Abstract
The coronavirus 2019 (COVID-19) pandemic has resulted in 168 million cases and about 3.5 million deaths (as of May 26, 2021) during the last 18 months. These 18 months of the COVID-19 pandemic have been characterized by phases or waves of new cases, the emergence of new variants of the deadly virus, and several new complications. After providing emergency approval to several drugs and adherence to several public health measures with frequent full and partial lockdowns, the incidence of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could not be contained till now on a global basis. Although prophylactic vaccines have inspired optimism, the scarcity of vaccines and several vaccine-related regulations indicate that the vaccine's benefit would not be reaching the people of developing countries anytime soon. In the course of our clinical practice, we used pegylated interferon (Peg-IFN) in 35 patients with chronic liver diseases (CLD), and we found that only two of them were infected with SARS-CoV-2 that was mild in nature. These two patients with CLD have a mild course of disease cured without any specific therapy. Patients with CLD are usually immune-compromised. However, three CLD patients remained free of SARS-CoV-2 although they had COVID-19 patients among their family members. Next, we accomplished two studies for assessing the immune-modulatory capacities of Peg-IFN, 1 and 12 injections following administration of Peg-IFN. The data revealed that peripheral blood mononuclear cells (PBMCs) of Peg-IFN-administered CLD patients produced significantly higher levels of some cytokines of innate immunity in comparison with the cytokines produced by PBMC of CLD patients before Peg-IFN intake. The pattern of cytokine responses and absence of infection of SARS-CoV-2 in 33 of 35 CLD patients represent some preliminary observations indicating a possible role of Peg-IFN in patients with CLD. The study may be extended to other chronic infections and cancers in which patients receive Peg-IFN. The role of Peg-IFN for pre- or postexposure prophylaxis in the acquisition of SARS-CoV-2 infection and influencing the natural course of COVID-19 remains to be clarified.
How to cite this article
Akbar SMF, Mahtab MA, Aguilar JC, et al. Role of Pegylated Interferon in Patients with Chronic Liver Diseases in the Context of SARS-CoV-2 Infection. Euroasian J Hepato-Gastroenterol 2021;11(1):27–31.
Keywords: Chronic liver disease, Innate immunity, Peg-IFN, SARS-CoV-2
Introduction
The novel human severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that caused widespread deaths across the globe [coronavirus 2019 (COVID-19)] was reported in December 2019. It has caused about 168 million infections and 3.5 million deaths (as of May 25, 2021) in almost all regions, countries, and territories of the world.1 The virus is an enveloped β-coronavirus. The full genome sequence reveals a genetic sequence very similar to SARS-CoV-1 (80%) and bat coronavirus RaTG13 (96.2%).2 Three main proteins coat the viral envelope: Spike (S) glycoprotein, envelope (E), and membrane (M) proteins. Among these proteins, S protein seems to be essential for binding to host cells. The subunit of the S proteins (S1) is responsible for receptor binding to angiotensin-converting enzyme 2, and the other one (S2) is a potential antiviral target.3
The virus has been declared to induce a pandemic on March 11, 2020, by the World Health Organization (WHO). Although more than 1 year has passed after the initial outbreak of SARS-CoV-2, various factors related to the acquisition of infection and pathogenesis of SARS-CoV-2 are yet to be satisfactorily understood. However, the virus transmission is going on in full swing, and about one million (0.6–1.7 million) of new infections and thousands of new deaths (6,000–17,000 death) have been reported daily during the last 6 months. SARS-CoV-2 and the genesis of COVID-19 diseases become more complicated as most infectious people run an asymptomatic course path without any sign or symptoms.4 On the contrary, several patients develop mild, moderate, or severe COVID-19.5,6 However, the mechanisms underlying the acquisition of SARS-CoV-2, development of asymptomatic SARS-CoV-2 infection, the pathogenesis of mild to moderate COVID-19, and several forms of COVID-19 are mostly unclear.
The virus enters human hosts mainly via the nasal route, although other routes of viral entry remain to be elucidated. It is localized and establishes infection in the nasal mucosa and upper pulmonary tract that may continue for variable numbers of days. SARS-CoV-2 may be contained in the upper respiratory tract or may pass toward the lower pulmonary tree and may develop serious complications like pneumonia and several other pathological lesions.7 The virus has been detected in various body tissues, but it is still unclear if the virus bears replicative or pathogenic potentials in these tissues. It is not completely clear about the cytopathic effect of the virus, and little is known about the extent of cytopathogenicity and magnitude of tissue damages.8 However, high titers of the virus have been detected in severe cases of COVID-19. On the other side, aberrant immunity and cytokine storm have been regarded as the main pathological events relating to the severity of COVID-19.
Due to the prevalence of these different schools of thought about SARS-CoV-2 pathogenicity, evidence-based drugs are yet to be developed for the treatment of COVID-19, and the hallmarks of COVID-19 management are dependent on symptomatic aliments. However, any pandemic provides an opportunity for repurposing drugs. As SARS-CoV-2 is a ribonucleic acid (RNA) virus and replicates via reverse transcriptase, several drugs that had been developed for other pathological conditions previously have been repurposed. These drugs have received emergency authorization certificates to be used for COVID-19 patients in almost all countries of the world. Thus, from chloroquine to ivermectin, remdesivir and favipiravir have been extensively used to treat COVID-19 patients.9–13 We and others have also used immunomodulatory drugs for the treatment of severe COVID-19.14,15 However, recent studies have shown the limitations of these drugs, and several countries no longer recommend these drugs for the treatment of COVID-19.16–18
Several vaccines have been developed to contain the new infection by SARS-CoV-2. A vaccine is regarded as an antidote and a saver from the acquisition of a new virus. However, the vaccine developed against SARS-CoV-2 is not completely comparable with other vaccines that are generally used in developing countries, both regarding safety and efficacy as well the concept of prophylaxis by the vaccine.19,20 In reality, considerable time is taken to develop the protective capacities of vaccine recipients. As the pandemic is going on and several people are susceptible to be infected by this virus, there is a need to block new viral infection by some means other than vaccines.
Based on these realities about SARS-CoV-2 and COVID-19, we assumed that evidence-based prophylaxis and therapy development represent challenges to humanity. Our fundamental target focuses on repurposing evidence-based molecules for SARS-CoV-2 and COVID-19 for prophylaxis and therapeutic purposes. In line with this proposition, we have recently concentrated on a nasal usage of a therapeutic vaccine for hepatitis B [called NASVAC that induces innate immunity and shapes aberrant immunity in chronic hepatitis B (CHB) patients].21–23
In this article, we are proposing another immune modulator for the prophylaxis purpose of SARS-CoV-2. Type I interferons (IFN) represent a group of antiviral agents capable of inducing signals that may trigger the expression of several hundred antiviral proteins and chemokines with antiviral properties.24–26 The fundamental principle of this study was retrieved as there has been a group of patients with chronic diseases taking IFN for their primary illness. We checked their susceptibility to SARS-CoV-2 by critical observation and day-to-day surveillance. Finally, we checked the innate immune responses of pegylated interferon (Peg-IFN) in patients with chronic liver diseases (CLD).
Materials and Methods
Several hundred patients with CLD have been receiving Peg-IFN weekly. Out of them, 35 patients cooperated in this study and provided informed consent. In addition to their liver function status, they were critically checked for any evidence of SARS-CoV-2 infection. The study was conducted in Dhaka, Bangladesh. Dhaka is the capital of Bangladesh and is the main hotspot of COVID-19 in Bangladesh.
The second set of studies was done to assess if innate immune cytokines are produced by IFN treatment. A total of 10 patients with CLD were subjected to this study, and inform consent was obtained from them to participate in the study.
The third set of patients with CLD were selected, and peripheral blood mononuclear cells (PBMC) were isolated from them before starting Peg-IFN therapy and 12 weeks after the end of 12 injections of Peg-IFN therapy.
Treatment Strategy
All patients received Peg-IFN at a dose of 180 µg/week. The patients received other drugs for their management of CLD. The patients were periodically followed up for safety by assessing different parameters of liver function tests and abdominal imaging. Upper gastrointestinal endoscopy was also done in some patients to assess progression to complications.
Assessment of SARS-CoV-2
The presence of SARS-CoV-2 RNA was assessed using an automatic system and polymerase chain reaction (PCR) kits (Roche Diagnostics, Switzerland). However, the assay system did not allow the quantification of SARS-CoV-2.
Hematological Assessment
Clinical laboratory hematology and parameters of safety and liver parameters (C-reactive protein, transaminases, creatinine, glycemia, and blood pressure) were evaluated following hospital-validated procedures using standard assessment techniques.
Isolation of Circulating PBMC from Peripheral Blood
PBMCs were isolated and enriched by methods that have been previously described. PBMCs were isolated from freshly drawn heparinized whole blood by Ficoll–Hypaque (Sigma, St. Louis, Missouri) density gradient centrifugation (specific gravity: 1.077). The cells were retrieved from the interface and washed three times in phosphate-buffered saline. Finally, PBMCs were resuspended in RPMI 1640 (Nipro, Osaka, Japan) plus 10% autologous serum. The use of fetal calf serum (FCS) was avoided to prevent the immune stimulatory effect of FCS. The viability of PBMCs was checked with the trypan blue exclusion test, and cells those revealed more than 90% viability were used for immunological studies.27,28
Culture of PBMC
Cytokine production by PBMC was performed by adding 100 μL of cell suspension into quadruplicate wells of 96-well U-bottomed plates. PBMCs were either not activated or activated with 50 μL of concanavalin (Sigma-Aldrich, St. Lous, Missouri) at a final concentration of 5 μg/mL. The plates were incubated at 37°C and 5% CO2 in a humidified incubator for 72 hours. After incubation, plates were centrifuged at 1000g for 1 minute, and cell-free supernatants from each well were collected and stored at −20°C until cytokines were measured by enzyme-linked immunosorbent assay (ELISA).27,28
Assessment of Cytokine Production in Culture
We measured different cytokines to develop insights into the immunogenicity of Peg-IFN in CLD patients. Cytokines in culture supernatants were measured by ELISA method using commercial kits, according to the manufacturer's instruction (RD Bioscience System, Minneapolis, Minnesota).
Statistical Analysis
The data have been shown as median and range. For the statistical analysis, paired t-test was used for normally distributed data. When the distribution was skewed, the Wilcoxon signed-rank test was used.
Result
SARS-CoV-2 Infection of the Study Cohort
The age of the patients in this study cohort varied from 22 to 68 years. Ten of the patients were female, and the rest 25 were male. Out of the total of 35 patients who received Peg-IFN for CLD, follow-up was accomplished systematically for any possible SARS-CoV-2 infection. Within an observation period of 6 months, only 2 of 35 patients with CLD were infected with SARS-CoV-2. The extent of the SARS-CoV-2 was mild, and they did not need hospitalization or any specific treatment for the virus. They became negative for SARS-CoV-2 within 15 days, at which time two consecutive assessments revealed viral negativity. Interestingly, the family members of three patients in the study cohort were infected with SARS-CoV-2. However, the patients with CLD treated with Peg-IFN were not infected by SARS-CoV-2.
Peg-IFN-induced Increased Cytokine Production after a Single Injection in CLD Patients
Immunological assessment of cytokine production was possible in 10 patients with CLD. The patients were bled twice, once before the intake of Peg-IFN and the second one 7 days after Peg-IFN intake before getting second dose of Peg-IFN. SARS-CoV-2 did not infect these patients during the 6 months of follow-up. The mean levels of interleukin-2 (IL-2) and tumor necrosis factor (TNF-α) were significantly higher due to the intake of Peg-IFN compared to those before taking Peg-IFN (Fig. 1). On the contrary, the levels of IL-10 and IL-4 were not significantly changed in these patients between pre-Peg-IFN and post-IFN periods.
Fig. 1.
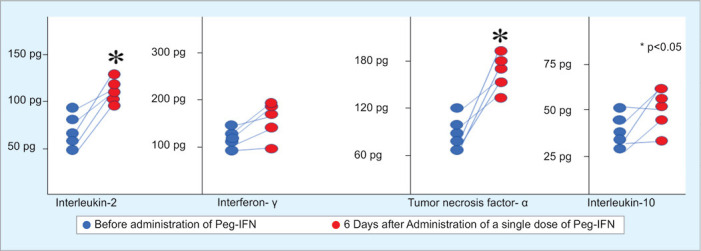
Increase in production of IL-2 and TNF-α in PBMC due to single administration of Peg-IFN
Inflammatory Cytokines Remained Elevated for 12 Weeks Receiving Weekly Peg-IFN
The levels of cytokines were measured in eight patients receiving 12 weekly administration of Peg-IFN. The cytokine levels of these patients were compared with the cytokine levels produced before the administration of Peg-IFN. Elevated levels of IL-2, IFN-γ, and TNF-α were detected due to the administration of Peg-IFN 12 times weekly (Fig. 2). However, no major change was found in IL-10 production by Peg-IFN. The levels of different cytokine before the start of IFN therapy were regarded as 100%, and the increased or decreased amounts were calculated from these basic levels as an increase in percentage.
Fig. 2.
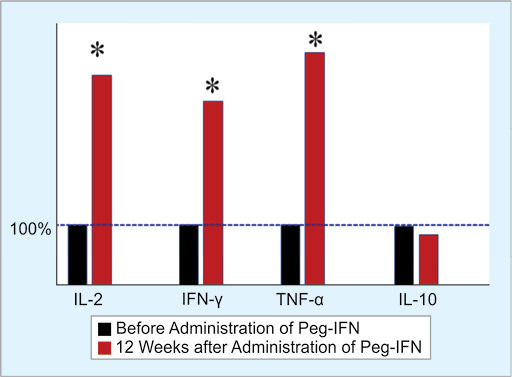
Increase in production of IL-2, IFN-γ, and TNF-α in PBMC due to 12 administrations of Peg-IFN
Discussion
Containment of new infection of SARS-CoV-2 and proper management of COVID-19 patients represent the major challenge of mankind. After its first appearance in December 2019 and declaration of a pandemic by WHO on March 11, 2020, a huge figure of 168 million people has been infected with SARS-CoV-2 with a death toll of 3.5 million. The real figure may be several times greater than this considering underreporting by various governments, house management of cases, and diagnosis limitation using a complicated assessment method like PCR. In addition, several thousand people of any country cannot reach the SARS-CoV-2 diagnosis center due to partial lockdown or complete lockdown and unavailability of transport. Also, the social discrimination of COVID-19 patients did not allow many of them to be diagnosed and get COVID-19 management opportunities. These realities are even more harsh and inhumane in developing and resource-constrained countries.29 Additionally, the political situations of many countries and the role of their leaders have ignited a fire to this pandemic.
Once COVID-19 progresses from moderate to severe or to critical forms, the management of these cases will be extremely hard. In addition, various new complications have surfaced recently that have made COVID-19 management a challenge.30 The repurposed antiviral drugs have shown limited or no efficacy in COVID-19 patients, and many of these drugs have lost the emergency marketing approach.17,18 Although the vaccine remains the strongest weapon to fight new COVID-19, the availability of vaccines is limited, and more than 2 to 3 years may be taken to bring the necessary population to vaccine coverage.
The incidence of new SARS-CoV-2 has been fluctuating over the last 15 months from country to country. However, the average infection rate is almost slipping near a point. Control of a pandemic is a matter of social, economic, and scientific development of a particular time. However, as we have learned from the flu pandemic of 1918 and other bigger epidemics of 20th century, it is never easy. Many of these public health measures (use of mask and handwashing) have not been properly followed in many countries, such as the USA and the EU, due to their inherent tendency to maintain a so-called Western lifestyle with individual choice of life. In Brazil and Mexico, the political system was unable to take the load of a pandemic. India entered an election spree, and this was added by the occurrence of several religious gatherings, making it an ongoing hot spot. Even in these scenarios, countries like Taiwan, Vietnam, China, Israel, Australia, Singapore, New Zealand, and some other countries adopted evidence-based approaches. They minimized the levels of new infection and brought it under control. Some countries adopted extra precautionary measures. Japan discarded and adopted some policies, such as discarding the 3C (closed space, crowded places, and close-contact settings) policy and adopting the 3T policy (testing, tracing, and treatment) of WHO. Even then, Japan has recorded total morbidity of 687,000 persons with SARS-CoV-2 with mortality of 11,591 (by mid May 2021).
As infection with SARS-CoV-2 is mediated via airborne infection mode, containment of the virus infection from infected persons to normal individuals remains a very difficult task. However, this is the only means to cut short the spread of the infection based on present realities and limitations. Within these realities about prevention and therapy regarding SARS-CoV-2 and COVID-19, the study presented here is a small observational study that indicates that Peg-IFN may be one of the drugs that can have a protective capacity for not acquiring SARS-CoV-2 of CLD patients. There are some limitations of this observational study. The first observational clinical study enrolled only 35 patients, and there is no control group. We are also not sure if infection of 2 of 35 CLD patients represents a meaningful containment strategy or not. However, this is an evidence-based concept related to the containment of SARS-CoV-2 by Peg-IFN in some chronic patients. IFN is related to the induction of innate immunity and SARS-CoV-2 bypassing natural innate immunity for infecting and inducing COVID-19 in normal individuals.31,32 Thus, the usage of IFN even for a short duration may benefit in controlling infection with SARS-CoV-2.
To get some insights into the underlying mechanisms, the second study in 10 patients that analyzed cytokine production after one injection of Peg-IFN revealed that a single injection of Peg-IFN induced significantly higher levels of cytokines of innate immunity. In addition, the third analytical study also revealed that after 12 weekly injections of Peg-IFN, the levels of cytokines were further accentuated in eight CLD patients. Taken together, it seems that if the situation of innate immunity can be exacerbated without notable side effects, it may have some effects on virus localization and replication at the upper respiratory tract.
Already, Peg-IFN has been used to combat the serious progression of COVID-19.33 The study inspired considerable optimism as a family member of three CHB patients was infected with SARS-CoV-2, but the CHB patients receiving Peg-IFN remained uninfected. Usually, patients with CHB represent one of the risky groups for acquiring SARS-CoV-2 due to specific defect of their immune system. However, the prophylactic capacity of Peg-IFN and postexposure prophylaxis or therapeutic effects of Peg-IFN remain to be checked in well-planned randomized studies.
Acknowledgments
The study was supported in part by a grant-in-aid from the Japan Agency for Medical Research and Development (AMED) to Sheikh MF Akbar (Grant number 20fk0310103h1904).
Sheikh MF Akbar and Mamun A Mahtab contributed equally to this work.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, Yang C, Xu X-F, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12–16. doi: 10.1016/j.jmii.2020.05.001. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie P, Ma W, Tang H, et al. Severe COVID-19: a review of recent progress with a look toward the future. Front Public Health. 2020;8:189. doi: 10.3389/fpubh.2020.00189. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Huang K, Jiang H, et al. Long-term existence of SARS-CoV-2 in COVID-19 patients: host immunity, viral virulence, and transmissibility. Virol Sin. 2020;35(6):793–802. doi: 10.1007/s12250-020-00308-0. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sungnak W, Huang N, Becan C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitjà O, Corbacho-Monné M, Ubals M, et al. A cluster-randomized trial of hydroxychloroquine for prevention of Covid-19. N Engl J Med. 2021;384(5):417–427. doi: 10.1056/NEJMoa2021801. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molento MB. Ivermectin against COVID-19: the unprecedented consequences in Latin America. One Health. 2021;13:100250. doi: 10.1016/j.onehlt.2021.100250. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Solidarity Trial Consortium; Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19-Interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Zhang C, Zhu Q, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: a multicenter, open-label, randomized trial. Int Immunopharmacol. 2021;97:107702. doi: 10.1016/j.intimp.2021.107702. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation; Krause PR, Fleming TR, et al. Placebo-controlled trials of Covid-19 vaccines – why we still need them. N Engl J Med. 2021;384(2):e2. doi: 10.1056/NEJMp2033538. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Islam MA, Mazumder MA, Akhter N, et al. Extraordinary survival benefits of severe and critical patients with COVID-19 by immune modulators: the outcome of a clinical trial in Bangladesh. Euroasian J Hepatogastroenterol. 2020;10(2):68–75. doi: 10.5005/jp-journals-10018-1327. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Peng T. Strategy, progress, and challenges of drug repurposing for efficient antiviral discovery. Front Pharmacol. 2021;12:660710. doi: 10.3389/fphar.2021.660710. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sultana J, Crisafulli S, Gabbay F, et al. Challenges for drug repurposing in the COVID-19 pandemic era. Front Pharmacol. 2020;6;11:588654. doi: 10.3389/fphar.2020.588654. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvathaneni V, Gupta V. Utilizing drug repurposing against COVID-19 – efficacy, limitations, and challenges. Life Sci. 2020;259:118275. doi: 10.1016/j.lfs.2020.118275. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Huang T, Guo C, et al. Genomic variation, origin tracing, and vaccine development of SARS-CoV-2: a systematic review. Innovation (N Y) 2021;2(2):100116. doi: 10.1016/j.xinn.2021.100116. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok HF. Review of Covid-19 vaccine clinical trials – a puzzle with missing pieces. Int J Biol Sci. 2021;17(6):1461–1468. doi: 10.7150/ijbs.59170. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Mahtab M, Akbar SM, Aguilar JC, et al. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol Int. 2013;7(4):981–989. doi: 10.1007/s12072-013-9486-4. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Mf Akbar S, Al-Mahtab M, I Khan S. Nature of host immunity during hepatitis B virus infection and designing immune therapy. Euroasian J Hepatogastroenterol. 2018;8(1):42–46. doi: 10.5005/jp-journals-10018-1256. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Mahtab M, Akbar SMF, Aguilar JC, et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment-controlled phase III clinical trial). PLoS One. 2018;13(8):e0201236. doi: 10.1371/journal.pone.0201236. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong MT, Chen SSL. Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection. Cell Mol Immunol. 2016;13(1):11–35. doi: 10.1038/cmi.2014.127. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crosse KM, Monson EA, Beard MR, et al. Interferon-stimulated genes as enhancers of antiviral innate immune signaling. J Innate Immun. 2018;10(2):85–93. doi: 10.1159/000484258. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King C, Sprent J. Dual nature of type 1 interferons in SARS-CoV-2 induced inflammation. Trends Immunol. 2021;42(4):312–322. doi: 10.1016/j.it.2021.02.003. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbar SM, Yamamoto K, Miyakawa H, et al. Peripheral blood T-cell responses to pyruvate dehydrogenase complex in primary biliary cirrhosis: Role of antigen-presenting dendritic cells. Eur J Clin Invest. 2001;31(7):639–546. doi: 10.1046/j.1365-2362.2001.00847.x. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Akbar SM, Horiike N, Chen S, et al. Mechanism of restoration of immune responses of chronic hepatitis B patients during lamivudine therapy; increased antigen processing and presentation by dendritic cells. J Viral Hepat. 2011;18(3):200–205. doi: 10.1111/j.1365-2893.2010.01300.x. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Maqbool A, Khan NM. Analyzing barriers for implementation of public health and social measures to prevent the transmission of COVID-19 disease using DEMATEL method. Diabetes Metab Syndr. 2020;14(5):887–892. doi: 10.1016/j.dsx.2020.06.024. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravani SA, Agrawal GA, Leuva PA, et al. Rise of the phoenix: mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69(6):1563–1568. doi: 10.4103/ijo.IJO_310_21. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez MA. Lack of effectiveness of repurposed drugs for COVID-19 treatment. Front Immunol. 2021;12:635371. doi: 10.3389/fimmu.2021.635371. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charting coronavirus vaccine around the world. https://vdata.nikkei.com/en/newsgraphics/coronavirus-vaccine-status/ Available from:
- 33.Pandit A, Bhalani N, Bhushan BLS, et al. Efficacy and safety of pegylated interferon alfa-2b in moderate COVID-19: a phase II, randomized, controlled, open-label study. Int J Infect Dis. 2021;105:516–521. doi: 10.1016/j.ijid.2021.03.015. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]


