Abstract
Background
High-altitude pulmonary edema (HAPE) is a common cause of hospitalization in high altitude areas with significant morbidity. The clinical presentation of HAPE can overlap with a broad spectrum of cardiopulmonary diseases. Also, it is associated with varied radiological manifestations mimicking other conditions and often leading to unnecessary and inappropriate treatment.
Patients and methods
The primary aim of the study was to study the various radiological manifestations of HAPE through real-world chest radiographs. We present six different chest X-ray patterns of HAPE as a pictorial assay, at initial presentation, and after the resolution of symptoms with supplemental oxygen therapy and bed rest alone.
Results
HAPE can present as bilateral symmetrical perihilar opacities, bilateral symmetrical diffuse opacities, unilateral diffuse opacities, bilateral asymmetrical focal opacities, and even lobar consolidation with lower zone or less commonly upper zonal predilection. These presentations can mimic many common conditions like heart failure, acute respiratory distress syndrome, pulmonary embolism, aspiration pneumonitis, pneumonia, malignancy, and tuberculosis.
Conclusion
A holistic clinical–radiological correlation coupled with analysis of the temporal course can help high-altitude physicians in differentiating true HAPE from its mimics.
How to cite this article
Yanamandra U, Vardhan V, Saxena P, Singh P, Gupta A, Mulajkar D, et al. Radiographical Spectrum of High-altitude Pulmonary Edema: A Pictorial Essay. Indian J Crit Care Med 2021;25(6):668–674.
Keywords: Chest X-ray, High-altitude pulmonary edema, Imaging, Radiograph
Introduction
The allure of the mountains and recreational activities attract hundreds of tourists every year to high altitude (HA). Sojourn in these altitudes for occupational reasons is also on the rise (i.e., military deployment, mining, railways, roadways, and bridge construction). The increased accessibility of air travel has also reduced travel times. These factors have contributed to the increased incidence of high-altitude illnesses (HAIs). Though acute mountain sickness resolves with conservative measures and rests over the next few days, high altitude pulmonary edema (HAPE) and high-altitude cerebral edema require urgent specialist treatment.
HAPE is a form of noncardiogenic pulmonary edema.1 It is the most frequent cause of hospital admissions in HA and is potentially fatal.2,3 The clinical presentation of HAPE can masquerade several other conditions like lower respiratory tract infections (pneumonia), pulmonary thromboembolism (PTE), and coronary artery disease (CAD). Extensive radiological studies such as computed tomography or chest ultrasonography are not always possible due to environmental, geographical, and resource constraints. Thus, in real-world HA settings, physicians often have to rely on conventional (nondigital) chest radiographs with limited resolution.
Healthcare facilities in HA are usually handled by young doctors who need to be sensitized to the different presentations of HAPE. This pictorial review covers the protean imaging manifestations of HAPE from real-world settings with limited resources.
Patients and Methods
We present a series of chest radiographs of HAPE patients managed at a secondary hospital located at an elevation of 3500 m. The radiographs have been chosen to represent the different patterns of HAPE presentation. Each pattern is represented by two radiographs, one at diagnosis and another at discharge. These patients were part of the studies published previously.4,5 These radiographs belong to individuals who ascended to HA as part of the occupational sojourn. All these individuals were young healthy males (age range of 24–32 years) who underwent rigorous clinical and laboratory evaluation before ascending to HA for any comorbidities (hematological, biochemical abnormalities, hypertension, diabetes, any past venous thrombosis, cardiovascular diseases, and life-threatening HAI in the past). All these individuals were subjected to the acclimatization for 7 days on the ascent to the HA. All these individuals developed HAPE within 5 days of arrival to HA during the acclimatization schedule in the absence of any known provocative factors (such as unaccustomed exertion, preceding respiratory tract infection, etc.). Clinical manifestations of all these patients included breathlessness and low oxygen saturation levels on pulse oximetry (SpO2). All patients fit into the diagnosis of HAPE as per the Lake Louis diagnostic criteria for HAPE.6 All patients were managed with only supplemental oxygen therapy and bed rest.4 Standard chest radiography was performed using floor-mounted projection radiography (nondigital equipment of 60 kV, 75 mAs exposure time, and film source distance of 6 ft). Standing posterior–anterior (PA) radiographs at end inspiration were done routinely. The study was conducted in accordance with the declaration of Helsinki, and informed consent was taken from all patients.
Results
The radiographical picture of HAPE has a diverse spectrum, as shown in Figures 1 to 10. We describe some of these which can often be confused with other cardiopulmonary conditions.
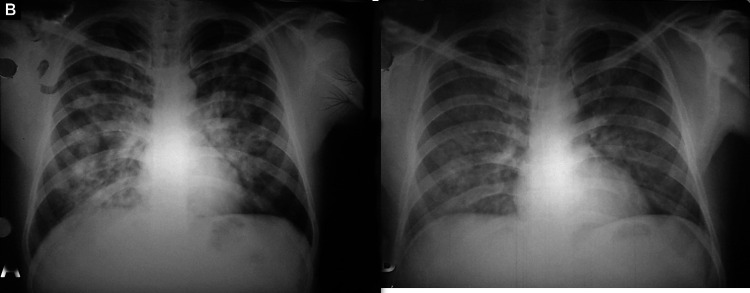
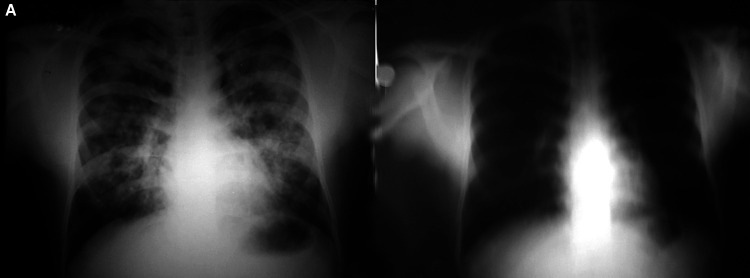
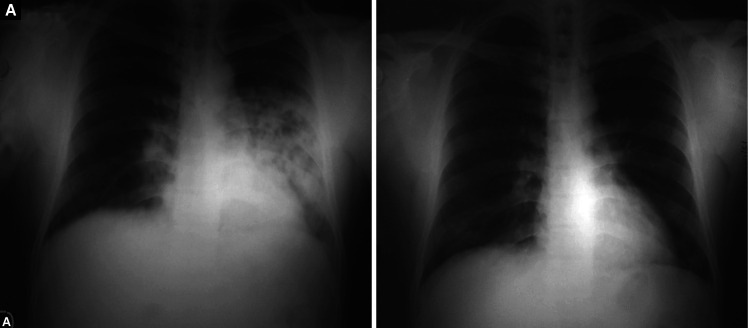
Figs. 1A and B.
(A) Bilateral symmetrical alveolo-interstitial opacities involving all zones with relative sparing of the periphery and apices. The margins of the opacities are ill-defined and distribution is patchy, with confluence in the perihilar region. Horizontal fissure is prominent. Costophrenic angles are sharp and clear and there is no cardiomegaly; (B) Significant resolution of opacities after treatment
Figs. 10A and B.
(A) Consolidation right upper lobe limited by horizontal fissure. Focal air-space opacities right mid-zone. The radiological appearance mimics tuberculosis. There is no cardiomegaly and the costophrenic angles are clear; (B) Significant resolution of opacities after treatment
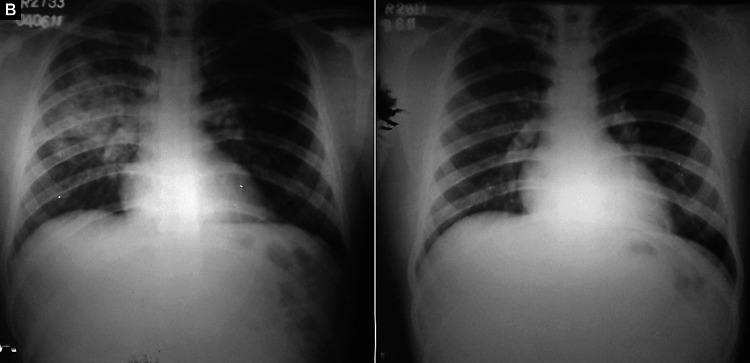
Bilateral symmetrical perihilar opacities (Figs. 1 and 2)
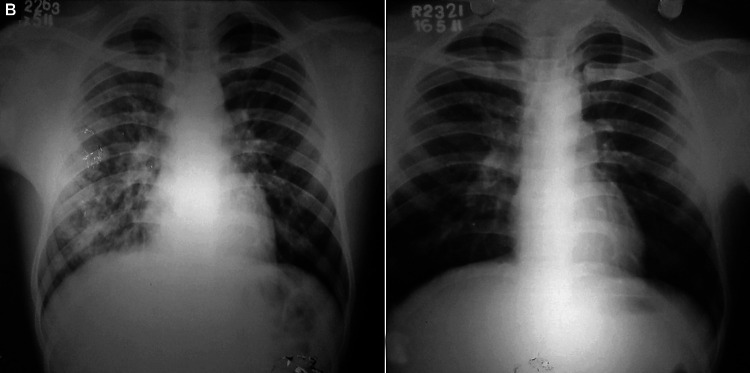
Figs. 2A and B.
(A) Bilateral (minimal) air-space opacities with central, perihilar distribution. Both hila appear prominent. Pulmonary arteries are enlarged with peripheral pruning and relative peripheral oligemia. Costophrenic angles are sharp and clear and there is no cardiomegaly; (B) Resolution of opacities after treatment. Pulmonary vasculature has normalized
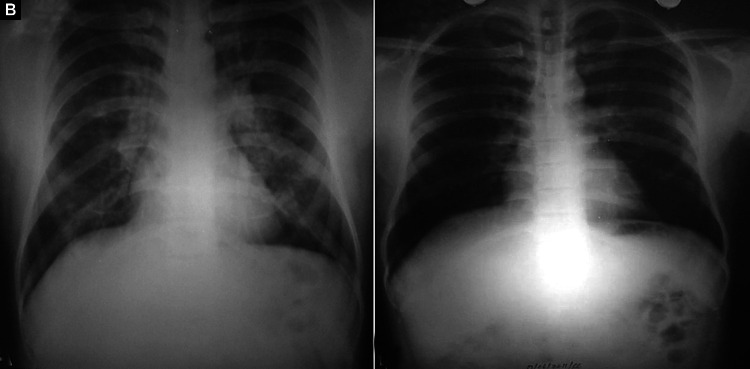
Bilateral symmetrical diffuse opacities (Fig. 3)
Figs. 3A and B.
(A) Bilateral diffuse alveolo-interstitial opacities with no zonal predilection. Unlike the first two figures, the opacities reach up to the periphery. The right descending pulmonary artery appears enlarged. Costophrenic angles are sharp and clear and there is no cardiomegaly. Static electricity artifacts are seen; (B) Resolution of opacities after treatment
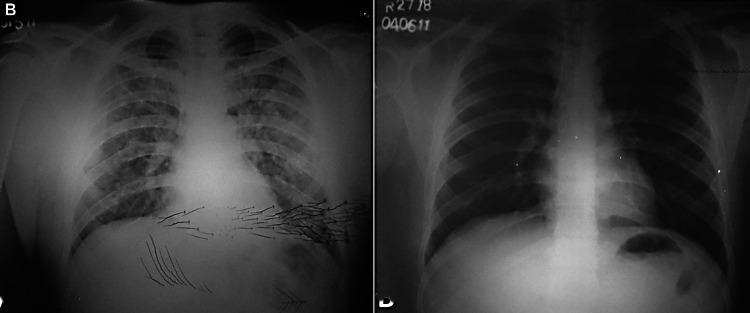
Unilateral diffuse opacities (Figs. 4 and 5)
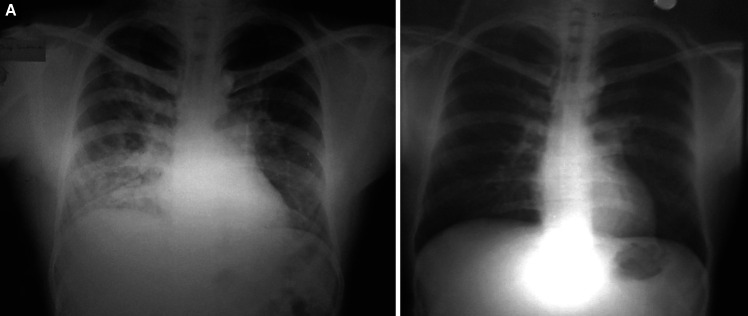
Figs. 4A and B.
(A) Diffuse airspace as well as reticular opacities involving all zones of right hemithorax. The hila and visible pulmonary vasculature are normal bilaterally. Costophrenic angles are sharp and clear; (B) Resolution of opacities after treatment
Fig. 5.
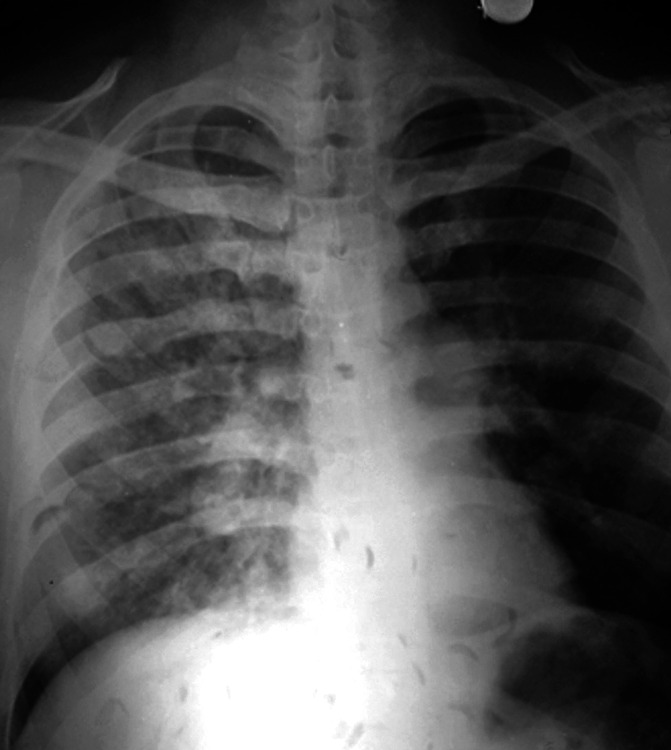
Diffuse alveolo-interstitial opacities involving all zones on the right side. The main pulmonary trunk is prominent (as seen below the aortic knuckle on the left edge of the mediastinal silhouette) and the right descending pulmonary artery is enlarged. Post-treatment radiograph is not available
Bilateral asymmetrical focal opacities (Figs. 6 to 8)
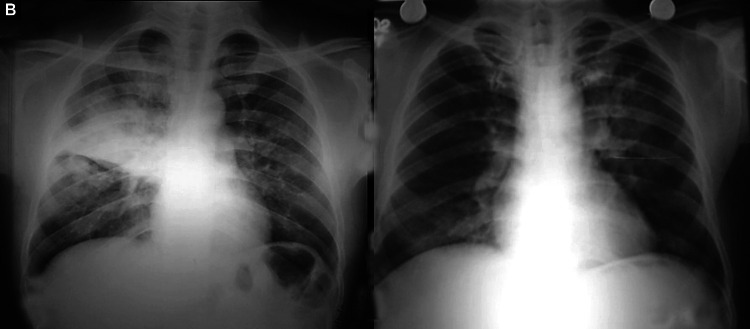
Figs. 6A and B.
(A) Focal patchy airspace consolidation involving right upper, bilateral middle, and left lower zones. Dense consolidation right lower zone with few air-bronchograms; (B) Significant resolution of opacities after treatment
Figs. 8A and B.
(A) Bilateral multifocal asymmetrical air-space opacities with patchy distribution and relative sparing of bilateral apices and costophrenic angles. Focal areas of confluence with some well-defined rounded opacities. Few air-bronchograms are seen. There is no cardiomegaly and the pulmonary vasculature appears normal; (B) Significant resolution of opacities after treatment
Figs. 7A and B.
(A) Dense consolidation right upper lobe limited by horizontal fissure. Patchy alveolo-interstitial opacities bilaterally with sparing of left upper zone and both costophrenic angles. There is no cardiomegaly and the pulmonary vasculature appears normal; (B) Significant resolution of opacities after treatment
Lobar consolidation with lower zone predilection (Fig. 9)
Figs. 9A and B.
(A) Focal consolidation involving left middle and lower zone, with areas of breakdown, mimicking necrotizing pneumonia. The upper cardiac border is silhouetted by the opacity. There is no cardiomegaly and the pulmonary vasculature appears normal; (B) Significant resolution of opacities after treatment
Lobar consolidation with upper zone predilection (Fig. 10)
Discussion
The diagnosis of HAPE as per Lake Louise criteria is based on pulmonary symptoms and signs.6 HAPE may present as cough, initially dry progressing to a productive cough, which may have whitish or blood-tinged sputum. Breathlessness (out of proportion to the amount of exertion), orthopnea, and chest pain are also seen. On examinatin, tachypnea, tachycardia, wheeze, and crackles on auscultation are noted. Often, these cases occur at remote locations with no access to necessary medical facilities. Delay in evacuation to the nearest healthcare facility can be detrimental to the patient. Making a timely correct diagnosis, therefore, assumes utmost importance.
Radiology remains a crucial cornerstone in the diagnosis and management of HAPE. These patients can have clinical–radiological discordance. Lung ultrasonography is a crucial evolving tool in diagnosing HAPE earlier, and its portability can help in field settings in the future.7 We have demonstrated different radiographical manifestations of HAPE. Several differentials for these radiographical patterns are tabulated in Table 1. Reports suggest radiographic evidence of early pulmonary edema even after prolonged high-intensity exertion at HA underlining the importance of chest radiography in this setting.8 Vock et al. also noted many different radio-morphological pictures in HAPE. Radiographic pictures may include unilateral or bilateral, central or peripheral, homogenous, or patchy fluffy opacities, predominantly in the dependent zones of lungs.9
Table 1.
Differential diagnosis of various radiological patterns in HAPE (only common causes enumerated with most important differentials listed first)
| Bilateral symmetrical perihilar opacities | Bilateral symmetrical diffuse opacities | Unilateral diffuse opacities | Bilateral asymmetrical focal opacities | Lobar consolidation |
|---|---|---|---|---|
| Cardiogenic pulmonary edema Diffuse alveolar hemorrhage ARDS Viral pneumonia PJP Acute interstitial pneumonia Acute eosinophilic pneumonia |
ARDS Diffuse alveolar hemorrhage Viral pneumonia PJP Cardiogenic edema Acute hypersensitivity pneumonitis Acute interstitial pneumonia Acute eosinophilic pneumonia |
Unilateral pulmonary edema (as in some cases of mitral regurgitation) Re-expansion pulmonary edema Aspiration pneumonitis Postobstructive pneumonia Bronchogenic carcinoma Pulmonary embolism (oligemia on the affected side may be misinterpreted as diffuse opacity on the unaffected side) |
Bronchopneumonia Tuberculosis Bronchogenic carcinoma with metastasis Pneumoconiosis Septic emboli Allergic bronchopulmonary aspergillosis Sarcoidosis Organizing pneumonia Vasculitis Aspiration pneumonitis |
Pneumonia Bronchogenic carcinoma Aspiration pneumonia Pulmonary embolism Zonal predilection: Upper-tuberculosis, pneumoconiosis, right upper lobe edema in acute mitral regurgitation Lower-aspiration pneumonia, asbestosis |
ARDS, acute respiratory distress syndrome;
PJP, Pneumocystis jirovecii pneumonia
Bilateral diffuse opacities is a common manifestation of HAPE seen in up to 40% patients by Vock et al., 81% patients by Li et al., and 85% patients by Kobayashi et al.9–11 Airspace opacification is sharply confined to central, perihilar regions of the lung with sparing of peripheral or subpleural regions in early cases and extending to the periphery in severe cases.12,13 It is essential to rule out differentials as all the patients with bilateral diffuse radiological opacities at HA need not be HAPE, as was reported in a patient of systemic lupus erythematosus with diffuse alveolar hemorrhage.14 Drowning or immersion pulmonary edema can mimic HAPE with bilateral diffuse opacities.13,15
Cardiogenic pulmonary edema is by far the commonest differential of HAPE (Figs. 1 and 2). The presence of an underlying heart disease, bilateral pleural effusion, and cardiomegaly should arouse the suspicion of this differential. Pleural effusion is rarely seen in HAPE but may be seen when patients have delayed presentations. It is vital to interpret cardiomegaly correctly in an anteroposterior film as may be required on certain occasions when a patient is too critical for a routine standing PA film. Increased cardiothoracic ratios have been reported in HAPE even in the absence of overt cardiogenic edema, especially right ventricle and prominence of the pulmonary vasculature (acute cor pulmonale), which rapidly reverses on recovery.10,16,17 Pulmonary hypertension may be appreciated as prominence of the pulmonary trunk (increased bulge below the aortic knuckle at the left mediastinal border) and central pulmonary arteries with peripheral pruning and enlargement of right descending pulmonary artery (interlobar artery) (Figs. 2, 3 and 5). Most importantly, for the same degree of radiological involvement or hypoxia, a patient with cardiogenic pulmonary edema is much more symptomatic than a patient with HAPE.
Less commonly, the opacities may be more diffuse without any central predilection (Fig. 3), as seen in most cases of acute respiratory distress syndrome and often in viral pneumonia and several noninfective interstitial pneumonitides (Table 1).
Unilateral pulmonary edema as a radiological presentation has been reported in HAPE previously, as also illustrated in Figures 4 and 5 above.18–20 Predominantly unilateral radiographic involvement was variably reported from 15 to 60%.9–11 Right lung predilection and predominance were shown in patients with unilateral and bilateral edema, respectively.9,11 Since HAPE presenting as unilobar or multilobar consolidation is rare, it is imperative to be aware of such a radiological picture (Figs. 6 to 10). Rounded opacities that may be misinterpreted as metastasis can sometimes be seen (Figs. 8). The mechanism of HAPE is uneven sheer stress of pulmonary capillaries along with capillary stress failure in areas of heterogeneous and over-perfused capillaries. Owing to the apicobasal pressure gradient, commonly involved zones are lower lung zones, i.e., the dependent zones. However, it is not unusual for HAPE to involve upper or mid-zone, as seen in Figure 10, and in endemic areas can mimic tuberculosis. In any given individual developing recurring HAPE, there is no predilection to any specific zone, as was reported by Kobayashi et al. in a patient with recurrent HAPE.10 There is no clear pathophysiological mechanism for atypical findings of HAPE. Some authors, though, have suggested a prior trauma or damaged lung tissue as a cause for zone predilection, but the same has not been proven. Asymmetrical perfusion due to a variety of causes like unilateral pulmonary artery agenesis/hypoplasia or unilateral embolism, prolonged lateral decubitus positioning, and cardiac disorders, mainly mitral regurgitation, have been suggested as plausible mechanisms for unilateral pulmonary edema in HAPE. Though none of the patients described in this manuscript had any forthcoming history, clinical findings, or echocardiography features to suggest any of the above.18,19,21–24
The clinical presentation of HAPE can overlap with a broad spectrum of cardiopulmonary diseases. While HAPE can be managed by supplemental oxygen therapy and bed rest with minimal or no pharmacotherapy, more severe disorders like PTE and CAD need urgent specialist care.4 Special caution is required in differentiating pneumonia from HAPE to avoid unnecessary overuse of antibiotics. Owing to the clinical overlap between HAPE and other cardiopulmonary conditions, relying solely on radiographic findings without emphasis on history and examination can lead to wrong diagnosis and poor outcomes. Despite severe hypoxia, HAPE patients do well with oxygen therapy and other conservative measures. Correct diagnosis can avoid unnecessary intubation and other potentially harmful therapeutic measures. A systematic approach is, therefore, warranted in all patients with suspected HAPE while maintaining a low threshold to seek specialist care. Such an approach has been described in detail in the supplement attached.
This study is limited by the relatively poor quality of radiographs with no incidence calculation of different radiological patterns, and the lack of supporting CT scan images. Most areas at such altitudes lack in healthcare infrastructure, and advanced imaging technology is often not available.
Conclusion
No fixed radiographical appearance is associated with HAPE, and it can masquerade many conditions. A high index of suspicion should be maintained in all patients visiting HA, particularly in the first few days of arrival. Unusual radiological presentation of HAPE should be kept in mind when the differential diagnosis is considered in HA settings.
Orcid
Uday Yanamandra https://orcid.org/0000-0002-0546-6585
Vasu Vardhan https://orcid.org/0000-0002-2180-218X
Puneet Saxena https://orcid.org/0000-0003-1111-1008
Priyanka Singh https://orcid.org/0000-0002-2468-8394
Amul Gupta https://orcid.org/0000-0002-0076-9317
Deepak Mulajkar https://orcid.org/0000-0001-9775-0032
Rajan Grewal https://orcid.org/0000-0001-5305-0990
Velu Nair https://orcid.org/0000-0002-8710-1925
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Pennardt A. High-altitude pulmonary edema: diagnosis, prevention, and treatment. Curr Sports Med Rep. 2013;12(2):115–119. doi: 10.1249/JSR.0b013e318287713b. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Luks AM. Do we have a “best practice” for treating high altitude pulmonary edema? High Alt Med Biol. 2008;9(2):111–4. doi: 10.1089/ham.2008.1017. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Swenson ER, Bärtsch P. High-altitude pulmonary edema. Compr Physiol. 2012;2(4):2753–2773. doi: 10.1002/cphy.c100029. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Yanamandra U, Nair V, Singh S, Gupta A, Mulajkar D, Yanamandra S, et al. Managing high-altitude pulmonary edema with oxygen alone: results of a randomized controlled trial. High Alt Med Biol. 2016;17(4):294–299. doi: 10.1089/ham.2015.0120. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Yanamandra U, Sharma M, Katoch D, Yanamandra S, Bhattachar SA, Gupta A, et al. High-altitude pulmonary oedema: Newer treatment modalities for an age-old problem. Indian J Med Res. 2019;149(6):778. doi: 10.4103/ijmr.IJMR_1981_17. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton JR, editor. International Hypoxia Symposium 1991. Lake Louise, Canada: Pergamon Press; 1992. Hypoxia and mountain medicine. [Google Scholar]
- 7.Yang W, Wang Y, Qiu Z, Huang X, Lv M, Liu B, et al. Lung ultrasound is accurate for the diagnosis of high-altitude pulmonary edema: a prospective study. Can Respir J. 2018;2018:5804942. doi: 10.1155/2018/5804942. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anholm JD, Milne EN, Stark P, Bourne JC, Friedman P. Radiographic evidence of interstitial pulmonary edema after exercise at altitude. J Appl Physiol (Bethesda, Md : 1985) 1999;86(2):503–509. doi: 10.1152/jappl.1999.86.2.503. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Vock P, Brutsche MH, Nanzer A, Bärtsch P. Variable radiomorphologic data of high altitude pulmonary edema: features from 60 patients. Chest. 1991;100(5):1306–1311. doi: 10.1378/chest.100.5.1306. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Koyama S, Kubo K, Fukushima M, Kusama S. Clinical features of patients with high-altitude pulmonary edema in Japan. Chest. 1987;92(5):814–821. doi: 10.1378/chest.92.5.814. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Li ZB, Chen HY, Li JY, Li GY, Liu CW, Chen YD. Clinical, laboratory and imaging features of high altitude pulmonary edema in Tibetan Plateau. Chin Med Sci J. 2018;33(3):160–173. doi: 10.24920/11813. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Gallagher SA. Images in emergency medicine. High-altitude pulmonary edema (HAPE). Ann Emerg Med. 2004;44(2):177–187. doi: 10.1016/j.annemergmed.2004.05.010. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Gluecker T, Capasso P, Schnyder P, Gudinchet F, Schaller MD, Revelly JP, et al. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19(6):1507–1531;. doi: 10.1148/radiographics.19.6.g99no211507. discussion 32–33. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Li S, Wang Y, Huang X, Cao J, Yang D. Diffuse alveolar hemorrhage from systemic lupus erythematosus misdiagnosed as high altitude pulmonary edema. High Alt Med Biol. 2015;16(1):67–70. doi: 10.1089/ham.2014.1094. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Lindholm P, Swenson ER, Martínez-Jiménez S, Guo HH. From ocean deep to mountain high: similar computed tomography findings in immersion and high-altitude pulmonary edema. Am J Respir Crit Care Med. 2018;198(8):1088–1089. doi: 10.1164/rccm.201803-0581IM. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Koizumi T, Kawashima A, Kubo K, Kobayashi T, Sekiguchi M. Radiographic and hemodynamic changes during recovery from high-altitude pulmonary edema. Intern Med (Tokyo, Japan) 1994;33(9):525–528. doi: 10.2169/internalmedicine.33.525. DOI: [DOI] [PubMed] [Google Scholar]
- 17.Lankford HV, Swenson ER. Dilated hearts at high altitude: words from on high. High Alt Med Biol. 2014;15(4):511–519. doi: 10.1089/ham.2014.1047. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Cherian SV, Estrada YMRM. Unilateral pulmonary edema after visiting high altitude. Ann Am Thorac Soc. 2017;14(4):589–593. doi: 10.1513/AnnalsATS.201609-726CC. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Domej W, Tilz GP, Dimai HP, Friedl K, Berghold F, Lang JK, et al. Unilateral high-altitude pulmonary edema (HAPE): a case report and discussion of pathophysiology. Wien Klin Wochenschr. 2001;113(3–4):130–133. PMID: [PubMed] [Google Scholar]
- 20.Koul PA, Khan UH, Hussain T, Koul AN, Malik S, Shah S, et al. High altitude pulmonary edema among “Amarnath Yatris”. Lung India. 2013;30(3):193–198. doi: 10.4103/0970-2113.116254. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorenzano G, Rastelli V, Greco V, Di Stefano A, Dottorini M. Unilateral high-altitude pulmonary edema in a subject with right pulmonary artery hypoplasia. Respiration. 1994;61(1):51–54. doi: 10.1159/000196304. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Hackett PH, Creagh CE, Grover RF, Honigman B, Houston CS, Reeves JT, et al. High-altitude pulmonary edema in persons without the right pulmonary artery. N Engl J Med. 1980;302(19):1070–1073. doi: 10.1056/NEJM198005083021907. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Handagala R, Ralapanawa U, Jayalath T. Unilateral pulmonary edema: a case report and review of the literature. J Med Case Rep. 2018;12(1):219. doi: 10.1186/s13256-018-1739-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nana-Sinkam P, Bost TW, Sippel JM. Unilateral pulmonary edema in a 29-year-old man visiting high altitude. Chest. 2002;122(6):2230–2233. doi: 10.1378/chest.122.6.2230. DOI: [DOI] [PubMed] [Google Scholar]