, PIRRO M & SAHEBKAR A 2018. Biological and pharmacological evaluation of dimethoxycurcumin: A metabolically stable curcumin analogue with a promising therapeutic potential. J Cell Physiol, 233, 124–140. [DOI] [PubMed] [Google Scholar]
- THOMAS SL, ZHAO J, LI Z, LOU B, DU Y, PURCELL J, SNYDER JP, KHURI FR, LIOTTA D & FU H 2010. Activation of the p38 pathway by a novel monoketone curcumin analog, EF24, suggests a potential combination strategy. Biochem Pharmacol, 80, 1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS SL, ZHONG D, ZHOU W, MALIK S, LIOTTA D, SNYDER JP, HAMEL E & GIANNAKAKOU P 2008. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle, 7, 2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEHARA Y, INOUE M, FUKUDA K, YAMAKOSHI H, HOSOI Y, KANDA H, OSHIMA M, IWABUCHI Y & SHIBATA H 2015. Inhibition of β-catenin and STAT3 with a curcumin analog suppresses gastric carcinogenesis in vivo. Gastric Cancer, 18, 774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VYAS A, DANDAWATE P, PADHYE S, AHMAD A & SARKAR F 2013. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr Pharm Des, 19, 2047–69. [PMC free article] [PubMed] [Google Scholar]
- WANG Y, XIAO J, ZHOU H, YANG S, WU X, JIANG C, ZHAO Y, LIANG D, LI X & LIANG G 2011. A novel monocarbonyl analogue of curcumin, (1E,4E)-1,5-bis(2,3-dimethoxyphenyl)penta-1,4-dien-3-one, induced cancer cell H460 apoptosis via activation of endoplasmic reticulum stress signaling pathway. J Med Chem, 54, 3768–78. [DOI] [PubMed] [Google Scholar]
- WANG Z, SAU S, ALSAAB HO & IYER AK 2018. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomedicine, 14, 1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU X, TANG W, MARQUEZ RT, LI K, HIGHFILL CA, HE F, LIAN J, LIN J, FUCHS JR, JI M, LI L & XU L 2016. Overcoming chemo/radio-resistance of pancreatic cancer by inhibiting STAT3 signaling. Oncotarget, 7, 11708–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YADAV VR, SAHOO K & AWASTHI V 2013. Preclinical evaluation of 4-[3,5-bis(2-chlorobenzylidene)-4-oxo-piperidine-1-yl]-4-oxo-2-butenoic acid, in a mouse model of lung cancer xenograft. Br J Pharmacol, 170, 1436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI M, MOORE TW, SUN A, SNYDER JP & SHOJI M 2012. Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-kappaB inhibition. Integr Biol (Camb), 4, 905–13. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI M, ZHU S, ZHANG S, WU D, MOORE TM, SNYDER JP & SHOJI M 2014. Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: involvement in osteoblastogenesis and osteoclastogenesis. Cell Tissue Res, 357, 245–52. [DOI] [PubMed] [Google Scholar]
- YAN J, WANG Q, ZOU K, WANG L, SCHWARTZ EB, FUCHS JR, ZHENG Z & WU J 2015. Inhibition of the JAK2/STAT3 signaling pathway exerts a therapeutic effect on osteosarcoma. Mol Med Rep, 12, 498–502. [DOI] [PubMed] [Google Scholar]
- YANG CH, YUE J, SIMS M & PFEFFER LM 2013. The curcumin analog EF24 targets NF-kappaB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS One, 8, e71130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG JS, LIN RC, HSIEH YH, WU HH, LI GC, LIN YC, YANG SF & LU KH 2019a. CLEFMA Activates the Extrinsic and Intrinsic Apoptotic Processes through JNK1/2 and p38 Pathways in Human Osteosarcoma Cells. Molecules, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG X, TANG Z, ZHANG P & ZHANG L 2019b. [Research Advances of JAK/STAT Signaling Pathway in Lung Cancer]. Zhongguo Fei Ai Za Zhi, 22, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN DL, LIANG YJ, ZHENG TS, SONG RP, WANG JB, SUN BS, PAN SH, QU LD, LIU JR, JIANG HC & LIU LX 2016. EF24 inhibits tumor growth and metastasis via suppressing NF-kappaB dependent pathways in human cholangiocarcinoma. Sci Rep, 6, 32167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOON MJ, KANG YJ, LEE JA, KIM IY, KIM MA, LEE YS, PARK JH, LEE BY, KIM IA, KIM HS, KIM SA, YOON AR, YUN CO, KIM EY, LEE K & CHOI KS 2014. Stronger proteasomal inhibition and higher CHOP induction are responsible for more effective induction of paraptosis by dimethoxycurcumin than curcumin. Cell Death Dis, 5, e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN S, CAO S, JIANG R, LIU R, BAI J & HOU Q 2014. FLLL31, a derivative of curcumin, attenuates airway inflammation in a multi-allergen challenged mouse model. Int Immunopharmacol, 21, 128–36. [DOI] [PubMed] [Google Scholar]
- YUAN S, ZHANG S, ZHUANG Y, ZHANG H, BAI J & HOU Q 2015. Interleukin-17 Stimulates STAT3-Mediated Endothelial Cell Activation for Neutrophil Recruitment. Cell Physiol Biochem, 36, 2340–56. [DOI] [PubMed] [Google Scholar]
- ZANETTI TA, BIAZI BI, COATTI GC, BARANOSKI A, MARQUES LA, CORVELONI AC & MANTOVANI MS 2019. Mitotic spindle defects and DNA damage induced by dimethoxycurcumin lead to an intrinsic apoptosis pathway in HepG2/C3A cells. Toxicol In Vitro, 61, 104643. [DOI] [PubMed] [Google Scholar]
- ZHANG X, ZHANG HQ, ZHU GH, WANG YH, YU XC, ZHU XB, LIANG G, XIAO J & LI XK 2012. A novel mono-carbonyl analogue of curcumin induces apoptosis in ovarian carcinoma cells via endoplasmic reticulum stress and reactive oxygen species production. Mol Med Rep, 5, 739–44. [DOI] [PubMed] [Google Scholar]
- ZHOU B, ZUO Y, LI B, WANG H, LIU H, WANG X, QIU X, HU Y, WEN S, DU J & BU X 2013a. Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analog AC17 causes NF-kappaB inhibition and p53 reactivation in human lung cancer cells. Mol Cancer Ther, 12, 1381–92. [DOI] [PubMed] [Google Scholar]
- ZHOU B, ZUO Y, LI B, WANG H, LIU H, WANG X, QIU X, HU Y, WEN S, DU J & BU X 2013b. Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analog AC17 causes NF-κB inhibition and p53 reactivation in human lung cancer cells. Mol Cancer Ther, 12, 1381–92. [DOI] [PubMed] [Google Scholar]
- ZHU S, MOORE TW, LIN X, MORII N, MANCINI A, HOWARD RB, CULVER D, ARRENDALE RF, REDDY P, EVERS TJ, ZHANG H, SICA G, CHEN ZG, SUN A, FU H, KHURI FR, SHIN DM, SNYDER JP & SHOJI M 2012. Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr Biol (Camb), 4, 633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU S, MOORE TW, MORII N, HOWARD RB, CULVER D, ARRENDALE RF, REDDY P, EVERS TJ, ZHANG H, SICA G, CHEN ZG, SUN A, FU H, KHURI FR, SHIN DM, SNYDER JP & SHOJI M 2014. Synthetic curcumin analog UBS109 inhibits the growth of head and neck squamous cell carcinoma xenografts. Curr Cancer Drug Targets, 14, 380–93. [DOI] [PubMed] [Google Scholar]


