Abstract
Aim and objective
Pediatric acute liver failure (PALF) is a life-threatening condition. Extracorporeal support has been applied for toxic metabolite clearance and serves as a bridging therapy to liver transplantation (LT) or to the regeneration of the liver, but evidence for treatment approaches is still lacking in the pediatric population. We aim to report our experience on therapeutic plasma exchange with high-volume continuous renal replacement therapy (TPE + HV-CRRT) as a promising supportive treatment for PALF.
Materials and methods
A total of eight PALF cases aged 9 months to 14 years, weighing 10–50 kg., who were admitted to PICU King Chulalongkorn Memorial Hospital, Thailand and treated with TPE + HV-CRRT from January 2016 to September 2019 were reviewed. Patient demographic data, indications, technical aspects, and clinical outcomes were recorded.
Results
All patients who underwent TPE + HV-CRRT showed clinical improvement regarding serum bilirubin levels and coagulation studies after the therapy. Complications from the therapy were hemodynamic instability, symptomatic fluid overload, and bleeding from catheter sites. Among these, 6 (75%) patients survived with 4 (50%) successful LTs and 2 (25%) spontaneous recovery. Two children (25%) died while on the transplantation list.
Conclusion
TPE + HV-CRRT can be used safely as a bridging therapy in children with PALF. As opposed to the adult population, higher volume of TPE or higher blood flow rate in pediatric patients might associate with hemodynamic instability during the procedure.
How to cite this article
Trepatchayakorn S, Chaijitraruch N, Chongsrisawat V, Chanakul A, Kongkiattikul L, Samransamruajkit R. Therapeutic Plasma Exchange with Continuous Renal Replacement Therapy for Pediatric Acute Liver Failure: A Case Series from Thailand. Indian J Crit Care Med 2021;25(7):812–816.
Keywords: Acute liver failure, Dialysis supports, Extracorporeal removal, Extracorporeal therapy, Liver transplantation, Pediatric critical care, Pediatric intensive care, Plasma exchange, Poisoning, Therapeutic Plasma Exchange, Yellow phosphorus
Introduction
Pediatric acute liver failure (PALF) is a rare, life-threatening condition without liver transplantation (LT). The accumulation of toxins leads to multiple organ failure and death. Effective extracorporeal liver support (ELS) is needed to bridge for LT or spontaneous recovery of the native liver. Many ELS, such as conventional dialysis, molecular adsorbent recirculation system (MARS), fractionated plasma separation and absorption system, and single-pass albumin dialysis (SPAD), have been investigated over the past decades but clinical outcomes regarding transplant-free survival have not been demonstrated. Recently, therapeutic plasma exchange in combination with high-volume continuous renal replacement therapy (TPE + HV-CRRT) shows promising results.1–7 Theoretically, TPE has multiple potential benefits for PALF, such as toxin removal and supplementation of coagulation proteins. The proposed mechanisms are the removal of albumin-bound and unbound toxins,8 aiming to prevent complications related to an impaired detoxification function of the failing liver. In addition, performing TPE with fresh frozen plasma might improve coagulopathy, which cannot be corrected by simple albumin dialysis. When combined with HV-CRRT, additional effects of toxin clearance can be achieved via convection.9 Emphasizing that dialysis dose must be high enough to create transmembrane pressure needed for successful blood purification.
Materials and Methods
We described our experience of TPE + HV-CRRT used at King Chulalongkorn Memorial Hospital, a major pediatric LT center in Thailand, from 2016 to 2019. We used PALF diagnosis criteria according to the PALF study group 2006.10 Indications for TPE?HV-CRRT in our institution were (1) PALF with encephalopathy grade ≥ 3 or (2) transplantation candidates. Therapy was continued until LT or spontaneous recovery occurred.
Therapeutic Plasma Exchange
Dialysis catheter insertion was performed using the full sterile technique at the bedside. A curved dialysis catheter was inserted into the right internal jugular vein by ultrasound-guided Seldinger technique until the tip of the catheter reached midatrium position. We used heparin solution at 2,500 unit/mL concentration in NSS as lock solution to maintain patency of the catheter. Circuit priming was done using leukocyte-depleted packed red cells 180–200 mL for pediatric patients. TPE solution of either 5% albumin or fresh frozen plasma was chosen regarding the severity of coagulopathy.
The volume of TPE solution was 40–75 mL/kg. TPE was performed once daily, 4–6 hours/session, total 1–6 days before transplantation or discharge depending on the severity of coagulopathy and encephalopathy. Significant TPE protocol change was made after the 2016 and 2019 American Society for Apheresis Guidelines8,11 were released. Our center now performs TPE at a higher dose, 150–200 mL/kg (2–3 × total plasma volume, TPV). The therapy session was also reduced to 1.5–2 hours/session, caused by an increase in blood flow rate. These changes were based on the experience of our blood bank on adult ALF patients.
High-volume Continuous Renal Replacement Therapy
After completion of TPE, HV-CRRT (dialysis dose greater than 2,000 mL/1.73m2/hour) was performed. Circuit priming was done using 5% human albumin solution. The usual anticoagulant was 4% trisodium citrate or anticoagulant free circuit with NSS flushing hourly. Dialysis solution was usually a custom-made solution to restore normal serum electrolyte levels and comprised of sodium of 135–140 mmol/L, potassium 0–3 mmol/L, chloride 108–112 mmol/L, bicarbonate 25–40 mmol/L, magnesium 1.5 mmol/L, and glucose 100–150 mg/dL.
Result
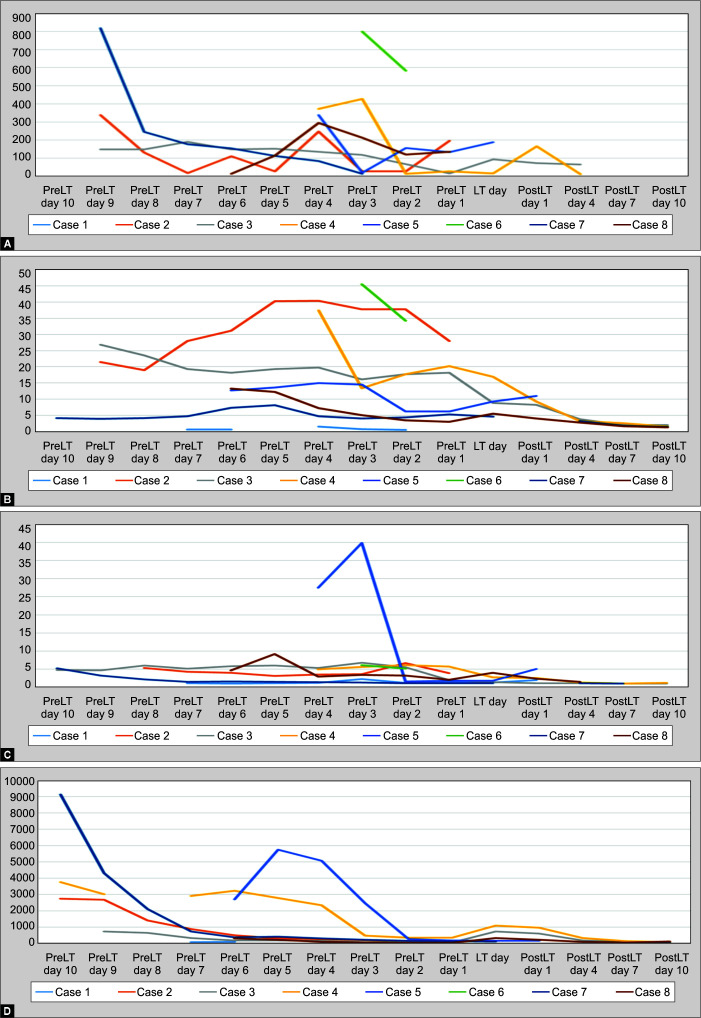
Eight PALF cases were included in this study. The youngest patient was 5 months old and weighed 4.8 kg. Median age 4 years (P25–75 10 months–10 years), bodyweight 14 kg (P25–75 11.4–30.5 kg.) Severity scores at admission were PELODS-II score 2.5 points (P25–75 1–9 points) and PRISMS-III score 11 points (P25–75 8–14.5 points). Details of each case are presented in Table 1. Initial laboratory data are shown in Table 2. SPAD was done in one patient due to feasibility and availability at that time. Among eight cases, six (75%) survived to discharge, four (50%) had successful LTs, and two (25%) had spontaneous recoveries. The other two (25%) died on the transplantation list. Details of pretherapy and post-therapy laboratory data are demonstrated in Table 3 (Fig. 1).
Table 1.
Details study cases
| Case no. | PALF cause | Apheresis date | LT/DC date | TPE volume | Total No. of treatment sessions | CRRT mode |
|---|---|---|---|---|---|---|
| 1 | SLE w/septic shock from S. pyogenes | August 11–13, 2016 | August 16, 2016 (toward) | 5% albumin 30 mL/kg + FFP 10 mL/kg | 2 | CVVH |
| 2 | Hepatic failure, unspecified with coma | SPAD: June 1–6, 2017 TPE + HV-CRRT: June 7, 2017 | June 9, 2017 (dead) | SPAD: 4% albumin 110 mL/kg TPE+HV-CRRT: FFP 60 mL/kg | 4 SPAD 1 TPE + HV-CRRT | CVVH |
| 3 | Hepatic failure, unspecified with coma | September 28–October 5, 2017 | October 6, 2017 (LT) | 5% albumin 40 mL/kg + FFP 10 mL/kg | 6 | CVVHDF |
| 4 | Hepatic failure, unspecified with coma | February 1–4, 2018 | February 5, 2018 (LT) | 5% albumin 50 mL/kg + FFP 15 mL/kg | 4 | CVVHD |
| 5 | Toxic effect of ingested mushrooms | August 6–8, 2018 | August 9, 2018 (LT) | FFP 200 mL/kg | 3 | CVVHDF |
| 6 | NEC w/septic shock | February 6, 2019 | February 8, 2019 (dead) | FFP 179 mL (failed at 70 mL) | 1 | CVVHDF |
| 7 | Acetaminophen overdose | February 27–March 1, 2019 | March 8, 2019 (to ward) | FFP 150 mL/kg | 3 | CVVHDF |
| 8 | Acetaminophen overdose | March 13–17, 2019 | March 18, 2019 (LT) | FFP 150 mL/kg | 5 | CVVHDF |
PALF, pediatric acute liver failure; TPE, therapeutic plasma exchange; HV-CRRT, high-volume continuous renal replacement therapy; FFP, fresh frozen plasma; CVVHDF, continuous venovenous hemodiafiltration; CVVHD, continuous venovenous hemodialysis; CVVH, continuous venovenous hemofiltration; SPAD, single-pass albumin dialysis; LT, liver transplantation; DC, patient discharge from PICU; NEC, necrotizing enterocolitis; SLE, systemic lupus erythematosus
Table 2.
Initial laboratory data
| Median | P25–75 | |
|---|---|---|
| Alanine aminotransferase (ALT) | 1487.5 U/mL | 134.75–3077.5 U/mL |
| Total bilirubin | 24.15 mg/dL | 6.5–35.1 mg/dL |
| Ammonia | 365 µg/dL | 290.75–803.5 µg/dL |
| International normalized ratio (INR) | 4.84 | 2.85–6.62 |
| Creatinine | 0.35 mg/dL | 0.29–0.97 mg/dL |
Table 3.
Pre-TPE and post-TPE laboratory data comparisons [median (IQR)]
| Laboratory data | Pre-TPE | Post-TPE | p-value |
|---|---|---|---|
| Creatinine (mg/dL) | 0.31 (0.23, 0.79) | 0.39 (0.26, 0.95) | 0.082 |
| Serum bilirubin (mg/dL) | 13.41 (6.22, 19.71) | 9.85 (3.46, 12.45) | 0.000 |
| Alanine transaminase (U/mL) | 174 (74, 648) | 72 (40, 238) | 0.000 |
| Serum ammonia (µg/dL) | 428 (182, 667) | 236 (135, 413) | 0.043 |
| INR | 4.42 (2.95, 6.04) | 1.86 (1.48, 3.50) | 0.001 |
Figs 1A to E.
Trend of daily highest laboratory data. LT day; day of liver transplantation or PICU discharge/death
All patients demonstrated improvement regarding coagulation studies and serum bilirubin after the therapy, but these lasted for only 24 hours. Clinical encephalopathy was not evaluated in our review due to the use of sedation to prevent catheter displacement. During the previous protocol therapy, all hemodynamic parameters were stable. Details of pre-TPE and post-TPE laboratory data after each therapeutic session are shown in Table 2.
After the protocol change in mid-2018, all four patients developed hypotension and required resuscitation with NSS bolus early at therapy initiation. One had severe hypotension and could not tolerate the therapy. One developed clinical symptoms of fluid overload immediately after the therapy ended. This prompted protocol adjustment to not return circuit volume to patients after the sessions and to lower blood flow rate. Another complication found was bleeding from the dialysis catheter site in one patient, which was managed by adrenaline gauze packing.
Discussion
From our experience, TPE + HV-CRRT is an effective treatment for children with acute fulminant hepatic failure. TPE + HV-CRRT could decrease serum ALT, bilirubin, ammonia, and reverse severe coagulopathy. However, these effects were transient. Thus, TPE + HV-CRRT should be performed consecutively while waiting for liver recovery or transplantation.
Ide et al.12 and Arikan et al.13 have previously demonstrated successful TPE + HV-CRRT and MARS for children with ALF. Nevertheless, the procedures in their studies were slightly different from our institution, using higher dialysis flow and higher volume TPE. They also reported improvement of encephalopathy. In contrast with our review, both studies reported no serious complications.
Both TPE and HV-CRRT require skilled physicians to perform catheter insertion and experienced staff for maintenance. Common complications of extracorporeal support can be found including clotting, bleeding, temperature instability, infection, fluid overload, and hemodynamic instability, especially when performed on small patients at large volumes and high blood flow rate. These complications were different from those described in adult populations. Explanation might be higher blood flow rate, higher volume of distribution for vasoactive drugs, and higher circuit volume per bodyweight. Other factors to be taken into consideration are financial costs and a clear communication with the family. Because this therapy itself is not a definitive treatment of PALF, duration of therapy, schedule for transplantation, and termination of therapy if the patient enters a palliative stage should be discussed within the medical team and family members before the therapy.
Our study has limitations. In this review, only one adolescent girl showed improvement of encephalopathy after receiving repeated sessions of TPE + HV-CRRT. Most of our patients were deeply sedated and encephalopathy could not be assessed clinically.
Conclusion
TPE + HV-CRRT is beneficial bridging therapy in PALF. The therapy can be used safely in resource-limited developing countries and can be performed on small infants to adolescents. Complications associated with this therapy are hemodynamic instability requiring resuscitation and bleeding at the catheter site. Individual institutes can develop their own protocols for this therapy to prevent possible complications and to improve quality of care for PALF patients.
Highlight
TPE + HV-CRRT for pediatric patients requires judicious monitoring as hemodynamic instability can complicate the treatment, contrary to stable vitals reported on adult counterparts.
Orcid
Sirawut Trepatchayakorn https://orcid.org/0000-0002-2364-4154
Nataruks Chaijitraruch https://orcid.org/0000-0002-6665-8046
Voranush Chongsrisawat https://orcid.org/0000-0002-6106-0504
Ankanee Chanakul https://orcid.org/0000-0002-9382-8151
Lalida Kongkiattikul https://orcid.org/0000-0002-4150-039X
Rujipat Samransamruajkit https://orcid.org/0000-0001-6536-7709
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Biancofiore G, Bindi LM, Urbani L, Catalano G, Mazzoni A, Scatena F, et al. Combined twice-daily plasma exchange and continuous veno-venous hemodiafiltration for bridging severe acute liver failure. Transplant Proc. 2003;35(8):3011–3014. doi: 10.1016/j.transproceed.2003.10.077. DFOI: [DOI] [PubMed] [Google Scholar]
- 2.Yokoi T, Oda S, Shiga H, Matsuda K, Sadahiro T, Nakamura M, et al. Efficacy of high-flow dialysate continuous hemodiafiltration in the treatment of fulminant hepatic failure. Transfus Apher Sci. 2009;40(1):61–70. doi: 10.1016/j.transci.2008.11.006. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Stenbog P, Busk T, Larsen FS. Efficacy of liver assisting in patients with hepatic encephalopathy with special focus on plasma exchange. Metab Brain Dis. 2013;28(2):333–335. doi: 10.1007/s11011-013-9403-5. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Hilal T, Morehead RS. Fulminant Wilson's disease managed with plasmapheresis as a bridge to liver transplant. Case Rep Med. 2014;2014:672985. doi: 10.1155/2014/672985. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Wang Z, Wang Y, Du C, Li S, Shi Z, et al. Part of plasmapheresis with plasma filtration adsorption combined with continuous hemodiafiltration in the treatment of severe acute liver failure. Exp Ther Med. 2016;12(4):2582–2584. doi: 10.3892/etm.2016.3633. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiklund RA. Preoperative preparation of patients with advanced liver disease. Crit Care Med. 2004;32(4 Suppl.):S106–S115. doi: 10.1097/01.ccm.0000115624.13479.e6. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35(11):2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 2016;31(3):149–162. doi: 10.1002/jca.21470. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Rimmele T, Kellum JA. High-volume hemofiltration in the intensive care unit: a blood purification therapy. Anesthesiology. 2012;116(6):1377–1387. doi: 10.1097/ALN.0b013e318256f0c0. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Squires RH Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652–658. doi: 10.1016/j.jpeds.2005.12.051. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354. doi: 10.1002/jca.21705. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Ide K, Muguruma T, Shinohara M, Toida C, Enomoto Y, Matsumoto S, et al. Continuous veno-venous hemodiafiltration and plasma exchange in infantile acute liver failure. Pediatr Crit Care Med. 2015;16(8):e268–e274. doi: 10.1097/PCC.0000000000000511. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Akcan Arikan A, Srivaths P, Himes RW, Tufan Pekkucuksen N, Lam F, Nguyen T, et al. Hybrid extracorporeal therapies as a bridge to pediatric liver transplantation. Pediatr Crit Care Med. 2018;19(7):e342–e349. doi: 10.1097/PCC.0000000000001546. DOI: [DOI] [PubMed] [Google Scholar]