Abstract
Background
Data are lacking on the role of cellular components of hematological system as biomarkers for prognosis of sepsis. We planned to identify if these parameters measured at admission to ICU and at 72 hours can be useful as prognostic marker in septic critically ill patients.
Materials and methods
In this prospective observational study, 130 adult patients with sepsis were recruited. Various hematological study parameters (total, differential, and absolute leukocyte count, platelet count, platelet distribution width, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio) were noted at day 1 and day 3 of admission. Primary outcome was 28-day mortality, and secondary outcomes were duration of mechanical ventilation, vasopressor requirement, ICU length of stay, and requirement of renal replacement therapy. The variables were compared between two groups and using binary regression model and were evaluated as prognostic markers for 28-day mortality.
Results
Data from n = 129 were analyzed. At day-28, n = 58 (44.96%) patients survived. Baseline and demographic parameters were comparable between survivors and nonsurvivors. Admission Sequential Organ Failure Assessment score was more in nonsurvivors than survivors [8 (6–8) vs 6 (4–8); p = 0.002]. In nonsurvivors, monocyte, lymphocyte, basophil, eosinophil, and platelet count were significantly less at day 1 and lymphocyte, eosinophil, basophil and platelet count were significantly less at day 3. NLR and PLR at day 3 were significantly more in nonsurvivors. On logistic regression analysis, age, thrombocytopenia on day 1, and low eosinophil count on day 3 predicted 28-day mortality (p = 0.006, p = 0.02, and p = 0.04, respectively).
Conclusion
Thrombocytopenia on day 1 and eosinopenia on day 3 may predict 28-day mortality in sepsis.
How to cite this article
Sinha H, Maitra S, Anand RK, Aggarwal R, Rewari V, Subramaniam R, et al. Epidemiology and Prognostic Utility of Cellular Components of Hematological System in Sepsis. Indian J Crit Care Med 2021;25(6):660–667.
Keywords: Critically ill adults, Mortality prediction, Sepsis, Thrombocytopenia
Introduction
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Diagnosis and proper prognostication of sepsis are of paramount importance as with every additional organ failure, mortality increases by approximately 20%.2
Organ dysfunction in sepsis is indicated by an increase in the Sequential Organ Failure Assessment (SOFA) score by two points or more.1 For hematological system, platelet count is used in SOFA scoring, and thrombocytopenia is represents worse prognosis. However, another prognostic ICU scoring system known as Simplified Acute Physiology II score uses total leukocyte count as a parameter.3
Hematological system is involved early during the pathogenesis of sepsis and is considered to play a significant role in the progression and resolution phase of sepsis.4 In the cellular component of hematological system, the most commonly seen abnormalities are anemia, thrombocytopenia, and leukocytosis (although erythrocytosis, thrombocytosis, and leukopenia may also be seen) while in the fluid phase components, dysregulated hemostasis is commonly observed.5
A sizable amount of research has been done on the soluble components of blood—the coagulation proteins, antithrombins, and fibrinolytic system to establish the pathophysiology and clinical course of coagulopathy in sepsis.6,7 However, only recently, studies have been performed on the cellular components of hematological system and their role as biomarkers for early detection and prognosis of sepsis. Kim et al. in their prospective study found that change in red cell distribution width (RDW) at 72 hours with respect to RDW on admission significantly predicts 28–90-day mortality in sepsis.8 Hubert et al. found that immature platelet fraction (IPF) had the highest diagnostic accuracy and correlated significantly with sepsis severity score.9 Seok et al. observed that the immature granulocyte fraction was able to predict mortality in sepsis.10
The available literature suggests the importance of conducting further research into the cellular component of hematological system to identify exact role of various parameters and their prognostic value as markers in sepsis. We, therefore, performed this prospective observational study to identify if the parameters derived from cellular components of hematological system [hematocrit, RDW, evidence of hemolysis in peripheral blood smear, immature granulocytes, thrombocytopenia, IPF, platelet distribution width (PDW)] in isolation or in combination, measured at admission to ICU and at 72 hours can be useful prognostic markers in septic critically ill patients. The primary outcome was 28-day mortality and secondary outcomes were length of ICU stay, days on mechanical ventilation, vasopressor support, and requirement of renal replacement therapy (RRT).
Materials and Methods
Study Design
Prospective observational study.
Study Setting
The study was conducted in the ICU of Department of Anaesthesiology and Critical Care, All India Institute of Medical Sciences, New Delhi between January 2017 and June 2018. Institute Ethics Committee clearance was obtained (Ref No. IECPG-603/08.12.2016), and the study was prospectively registered in the Clinical Trials Registry of India ({{a href='http://www.ctri.nic.in/'}}www.ctri.nic.in{{/a}} CTRI/2017/11/010458).
Study Population
Adult patients aged 18–65 years with diagnosis of sepsis were included in the study within 24 hours of admission after obtaining informed written consent from patients or their legally authorized representatives. Exclusion criteria were (i) patient's/their relative's refusal to consent for study, (ii) preexisting hematological disorder/malignancy, (iii) previous history of sepsis, (iv) history of ICU stay within last 6 months, (v) any chronic disease, which can impair hematological system, and (vi) history of blood transfusion in the last 2 weeks before ICU admission or within first 3 days of ICU admission.
Sample Size
Assuming an incidence of mortality of 50% in sepsis11 and anticipated decrease in mortality by 15% by early prediction, a total of 113 patients would be required with an alpha error of 0.05 and power of 90%. Anticipating a drop put of 10% approximately 125 would be required.
Study Protocol
Sepsis was diagnosed on admission based on the sepsis-3 definition.1 Demographic parameters, diagnosis, SOFA and APACHE II scores were noted at admission. Additional parameters were observed daily starting from day 1 of admission till day-28/death/discharge whichever was earlier. These parameters were daily SOFA scores, mean arterial pressure, vasopressor requirement, lactate, base deficit, coagulation profile, urine output, electrolytes, cumulative fluid balance, renal replacement therapy, kidney function test, liver function test, presence of hypoglycemia, mechanical ventilation with mode, presence of ventilator-associated pneumonia, PaO2/FiO2 ratio, Glasgow Coma Scale, sedation, type of nutritional support.
On admission and on day 3, the following parameters were be noted—hemoglobin, hematocrit, red blood cell count, mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration, RDW, total leukocyte count (TLC), differential leukocyte count (DLC), peripheral blood smear for immature granulocytes, band cells, schistocytes, burr cells, megakaryocytes and immature platelets, platelet count, and PDW.
Statistical Analysis
All recorded variables including outcome data were entered in the Microsoft Excel datasheet. Shapiro–Wilk normality test was used to assess the distribution of all study variables. Continuous variables following normal distribution (such as age, BMI, etc.) were expressed as mean ± standard deviation (SD). Continuous variables not following normal distribution and categorical variables (SOFA score, duration of ICU stay, etc.) were expressed as median with interquartile range (IQR). Binary variables (such as survival at 28 days and requirement of RRT) were expressed as numbers and proportions. Independent sample t-test was used to compare normally distributed continuous variables. Mann–Whitney U test was used for categorical variables and continuous variables not following a normal distribution. Fisher's exact test or chi-square test was used for all binary variables. p-value of less than 0.05 was considered as statistically significant for all tests.
Non-normally distributed variables (duration of ICU stay, duration of vasopressor therapy, duration of mechanical ventilation, platelet count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and differential count of all WBC lineage) were log transformed (log10) for inclusion in the regression model.
Hematological parameters (both at day 1 and day 3) were compared in survivors and nonsurvivors. Parameters where a univariate association was obtained (p <0.20) were included in the binary logistic regression model to identify risk factors of mortality. Adjusted odds ratio with 95% confidence interval and Z score was reported for all variables included in the final model.
For prediction of duration of ICU stay, mechanical ventilation days, and vasopressor days, a stepwise regression model was used with the best predictive model (on the basis of adjusted R2) was used. A generalized regression model with Gaussian distribution was used. The performance of the final model for mortality prediction was analyzed by the receiver operating characteristic (ROC) curve and goodness of fit statistics. All statistical analyzes were conducted in STATA 12 software for Mac OS (Stata Statistical Software: Release 12. College Station, Texas: StataCorp LP.).
Results
A total of 130 patients were recruited and data from n = 129 were analyzed (one patient had day 1 blood sample clotted and hence excluded from analysis). At day-28, n = 58 (44.96%) patients survived. Demographic parameters, severity of illness score, and outcome data are provided in Table 1. Nonsurvivors had longer ICU stay and increased requirement of RRT. Vasopressor days and mechanical ventilation days were similar in both survivors and nonsurvivors. Hematological parameters on day 1 and day 3 are provided in Tables 2 and 3, respectively.
Table 1.
Baseline and outcome data of all patients; data expressed as mean ± SD, median (IQR) or proportions as applicable
| Variable | All patients (n ± 129) | Survivors at day 28 (n ± 58) | Nonsurvivors at day 28 (n ± 71) | Significance (p value) |
|---|---|---|---|---|
| Age (years) | 47.49 ± 18.32 | 45.27 ± 18.61 | 49.30 ± 18.01 | 0.21 |
| Sex | ||||
| Male | 72 (55.81%) | 28 (48.27%) | 45 (61.97%) | 0.06 |
| Female | 57 (44.19%) | 31 (51.73%) | 26 (38.03%) | |
| Bodyweight (kg) | 58.26 ± 9.11 | 57.05 ± 7.86 | 59.25 ± 9.96 | 0.17 |
| Height (cm) | 157.65 ± 8.07 | 156.92 ± 7.61 | 158.19 ± 8.44 | 0.39 |
| BMI (kg/m−2) | 23.35 ± 2.37 | 23.10 ± 2.30 | 23.58 ± 2.44 | 0.25 |
| SOFA at admission | 8 (6–8) | 6 (4–8) | 8 (6–8) | 0.0002 |
| Length of ICU stay (days) | 10 (5–15.5) | 6 (11–20) | 8.5 (4–13) | 0.02 |
| Mechanical ventilation days | 7 (3–12) | 6.5 (3–12) | 8 (4–12) | 0.67 |
| Vasopressor days | 7 (3–12) | 5 (3–12) | 8 (4–12) | 0.21 |
| Requirement of RRT (yes/no) | 43/85 | 14/44 | 29/41 | 0.04 |
Table 2.
Hematological parameters on day 1
| Variable | All patients (n ± 129) | Survivors at day 28 (n ± 58) | Nonsurvivors at day 28 (n ± 71) | Significance |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 9.88 ± 2.16 | 9.94 ± 2.07 | 9.82 ± 2.25 | 0.75 |
| Hematocrit (%) | 30.42 ± 6.88 | 30.24 ± 7.00 | 30.56 ± 6.82 | 0.79 |
| Mean cell volume (fL) | 89.84 ±7.73 | 88.17 ± 8.13 | 91.4 ± 7.05 | 0.02 |
| Mean cell hemoglobin (pg) | 27.50 ± 3.35 | 26.90 ± 2.97 | 28.05 ± 3.60 | 0.06 |
| Red cell count (106/µL) | 3.4 (2.73–4.08) | 3.44 (3.12–4.16) | 3.27 (2.66–3.79) | 0.08 |
| Red cell distribution width (%) | 16.8 (15.25–19) | 16.4 (15–18.5) | 17 (15.6–19.7) | 0.38 |
| Total leukocyte count (103/dL) | 12.70 (7.38–21.10) | 13.35 (9.26–19.12) | 12.16 (5.70–22.09) | 0.78 |
| Differential leukocyte count (%) Neutrophils Lymphocytes (%) Monocytes (%) Basophils (%) Eosinophils (%) |
81.91 ± 14.07 10.36 ± 10.81 6.71 ± 7.68 0.25 ± 0.90 0.62 ± 1.07 |
79.53 ± 14.48 11.25 ± 11.11 7.67 ± 8.22 0.39 ± 1.27 0.868 ± 1.36 |
84.10 ± 13.42 9.54 ± 10.55 5.83 ± 7.10 0.12 ± 0.21 0.39 ± 0.64 |
0.07 0.38 0.18 0.09 0.01 |
| Absolute leukocyte count (cells/dL) Neutrophils Lymphocytes Monocytes Basophils Eosinophils |
10531.5 (6184.1–16712.5) 848.4 (520.46–1579.6) 615.96 (316–954) 8.1 (0–26) 22.4 (0–98.8) |
10755.75 (6600–15931) 1073.2 (557.2–1779.4) 745.2 (396–1174.1) 16.025 (0–33.82) 25.905 (9.1–190.5) |
9537 (4674–20339.2) 728.9 (403.3–1376) 543 (261.82–880) 0 (0–17.3) 15.3 (0–66) |
0.93 0.04 0.04 0.005 0.01 |
| Platelet (103/µL) | 142.5 (83.5–234) | 156.5 (109–282) | 136.5 (53–191) | 0.05 |
| Platelet distribution width (%) | 16.5 (15–18) | 16.55 (15–18) | 16.5 (15–18) | 0.89 |
| Neutrophil–lymphocyte ratio | 17.20 (±17.67) | 14.38 (±12.61) | 19.80 (±21.07) | 0.09 |
| Platelet–lymphocyte ratio | 37.17 (±55.30) | 30.14 (±27.93) | 43.63 (±71.50) | 0.18 |
Table 3.
Hematological parameters on day 3
| Variable | All patients (n ± 129) | Survivors at day 28 (n ± 58) | Nonsurvivors at day 28 (n ± 71) | Significance |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 9.42 ± 1.95 | 9.52 ± 2.01 | 9.31 ± 1.91 | 0.56 |
| Hematocrit (%) | 29.09 ± 6.22 | 29.06 ± 6.29 | 29.13 ± 6.20 | 0.94 |
| Mean cell volume (fL) | 91.09 ± 20.47 | 92.36 ± 28.31 | 89.86 ± 7.36 | 0.51 |
| Mean cell hemoglobin (g/dL) | 27.02 ± 3.04 | 26.73 ± 3.05 | 27.03 ± 3.03 | 0.31 |
| Red cell count (106/µL) | 3.2 (2.87–3.74) | 3.43 (3.08–3.74) | 3.13 (2.7–3.7) | 0.17 |
| Red cell distribution width (%) | 16.8 (15.1–20.1) | 16.45 (15.1–19.0) | 17.2 (15.1–21.4) | 0.30 |
| Total leukocyte count (103/dL) | 12.3 (8.69–18.30) | 12.64(10.5–15.90) | 10.1 (7.17–18.33) | 0.33 |
| Differential leukocyte count (%) Neutrophil Lymphocyte Monocyte Basophil Eosinophil |
80.56 ± 13.14 11.29 ± 9.04 5.99 ± 5.32 0.32 ± 1.06 1.01 ± 1.73 |
79.1 ± 13.86 11.79 ± 7.29 5.73 ± 4.04 0.45 ± 1.35 1.45 ± 1.86 |
81.98 ± 12.36 10.80 ± 10.49 6.24 ± 6.33 0.20 ± 0.66 0.60 ± 1.50 |
0.23 0.55 0.60 0.20 0.00 |
| Absolute leukocyte count (cells/dL) Neutrophils Lymphocytes Monocytes Basophils Eosinophils |
10428.55 (6615–15732) 1117.3 (507–1855.9) 571.95 (314.75–929.25) 0 (0–28.9) 32.85 (0–126.5) |
10428.55 (7592.4–13436.8) 1504.2 (858–1992.6) 666 (413.4–929.25) 13.55 (0–46) 98.35 (14–292) |
10131.35 (5216.26–16683.44) 672.33 (329.34–1574.75) 516.1 (288.5–914.25) 0 (0–15.03) 18.76 (0–65.4) |
0.61 0.001 0.20 0.03 0.001 |
| Platelet (103/dL) | 129 (80–223) | 151 (90–262) | 120 (56–188) | 0.05 |
| Platelet distribution width (%) | 16.1 (14.2–18.0) | 16.1 (14.2–17.5) | 16.35 (14.15–18.2) | 0.67 |
| Neutrophil–lymphocyte ratio | 14.74 ± 15.15 | 10.72 ± 10.30 | 18.62 ± 17.94 | 0.004 |
| Platelet–lymphocyte ratio | 29.68 ± 38.63 | 22.54 ± 20.42 | 36.60 ± 49.58 | 0.04 |
| Delta red cell distribution width (%) | −5.11 ± 55.40 | −10.46 ± 79.36 | −0.02 ± 0.17 | 0.30 |
| Delta platelet distribution width (%) | −0.00 ± 0.12 | 0.00 ± 0.12 | −0.00 ± 0.12 | 0.62 |
On day 1 MCV, platelets, eosinophil percentage in DLC, and absolute counts of lymphocyte, monocyte, basophil, and eosinophil were significantly different between survivors and nonsurvivors. On day 3, platelets, eosinophil percentage in DLC, and absolute counts of lymphocyte, basophil, and eosinophil and NLR and PLR were significantly different.
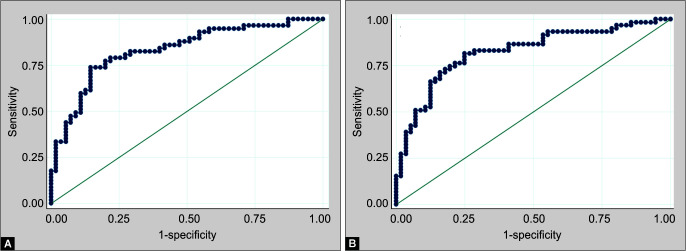
A binary logistic regression model was constructed to predict mortality at 28 days (Table 4; Fig. 1A). Age and low platelet count on day 1 had significant predictive value for 28-day mortality and the model had a sensitivity of 80.7% and specificity of 72.70%.
Figs 1A and B.
ROC curves of binary logistic regression model for predicting 28-day mortality: (A) Model based on day 1 parameters; (B) Model based on day 3 parameters
Table 4.
Predictors of 28-day mortality and other secondary outcomes (binary/generalized logistic regression model)
| Variables | Adjusted odds ratio (95% CI) | Z score | p value |
|---|---|---|---|
| Predictors of 28-day mortality at day 1 | |||
| Age (years) | 1.04 (1.01–1.07) | 2.74 | 0.006 |
| Platelet (103/dL) | 0.99 (0.99–0.99) | –2.22 | 0.02 |
| Predictors of 28-day mortality at day 3 | |||
| Age (years) | 1.02 (1.00–1.05) | 2.30 | 0.02 |
| Absolute eosinophil count (cells/dL) | 0.99 (0.98– 0.99) | −1.99 | 0.04 |
| Predictor of need for renal replacement therapy | |||
| Mean cell volume (fL) | 1.07 (1.00–1.15) | 2.11 | 0.03 |
| Predictor of mechanical ventilation days | |||
| Hemoglobin (g/dL) | 0.91 (0.83–0.99) | −2.01 | 0.04 |
| Neutrophil–lymphocyte ratio | 1.00 (1.00–1.01) | 2.86 | 0.004 |
| Predictor of ICU length of stay | |||
| Mean cell volume (fL) | 1.01 (1.00–1.02) | 2.13 | 0.03 |
| Red cell count (106/dL) | 1.36 (1.10–1.68) | 2.91 | 0.004 |
| Hemoglobin (g/dL) | 0.88 (0.82–0.95) | −3.11 | 0.002 |
| Platelet distribution width (%) | 0.97 (0.95–0.99) | −2.27 | 0.02 |
| Neutrophil–lymphocyte ratio | 1.00 (1.00–1.00) | 2.36 | 0.018 |
| Predictor of vasopressor days | |||
| Mean cell volume (fL) | 1.01 (0.99–1.02) | 1.89 | 0.05 |
| Red cell count (106/µL) | 1.27 (1.00–1.60) | 2.03 | 0.04 |
| Hemoglobin (g/dL) | 0.90 (0.82–0.97) | −2.44 | 0.015 |
| Neutrophil–lymphocyte ratio | 1.00 (1.00–1.01) | 2.95 | 0.003 |
In a binary logistic regression model based on day 3 parameters, age and absolute eosinophil count were found to be significant predictors of 28-day mortality with a sensitivity and specificity of 80.7 and 72.70%, respectively (Table 4, Fig. 1B).
Similarly, logistic regression models were created to predict the requirement of RRT, mechanical ventilation days, vasopressor days, and length of ICU stay (Table 4). MCV was a significant predictor of the RRT requirement. In generalized linear regression model, hemoglobin and NLR at day 1 predicted mechanical ventilation days; hemoglobin, red cell count, MCV, NLR, and PDW on day 1 predicted ICU length of stay and hemoglobin, red cell count, MCV, and NLR on day 1 predicted vasopressor days (Table 4).
Discussion
In this prospective observational study, we identified age, thrombocytopenia at day 1, and decreased absolute eosinophil count at day 3 as the most important prognostic factors for 28-day mortality in sepsis patients.
Martin et al.12 in a retrospective longitudinal study of 10,422,301 adults hospitalized for sepsis over 24 years, found that age could independently predict mortality and case fatality rate increased linearly with age. Moreover, the elderly nonsurvivors of sepsis had a lesser duration of ICU stay implying that they die earlier during their hospital stay. Cohen et al.13 in their study on the effect of age on outcomes of mechanical ventilation in 41,848 patients, observed that elderly population had a poorer outcome of mechanical ventilation when compared to patients <65 years. Yang et al.,14 in a retrospective observational study of 6,929 patients admitted with critical illness, found that patients aged above 65 years had increased mortality and 1.5 times longer hospital stay compared to patients aged 18–54 years. In this study, age was found to be a predictor of 28-day mortality. However, the mean age was 47 years and age did not predict other secondary outcomes.
In the current study, various indices of red blood cells had significance in the prognosis of sepsis. In 815 sepsis patients, Muady et al.15 found that initial hemoglobin at presentation correlated with in-hospital mortality. In the current study, however, presenting hemoglobin was as good as 9.8 g/dL and it did not correlate with mortality at 28 days. However, low hemoglobin on day 1 significantly correlated with the duration of mechanical ventilation, ICU stay, and vasopressor days. In patients with acute respiratory failure, Khamiees et al.16 observed that patients with anemia (Hb <10 g/dL) were five times more likely to be reintubated after initial successful extubation. Anemia is multifactorial in sepsis with various causes being inflammation, reduced erythropoietin production, decreased response of bone marrow to erythropoietin and decreased red cell survival, repeated blood sampling, blood loss due to surgery/trauma, new-onset liver and kidney disease, disseminated intravascular coagulation, hemolysis, hypoadrenalism, hemodilution, and nutritional deficiencies.5
In our study, nonsurvivors had significantly higher MCV compared to the survivors, and MCV was associated with longer ICU stay, RRT requirement, and vasopressor days. No such correlation with mortality was found. On the contrary, Meynaar et al. found that MCV and MCH had no significant correlation with mortality or longer ICU stay or longer mechanical ventilation in 2,915 critically ill patients. Aging RBCs in normal patients shrink due to exposure to oxygen radicals and shear stress17 and an increased MCV may indicate increased reticulocytosis from bleed or hemolysis, vitamin B12 or folate deficiency or liver disease.5 Probably oxidative stress-induced RBC membrane changes leading to loss of deformability and early RBC death in sepsis,18 lead to increased average volume of RBCs. However, further research may be needed to substantiate this rationale. Moreover, survivors had higher red cell count on both day 1 and day 3 and in linear regression models; low red cell count significantly predicted prolonged ICU stay and number of days of vasopressors. As per hemoglobin concentration studies, low red cell count is expected to have poorer prognostication in sepsis.
RDW is an objective measure of the spectrum of RBC sizes and higher RDW reflects more variation of RBC sizes (anisocytosis). RDW is expected to increase in sepsis due to oxidative stress and inflammation-induced changes in RBC characteristics like inhibition of maturation, reduced survival, altered membrane function, and nucleated cells releasing into circulation.18 Moreover, arterial underfilling in sepsis may activate the sympathetic nervous system and renin-angiotensin-aldosterone system, which accelerate erythropoiesis leading to skipped cell division and release of macrocytes.19 Small erythrocytes may reflect shredded red cell vesicles in the deranged microcirculation while the large cells are actually the nucleated early RBCs that have been driven into circulation to compromise for anemia. Bazick et al.,19 in a multicenter study of 51,413 critically ill patients, observed that RDW was a robust predictor of all-cause 30-day mortality, and the mortality risk increased with increasing RDW values in a graded manner above 13.3% with the highest risk observed above 15.8% (OR, 2.61; 95% CI, 2.37–2.86; p <0.001). Kim and colleagues in a prospective observational study on 329 patients of sepsis found that the 28- and 90-day mortality were highest in the subgroup with delta RDW >0.2 % at 72 hours.8 In our study, RDW at day 1 and day 3 and delta RDW did not predict mortality or any other outcome. However, RDW values in both the groups were high (16–17%); nonsurvivors had nonsignificantly higher median RDW values as compared to survivors on both day 1 and day 3. Moreover, delta RDW was 0.2% in nonsurvivors (compared to 0.05% in survivors) but failed to reach any predictive power. Similarly, Fontana et al. in a retrospective study of 122 septic patients did not find any association of RDW with microcirculatory dysfunction or prognosis in sepsis.20 Probably a larger dataset is required to establish the prognostic significance of RDW in sepsis.
Anisopoikilocytosis on day 3 was almost a significant predictor of mortality [OR 9.70 (0.99–95.01); p = 0.05] in the current study. Anisopoikilocytosis in a peripheral smear is suggestive of varied RBC shapes in addition to varied RBC sizes and this could be suggestive of ongoing insults in microcirculation or oxidative membrane stress5 and decreased erythrocyte deformability as a result of anisopoikilocytosis in sepsis may be associated with organ dysfunction and patient outcome.18
Thrombocytopenia may occur in as many as 35 to 59% of patients in sepsis and as per SOFA score denotes deterioration of the function of the overall hematological system.4,5 Primary cause of thrombocytopenia in sepsis is nonimmune destruction and other causes like increased adhesiveness, hemodilution, consumption, sequestration, phagocytosis of platelet precursors in bone marrow by reticuloendothelial cells (hemophagocytic histiocytes), antibodies to platelet antigens like GpIIb/IIIa or Gp Ib/IX, hypoproliferative bone marrow due to cytokines/drugs/pre-existing disease may contribute.4,5 Initially in sepsis, platelet production increases to keep up with the destruction leading to the release of younger but larger platelets in the circulation, but subsequently, bone marrow repression occurs leading to thrombocytopenia.21 This is reflected by the increase of IPF and giant platelets in peripheral smears. In this study, nonsurvivors had a lower platelet count on presentation and on day 3, and thrombocytopenia on presentation predicted 28-day mortality. Similarly, in a prospective, multicenter study of 1,486 patients of septic shock,21 platelet count at day 1 predicted 28-day mortality and the risk of mortality progressively increased with severity of thrombocytopenia (hazard ratio, 1.65; 95% CI, 1.31–2.08 for platelet count <50 × 103/dL vs platelet count >1.50 × 103/dL; p <0.0001).
In the initial stage of sepsis, platelets may change from discoid shape to a spherical shape and form pseudopodia leading larger surface area and thus PDW may increase.22 Moreover, platelet destruction and increased IPF contribute to raised PDW. Guclu et al. found that PDW was more in sepsis than controls and more in nonsurvivors than survivors in a retrospective cohort study and concluded that PDW >18% predicted mortality.22
In our study, PDW on day 1 and day 3 in both survivors and nonsurvivors were within a normal range (10–17.9%) and did not predict mortality. The reason could be the different degrees of thrombocytopenia, the difference in the severity of sepsis (SOFA) score, or the type of patients. In the generalized linear model, however, PDW predicted length of ICU stay, implying the fact that higher PDW reflects a higher degree of inflammation, organ damage, and thereby prolonged ICU stay. In our study, the only day 3 PLR was significantly higher in nonsurvivors, and PLR at neither day 1 nor day 3 was predictive of mortality or other secondary outcomes. Similarly, Biyikli et al. found day 1 PLR was not different between survivors and nonsurvivors in elderly patients with sepsis.23
In our study, the NLR was significantly higher in nonsurvivors on day 3 but not on day 1. Moreover, NLR did not predict mortality but was a significant predictor of duration of mechanical ventilation, vasopressor requirement, and length of ICU stay. In previous studies also, NLR either did not predict24 or was a moderate predictor (AUROC = 0.695) of mortality in sepsis.25 However, NLR predicted weaning failure, increased ventilation days,26 prolonged ICU stay, and persistent organ failure similar to the current study.27
The explanation for this alteration in NLR is that neutrophils, which are the cells of the innate immune system and the first cellular line of defense, usually undergo apoptosis after killing the pathogen. However, if the infection persists, this apoptosis might not occur and simultaneously, the production of neutrophils continues. On the other hand, lymphocytes are involved in the adaptive immune response. Systemic catecholamines and cortisol cause more and more of lymphocytes to undergo apoptosis or migrate to the reticuloendothelial system while increasing the production of neutrophils to clear out pathogens, overall increasing NLR in sepsis.24 Persistently increased NLR, therefore, reflects ongoing sepsis and organ damage thereby increasing ICU morbidity.
TLC more than 12,000/dL or less than 4,000/dL or with >10% immature granulocytes, for long has been a part of diagnostic criteria for systemic inflammatory response syndrome (SIRS). Sepsis used to be diagnosed based on the fulfillment of the SIRS criteria along with a source of infection.28 Gardner et al. observed that leukocyte count <6,000 or >25,000 was predicted 7-day mortality in pneumococcal pneumonia.29 Sepsis in itself can cause bone marrow suppression resulting in leucopenia, which is a poor prognostic sign.29 In another study by Bermejo-Martin et al., level on circulating neutrophil count (CNC) was studied in 195 patients with sepsis.30 They found that patients with CNC <7,226 cells/dL had almost two-fold risk of death. Neutrophils in sepsis may be both harmful (by causing mediators related organ damage) and protective (by defending against pathogens) and lower circulating neutrophils in sepsis could be due to increased adhesion to vascular endothelium. In our study, neither total leukocyte count nor neutrophil count (differential or absolute count) could discriminate survivors from nonsurvivors. On multivariate analysis, the number of total leukocytes and neutrophils did not appear to prognosticate any of the worse outcomes.
In a retrospective observational study in 2,311 patients, Terradas et al. found that persistent eosinophil count below 45 cells/dL was an independent risk factor for mortality, and mean eosinophil count in survivors showed an increasing pattern over 2–3 days.31 Several other studies have also suggested that low eosinophil are suggestive of bacterial infection, even more reliable than CRP.31,32 Abidi et al., in their study of 200 critically ill patients, found that eosinopenia in the first week of admission predicted 28-day mortality and 7th day eosinophil count was significantly less in nonsurvivors.32 In another study in patients with acute exacerbation of COPD, Mat Holland et al. found that eosinopenia was a marker of prolonged ICU stay.33 In the current study, eosinophil count was significantly less in nonsurvivors in day 1 and day 3 and in the binary regression model, day 3 absolute eosinophil count predicted 28-day mortality. It appears that eosinophil count is a promising prognostic variable in sepsis. The pathophysiology of eosinopenia in sepsis includes its sequestration in extravascular tissues and stress hormone influenced migration.33
The role of basophils in sepsis is causing histamine-mediated dilation of capillaries that promotes phagocyte migration to the site of infection.34 In our study, however, survivors had significantly more basophils on both day 1 and day 3. The rationale could be a stronger inflammatory response to fend off infection in sepsis survivors.
In the current study, a significantly higher SOFA score (median score of 8) at presentation was observed in nonsurvivors. In a prospective, observational study in 352 critically ill patients, Ferreira et al. found that the initial or highest score of more than 11 or mean score of more than five corresponded to mortality of more than 80%.35 Both mean and highest SOFA score predicted outcome while an increase in SOFA score during the first 2 days of ICU stay predicted mortality rate of 50% or more.
Strengths and Limitations
All the cellular components of the hematological system were analyzed extensively in the current study and sizable number of patients was studied with almost no data loss in follow-up. Absolute counts of various leukocytes were recorded and analyzed.
Our study had certain limitations. The study population was heterogeneous with a case mix of medical and surgical cases from a single center. Timing of first blood sampling could be anytime within first 24 hours and could not be kept uniform. Some patients would have received antibiotics and fluid resuscitation before sampling. However, the extent of the effect of such intervention on hematological parameters remains uncertain.
Conclusion
Age, thrombocytopenia on day 1, and decreased eosinophil count on day 3 can predict 28-day mortality in adult patients with sepsis.
Orcid
Harsha Sinha https://orcid.org/0000-0002-1938-1785
Souvik Maitra https://orcid.org/0000-0002-2328-9201
Rahul Kumar Anand https://orcid.org/0000-0002-7852-1231
Richa Aggarwal https://orcid.org/0000-0002-4531-2759
Vimi Rewari https://orcid.org/0000-0001-9800-1367
Rajeshwari Subramaniam https://orcid.org/0000-0002-3830-5278
Anjan Trikha https://orcid.org/0000-0002-6001-8486
Mahesh K Arora https://orcid.org/0000-0002-1751-5006
Ravinder K Batra https://orcid.org/0000-0001-8879-0171
Renu Saxena https://orcid.org/0000-0003-1532-297X
Dalim K Baidya https://orcid.org/0000-0001-7811-7039
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Goyette RE, Key NS, Ely EW. Hematologic changes in sepsis and their therapeutic implications. Semin Respir Crit Care Med. 2004;25(6):645–659. doi: 10.1055/s-2004-860979. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Aird WC. The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clin Proc. 2003;78(7):869–881. doi: 10.4065/78.7.869. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83(3):536–545. doi: 10.1189/jlb.0607373. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Levi M, ten Cate H, van der Poll T, van Deventer SJ. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA. 1993;270(8):975–979. doi: 10.1001/jama.1993.03510080079035. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Park JT, Kim EJ, Han JH, Han JS, Choi JY, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care Lond Engl. 2013;17(6):R282. doi: 10.1186/cc13145. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enz Hubert RM, Rodrigues MV, Andreguetto BD, Santos TM, de Fátima Pereira Gilberti M, de Castro V, et al. Association of the immature platelet fraction with sepsis diagnosis and severity. Sci Rep. 2015;5:8019. doi: 10.1038/srep08019. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn C, Kim W, Lim TH, Cho Y, Choi KS, Jang BH. The delta neutrophil index as a prognostic marker for mortality in adults in sepsis: a systematic review and meta-analysis. Sci Rep. 2018;8(1):6621. doi: 10.1038/s41598-018-24211-7. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Cohen IL, Lambrinos J. Investigating the impact of age on outcome of mechanical ventilation using a population of 41,848 patients from a statewide database. Chest. 1995;107(6):1603–1680. doi: 10.1378/chest.107.6.1673. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Yang KS, Hsann YM, Lim V, Ong BC. The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J Crit Care. 2010;25(3):398–405. doi: 10.1016/j.jcrc.2009.09.001. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Muady GF, Bitterman H, Laor A, Vardi M, Urin V, Ghanem-Zoubi N. Hemoglobin levels and blood transfusion in patients with sepsis in Internal Medicine Departments. BMC Infect Dis. 2016;16:569. doi: 10.1186/s12879-016-1882-7. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001;120(4):1262–1270. doi: 10.1378/chest.120.4.1262. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Meynaar IA, Knook AHM, Coolen S, Le H, Bos MMEM, van der Dijs F, et al. Red cell distribution width as predictor for mortality in critically ill patients. Neth J Med. 2013;71(9):488–493. PMID: [PubMed] [Google Scholar]
- 18.Bateman RM, Sharpe MD, Singer M, Ellis CG. The effect of sepsis on the erythrocyte. Int J Mol Sci. 2017;18(9):1932. doi: 10.3390/ijms18091932. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39(8):1913–1921. doi: 10.1097/CCM.0b013e31821b85c6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana V, Savino S, Ottavia B, Cavicchi FZ, Annoni F, Donadello K, et al. No relationship between red blood cell distribution width and microcirculatory alterations in septic patients. Clin Hematol Microcircl. 2017;66(2):131–141. doi: 10.3233/CH-160154. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Thiery-Antier N, Binquet C, Vinault S, Meziani F, Boisramé-Helms J, Quenot J-P, et al. Is thrombocytopenia an early prognostic marker in septic shock? Crit Care Med. 2016;44(4):764–772. doi: 10.1097/CCM.0000000000001520. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. Afr Health Sci. 2013;13(2):333–338. doi: 10.4314/ahs.v13i2.19. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biyikli E, Kayipmaz AE, Kavalci C. Effect of platelet-lymphocyte ratio and lactate levels obtained on mortality with sepsis and septic shock. Am J Emerg Med. 2018;36(4):647–650. doi: 10.1016/j.ajem.2017.12.010. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Ergan B. Can neutrophil-lymphocyte ratio predict disease severity and mortality in sepsis and septic shock patients? Acta Med Mediterr. 2018;34:877. doi: 10.19193/0393-6384_2018_3_134. DOI: [DOI] [Google Scholar]
- 25.Liu X, Shen Y, Wang H, Ge Q, Fei A, Pan S. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediat Inflamm. 2016;2016:8191254. doi: 10.1155/2016/8191254. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Z, Zheng Y, Yang L, Liu S, Zhu J, Zhao N, et al. Neutrophil/lymphocyte ratio is helpful for predicting weaning failure: a prospective, observational cohort study. J Thorac Dis. 2018;10(9):5232–5245. doi: 10.21037/jtd.2018.08.68. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Xu X, Ni H, Deng H. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. J Crit Care. 2014;29(5):885.e1–885.e6. doi: 10.1016/j.jcrc.2014.04.020. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Gardner JG, Bhamidipati DR, Rueda AM, Nguyen DTM, Graviss EA, Musher DM. White blood cell counts, alcoholism and cirrhosis in pneumococcal pneumonia. Open Forum Infect Dis. 2017;4(2):ofx034. doi: 10.1093/ofid/ofx034. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bermejo-Martin JF, Tamao E, Ruiz G, Andaluz-Ojeda D, Herran-Monge R, Muriel-Bombin A, et al. Circulating neutrophil counts and mortality in septic shock. Crit Care. 2014;18(1):407. doi: 10.1186/cc13728. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One. 2012;7(8):e42860. doi: 10.1371/journal.pone.0042860. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12(2):R59. doi: 10.1186/cc6883. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland M, Alkhalil M, Chandromouli S, Janjua A, Babores M. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15(1):165–167. doi: 10.1111/j.1440-1843.2009.01651.x. DOI: [DOI] [PubMed] [Google Scholar]
- 34.Lichtenstein LM, Marone G, Thomas LL, Malveaux LJ. The role of basophils in inflammatory reactions. J Inves Dermatol. 1978;71(1):65–69. doi: 10.1111/1523-1747.ep12544308. DOI: [DOI] [PubMed] [Google Scholar]
- 35.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. DOI: [DOI] [PubMed] [Google Scholar]