Abstract
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) infections have a major effect on mortality as well as healthcare cost. Intensive care units (ICUs) in India, the epicenters for multidrug-resistant organisms, are facing a “postantibiotic era” because of very limited treatment options. A latest beta-lactam/beta-lactamase inhibitor ceftazidime–avibactam (CZA) new has a broad-spectrum antibacterial activity. CZA inhibits class-A and class-C beta-lactamases (as well Klebsiella pneumoniae carbapenemase (KPC)), along with some class-D carbapenems such as OXA-48-like enzymes that are seen in Enterobacteriaceae has recently become available. The current study aimed to assess and present the clinical response and patient outcome with infections due to CRE when treated with CZA alone or in combination with other drugs.
Materials and methods
This retrospective study reviews the experience recorded and analyzed at two tertiary care centers including only adult patients with CRE infection who had received CZA alone or in combination with other antibiotics over a period between February 2019 and January 2020.
Results
In the period from February 2019 to January 2020, 119 culture-confirmed CRE isolates were tested for Xpert Carba-R. The predominant genetic mechanism was a combination of NDM+OXA-48 in 45/119 (37.81%). Total 40/57 patients received CZA+aztreonam alone or in combination with other drugs with an overall cure rate of 77.5% while the rest 17 received CZA alone in combination with the cure rate of 82.35%. 41/57 (71.92%) patients were in ICU.
Conclusion
With overall mortality of 21%, these data suggest that CZA is a viable option for patients with CRE infections. To our knowledge, this is the first Indian study reporting CZA data in CRE infections.
How to cite this article
Nagvekar V, Shah A, Unadkat VP, Chavan A, Kohli R, Hodgar S, et al. Clinical Outcome of Patients on Ceftazidime–Avibactam and Combination Therapy in Carbapenem-resistant Enterobacteriaceae. Indian J Crit Care Med 2021;25(7):780–784.
Keywords: Carbapenem-resistant Enterobacteriaceae, Combination therapy, ICU mortality
Background
In India, the burden of carbapenem-resistant Enterobacteriaceae (CRE) infection is substantial, including the longer hospital stay, raised mortality cases, and higher direct and indirect healthcare costs. Previous studies have reported that the major independent risk factor for mortality is carbapenem resistance which is mainly because of inappropriate initial antimicrobial therapy.1–3 Carbapenems are the last-line effective antibiotics, and their resistance in gram-negative bacteria (GNB) has reached an alarming number (12%–83%) in Indian intensive care units (ICUs).4 ICUs are considered as the “hot spots” of multidrug-resistant organisms and here there are chances of deficient empirical antibiotic therapy leading to excess mortality.4 Based on the latest evidence of emergence of CRE along with the latest study reports on colistin resistance, ICUs are dealing with the possibility of “postantibiotic era.”
Management of a variety of infections due to CRE is challenging as very limited agents, such as colistin (CL), tigecycline, minocycline, and fosfomycin, are available.5,6 However, these drugs have limitations for use as monotherapy for a variety of reasons, so are better preferred as combination therapy.5,7
The combination of a novel beta-lactam/beta-lactamase inhibitor (BL-BLI) ceftazidime–avibactam (CZA) is a Food and Drug Administration approved combination used for treating the complicated intra-abdominal and urinary tract infections (UTIs) including pyelonephritis. Hospital-acquired pneumonia (HAP) (including ventilator-associated pneumonia) in adults, when treated with CZA, are found effective.8 CZA has a broad-spectrum antibacterial activity and is effective against class-A and class-C beta-lactamases (as well as KPC), and some class D carbapenemases such as OXA-48-like enzymes that are seen in Enterobacteriaceae. As per previous studies, CZA has demonstrated exceptional in vitro activity against Enterobacteriaceae and isolates producing OXA-48-like enzymes.9–11
Among the currently available various newer BL/BLI, CZA is the only agent active against OXA-48-like producers, which are being increasingly seen along with MBLs (NDM in India). A recent study reported OXA-48-like genes co-carried NDM, which limits the usefulness of CZA in these isolates.9
Another newer BL/BLI, aztreonam (ATM)/avibactam that has potential activity against NDM and OXA-48, is currently in clinical development.12 ATM is found to be stable against MBLs (NDM); however, its utility in the treatment is limited due to its inactivation by the presence of ESBLs and AmpCs along with MBLs (NDM). As CZA is not active against MBL-producing organisms, Hypothetically, ATM may be combined with CZA. This combination may have a strong inhibitory activity against CRE, expanding the coverage over NDM and OXA-48-like enzymes.
The study aims to assess and present the clinical response and patient outcome with infections due to CRE when treated with CZA alone or in combination with other drugs. We have also assessed the performance of CZA in combination with aztreonam against NDM as well as NDM and OXA-48 co-producers. With reference to the dosing recommended by the manufacturer, CZA was administered by the providers.13
Materials and Methods
This was a retrospective cohort study, conducted in the patients with CRE infections from February 2019 to January 2020 at two tertiary care centers of Mumbai, India. All adult patients (>18 years) were treated with CZA for at least 72 hours, and all the patient's cultures showing carbapenem-resistant isolates were screened. Clinically established CRE-infected patients were included in the study. The Vitek 2 system (make-bioMérieux, France) and N-280 card were used for the susceptibility testing. The Clinical Laboratory Standards Institute (CLSI) interpretation guidelines were followed.
Confirmed CRF isolates from the culture were used for the testing. For rapid detection and differentiation of the blaNDM, blaKPC, blaVIM, blaOXA-48, and blaIMP gene sequences linked to carbapenem resistance in gram-negative bacteria Xpert Carba-R kit was used. The procedure was performed as per the manufacture's recommendation.
Following detection of gene sequence, CZA susceptibility testing was performed. The interpretation was based on the CLSI guidelines. We demonstrated synergy with “Zone of Hope” by placing CZA and aztreonam disc at a distance of 20 mm also by putting AZT disck on CZA MIC strip and check for the clearing (Figs 1 to 3). We also performed sandwich technique by putting CZA and AZT disc on each other and thus observing the clearing of zone of >18 mm (Fig. 4). Except one patient of E. coli, the rest all had synergy and best with Klebsiella.
Fig. 1.
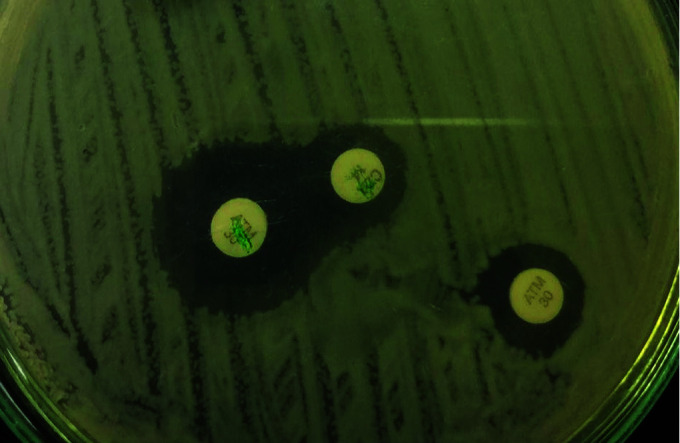
Synergy with zone of hope
Fig. 3.
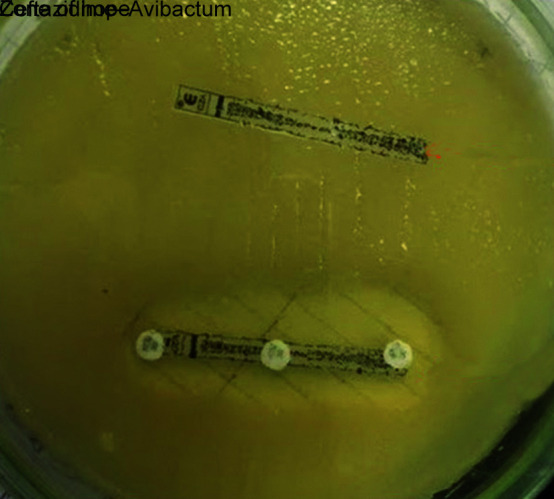
Zone of hope—ceftazidime–avibactam
Fig. 4.
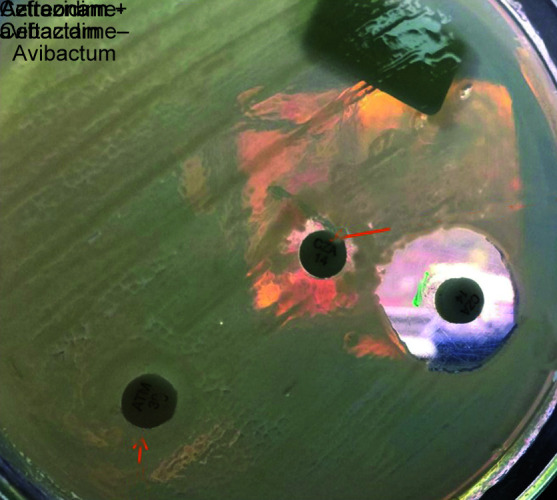
Zone of hope—ceftazidime-avibactam and aztreonam + ceftazidime-avibactam
Fig. 2.
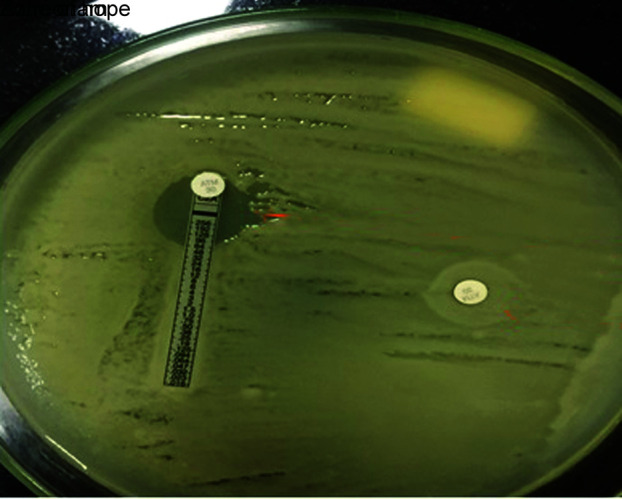
Zone of hope—aztreonam
Total 121 CRE culture isolates were tested using Xpert Carba-R kit for rapid detection of gene sequences. For CZA sensitivity determination, MIC strips were used in 69 patients while 50 patients were tested with 10/4 µg CZA disc as previously MIC strips were not available. Two patients were excluded as they did not show any resistance mechanism.
In this study, the cases who received CZA or with combination therapy were studied.
Total 57 out of 119 patients received CZA alone or in combination with other drugs like aztreonam, polymyxins, tigecycline, or fosfomycin based on their sensitivity pattern. Colisitn MIC was determined by using broth microdilution technique.
Results
We identified that a total of 121 patients were tested for Xpert Carba-R between February 2019 to January 2020. Out of these 121 patients, 57 patients received CZA alone or in combinations with other medications for the treatment of CRE infections depending upon the resistance pattern detected.
Xpert Carba-R showed 40 OXA-48 (38 Klebsiella pneumoniae, 2 E. coli), 45 NDM+OXA-48 (all 45 Klebsiella pneumoniae), while 34 were NDM (8 Klebsiella pneumoniae, 26 E. coli). Two isolates for Xpert Carba-R were negative suggesting an alternative mechanism of resistance as presented in Table 1.
Table 1.
XpertCarba-R negative results suggesting alternative mechanism of resistance
| Total | OXA 48 | NDM+OXA-48 | NDM |
|---|---|---|---|
| 119 | 40 (33.61%) | 45 (37.81%) | 34 (28.57%) |
| E. coli | 2 | 0 | 26 |
| KLEB | 38 | 45 | 8 |
Outcomes are listed in Table 2. The most common infection type with 18 (31.57%) patients was the intra-abdominal infection followed by nosocomial pneumonia with 15 (26.31%) patients. Patients (n = 5, 8.77%) with bloodstream infection (n = 5, 8.77%) with UTI, (n = 4, 7.01%) with wound infection, and (n = 4, 7.01%) with skin and soft tissue infection were other infections in which CZA was used for the treatment. Six patients had an established solid organ transplant. K. pneumonia was the predominant pathogen (n = 48).
Table 2.
Patient characteristics and outcome
| Male gender | 39 (68.42%) |
| Age (years) (median) | 60 |
| Type of infection | |
| Intra-abdominal infections | 18 (31.57%) |
| Nosocomial pneumonia | 15 (26.31%) |
| cUTI | 5 (8.77%) |
| Blood stream infection | 5 (8.77%) |
| Others | 14 (24.56%) |
| Infecting organism | |
| Klebsiella pneumoniae | 48 (84.21%) |
| E. coli | 9 (15.78%) |
| ICU admission | 41 (71.92%) |
| Non-ICU admission | 16 (28.07%) |
| Renal dose adjustment | |
| Yes | 37 (64.91%) |
| No | 20 (35.08%) |
| Clinical cure without relapse | |
| Or death within 30 days | 45 (78.94%) |
| 30 days mortality | 12 (21.05%) |
| Resistance mechanisms | |
| OXA-48 | 17 |
| NDM | 07 |
| NDM + OXA-48 | 33 |
High grade of acute infection was observed with 72% (41/57) of patients in the ICU during the treatment of CZA. Renal dose adjustment was needed in 37 patients.
The total hospital death rate observed was 21.05% (12/57). The ICU patients showed significantly higher rates of in-hospital deaths when compared to the non-ICU patients 24.39% (10/41) versus 1.5 % (2/16)] as presented in Table 2.
OXA-48 was detected in 17 patients (15 Klebsiella pneumoniae, 2 E. coli), NDM was detected in 7 patients (7 E. coli) while combination of NDM and OXA-48 was detected in 33 patients (33 Klebsiella pneumoniae) who had received CZA treatment. Many patients received an added gram-negative active agent while on CZA therapy. The most regularly used drugs were aztreonam, polymyxin, tigecycline, and fosfomycin.
Table 3 demonstrates the treatment options and clinical cure rate. For the treatment of pure OXA-48 group of patients CZA was used alone in four patients while in the combination of polymyxin in four patients, with tigecycline in seven patients and polymyxin and Fosfomycin combination along with CZA in two patients. The clinical cure rate in this group of patients is 14/17 (82.35%). Those patients who were on monotherapy were less sick patients and in reality, it was the beginning of using CZA. More studies on the usage of monotherapy in sicker patients required as well but invariably when there is a CRE sick patient, its usually a combination that is used. Also, they were all OXA 48.
Table 3.
Treatment options and clinical cure rate
| Resistance mechanisms | CZA | CZA+POL | CZA+TIGE | CZA+POLY+FOS | Total |
|---|---|---|---|---|---|
| OXA 48 | 4/4 | 3/4 | 5/7 | 2/2 | 14/17 |
| Clinical cure rate | 100% | 75% | 71% | 100% | 82.35% |
| CZA+AZT | CZA/AZT+POLY | CZA+AZT+FOS | – | – | |
| NDM +OXA-48 OR NDM | 11/12 | 14/21 | 6/7 | – | 31/40 |
| Clinical cure rate | 91.67% | 66.67% | 85.71% | – | 77.5% |
Those patients where combination of NDM+OXA or pure NDM were detected, as well as were more sicker, and were put on combination of CZA with available best option.
Treatment of NDM or combination of NDM and OXA-48, CZA was prescribed in combination with aztreonam alone in 12 patients while this combination was used along with polymyxin in 21 patients and along with fosfomycin in 7 patients. The clinical cure rate in these groups of patients is 31/40 (77.50%).
In our assessment of the use of CZA for the treatment of infections due to CRE in acutely ill patients, overall, the in-hospital death rate was found to be 21%. Various studies on CRE infections have shown high inconsistent outcomes. The results of several studies prior to the availability of CZA have demonstrated low mortality rates with combination therapy as compared to monotherapy. A case series of 37 patients with different CRE infections and who were treated with CZA showed a 30-day death rate of 24% with a clinical success rate of 59% and a microbiologic failure rate of 27%. CZA was given in combination with another agent to 30% of the cases.14 During therapy, CZA resistance was developed in 8% of cases. The occurrence of CZA-resistant strains during treatment can be a causative factor for the increased death rate among patients with OXA-48 type CRE infections.14 Resistance to CZA is described by Shields et al. as the emergence of microbiological failure following the treatment for 10 to 19 days in three out of ten patients.
CZA has evolved as an encouraging therapy for infections due to CRE. However, in most of the clinical studies patients with KPCs15,16 were enrolled. Lower all-cause death in the CZA group was observed in a prospective multicenter cohort study. This study assessed the clinical response for patients with CRE infections and compared 38 CZA treated patients with 99 patients treated with colistin for KPC-producing CRE.16 In another prospective study by Sousa et al., the effectiveness of CZA as a rescue treatment for infections due to OXA-48-producing Enterobacteriaceae was presented in 57 patients, where 81% received monotherapy of CZA.17
Alraddadi et al. demonstrated clinical usefulness of CZA for the treatment of infections due to CRE, including those caused by OXA-48 producing organisms, when compared with standard treatment. A majority of the patients in the study had HAP infections. All-cause mortality in this study was 50 to 57.1% compared to 21% in our cohort while majority of our patients had intra-abdominal infections.18
A multicenter assessment of the use of CZA was performed by King et al. for treatment of infections due to CRE in acutely ill patients. The overall, in-hospital death rate observed was 32%.19
Limitations of the Study
Limitations of this study include the retrospective design of this study and we were not able to limit for confounding factors. We have not determined infection-associated mortality and there are high chances that the deaths could be ascribed to other disease processes. Use of other added antibiotics which varied among patients, the dosage of the drugs and comorbidities is another limiting factor.
Conclusion
With an overall death of 21% in this patient population, the findings of the study suggest that CZA is a viable option for the treatment of infections due to CRE, as well as for acutely ill or posttransplant patients. To our knowledge, this is the first Indian study reporting CZA data in CRE infections. Additional data and clinical studies are urgently needed. In standard antibiogram susceptibility testing CZA should be incorporated. Additionally, because of the less sample size, our study fails to achieve the statistical power to detect significant differences in efficacy or tolerability of the treatment regimen. Further, more randomized trials are required to support the existing results and to guide the clinical practice.
Highlights
Infections due to CRE are having a major impact on mortality.
CZA is a better choice for treatment of the patients with CRE infections as well as for acutely ill or posttransplant patients.
Orcid
Vasant Nagvekar https://orcid.org/0000-0002-5603-9102
Anand Shah https://orcid.org/0000-0002-0079-8716
Vrajeshkumar P Unadkat https://orcid.org/0000-0002-5147-6944
Amol Chavan https://orcid.org/0000-0002-7803-8872
Ruhi Kohli https://orcid.org/0000-0002-6563-6101
Shailendra Hodgar https://orcid.org/0000-0003-3547-7020
Aashita Ashpalia https://orcid.org/0000-0001-8970-6584
Niranjan Patil https://orcid.org/0000-0001-8427-0355
Rahul Kamble https://orcid.org/0000-0003-3293-7554
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Escandon-Vargas K, Reyes S, Gutierrez S, Villegas MV. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev Anti-Infect Ther. 2017;15(3):277–297. doi: 10.1080/14787210.2017.1268918. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106. doi: 10.1086/592412. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Wattal C, Javeri Y, Goel N, Dhar D, Saxena S, Singh S, et al. Convergence of minds: for better patient outcome in intensive care unit infections. Indian J Crit Care Med. 2017;21(3):154–159. doi: 10.4103/ijccm.IJCCM_365_16. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calfee DP. Recent advances in the understanding and management of Klebsiella pneumoniae. F1000Res. 2017;6:1760. doi: 10.12688/f1000research.11532.1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar C, Nabarro LE, Anandan S, Ravi R, Babu P, Munusamy E, et al. Extremely high mortality rates in patients with carbapenem-resistant, hypermucoviscous Klebsiella pneumoniae blood stream infections. J Assoc Phys India. 2018;66:13. [PubMed] [Google Scholar]
- 7.Gomez-Simmonds A, Nelson B, Eiras DP, Loo A, Jenkins SG, Whittier S, et al. Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2016;60:3601–3607. doi: 10.1128/AAC.03007-15. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Duin D, Bonomo RA. Ceftazidime–Avibactam and ceftolozane/Tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis. 2016;63:234–241. doi: 10.1093/cid/ciw243. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazmierczak KM, Bradford PA, Stone GG, de Jonge BL, Sahm DF. In vitro activity of ceftazidime–avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the international network for optimal resistance monitoring (INFORM) global surveillance program from 2012 to 2015. Antimicrob Agents Chemother. 2018;62:e00592–18. doi: 10.1128/AAC.00592-18. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlowsky JA, Biedenbach DJ, Kazmierczak KM, Stone GG, Sahm DF. Activity of ceftazidime–avibactam against extended-spectrum- and AmpC β-lactamase-producing Enterobacteriaceae collected in the INFORM global surveillance study from 2012 to 2014. Antimicrob Agents Chemother. 2016;60:2849–2857. doi: 10.1128/AAC.02286-15. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin D, Wu S, Yang Y, Shi Q, Dong D, Zhu D, et al. Results from the China antimicrobial surveillance network (CHINET) in 2017 of the in vitro activities of ceftazidime–avibactam and ceftolozane-tazobactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63:e02431–18. doi: 10.1128/AAC.02431-18. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaiskos I, Galani I, Souli M, Giamarellou H. Novel β-lactam-β-lactamase inhibitor combinations: expectations for the treatment of carbapenem-resistant gram-negative pathogens. Expert Opin Drug Metab Toxicol. 2019;15:133–149. doi: 10.1080/17425255.2019. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime–avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis. 2016;63:1615–1618. doi: 10.1093/cid/ciw636. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, et al. Efficacy of ceftazidime–avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68(3):355–364. doi: 10.1093/cid/ciy492. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, et al. Colistin versus ceftazidime–avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa A, Perez-Rodriguez MT, Soto A, Rodriguez L, Perez-Landeiro A, Martinez-Lamas L, et al. Effectiveness of ceftazidime–avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3170–3175. doi: 10.1093/jac/dky295. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime–avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19:772. doi: 10.1186/s12879-019-4409-1. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, et al. Multicenter study of outcomes with ceftazidime–avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61:e00449–17. doi: 10.1128/AAC.00449-17. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]


