Abstract
Background
Tidal volume challenge pulse pressure variation (TVC-PPV) is considered one of the recent reliable dynamic indices of fluid responsiveness (FR); also, passive leg raising (PLR)-induced changes in cardiac output (CO) detected by echocardiography are considered a reliable reversible self-fluid challenge test; many patients share eligibility for both tests.
Objectives
The study aimed to compare the sensitivity and specificity of both tests for the prediction of FR in mechanically ventilated patients with hemodynamic instability.
Methods
We studied 46 patients. Hemodynamic parameters including PPV and CO (detected by velocity time integral (VTI) using echocardiography) recorded at tidal volume (VT) of 6 mL/kg/ideal body weight (IBW) in semi-recumbent position then recorded again after one-minute increase in TV from 6 to 8 mL/kg/IBW then recorded with PLR at TV of 6 mL/kg/IBW and finally with actual volume expansion in semi-recumbent position by 4 ml/kg bolus of crystalloid solution to define actual responders with increase of cardiac output of 15% or more.
Results
Sixteen patients were responders, and thirty patients were nonresponders; responders had significant increase in PPV with TVC 6 to 8 ml/kg/IBW with best cutoff value of 3.5 with a sensitivity of 93.8% and a specificity of 93.9%. PLR test-induced changes in CO had a sensitivity of 93.9% and a specificity of 86.7% with statistically best cutoff value of 6.5% increase in CO, but sensitivity was 75% at cutoff value of 10% increase in CO. Other parameters like PPV, PPV changes with PLR test, and PPV changes with fluid expansion were less sensitive indicators.
Conclusion
FR in patients with hemodynamic instability and mechanically ventilated with low tidal volume strategy can be efficiently predicted when PPV increases more than 3.5 with tidal volume challenge and when PLR induces 6.5% increase in CO monitored through VTI method by Doppler echocardiography, and both tests are equally reliable.
How to cite this article
Elsayed AI, Selim KAW, Zaghla HE, Mowafy HE, Fakher MA. Comparison of Changes in PPV Using a Tidal Volume Challenge with a Passive Leg Raising Test to Predict Fluid Responsiveness in Patients Ventilated Using Low Tidal Volume. Indian J Crit Care Med 2021;25(6):685–690.
Keywords: Fluid responsiveness, Passive leg raising, Pulse pressure variation, Tidal volume challenge
Introduction
Volume expansion is a cornerstone treatment of acute circulatory failure like during the early hours in patients with septic shock, but it can be a cause of therapeutic dilemma because fluid overload can lead to acute kidney injury, prolongation of mechanical ventilation, acute respiratory distress syndrome (ARDS), and higher mortality rates.1–4 Predicting fluid responsiveness with a reliable, easy, and rapid way is essential to know when to start and when to stop fluid therapy especially as patients may react differently to VE.5 Static hemodynamic parameters like inferior vena cava diameter and central venous pressure are unreliable and cannot predict FR precisely; contrarily, dynamic parameters that depend on cyclic changes in cardiac preload caused by mechanical ventilation, leading to variation of stroke volume (SV) or pulse pressure (PPV), have proven to be reliable when predicting FR in hemodynamically unstable patients.6,7
PPV is a common dynamic parameter that can predict FR reliably and can be recorded through most of recent bedside monitors easily using arterial lines; also, it does not need CO monitoring or any other maneuvers to be done.8,9 One of its important drawbacks, being inaccurate when using low tidal volume strategy, is considered a common ventilation strategy among critically ill patients.10 Some papers have recently shown that using the changes in PPV with tidal volume challenge (TVC-PPV) can overcome these limitations and improve PPV accuracy with low tidal volume strategy. Tidal volume challenge was done by elevating tidal volume from specificity 6 ml/kg IBW to 8 ml/kg IBW for only 60 seconds and noticing the change in PPV (ΔPPV6–8).11
PLR test is considered a reversible “preload challenge” of around 300 ml of blood that can be repeated frequently when required without infusing any fluids.12 It is reliable during low tidal volume ventilation with estimated sensitivity of 85% and of 91% according to many studies13 other reason behind. The popularity of PLR is being reliable in patients with arrhythmias or spontaneous breathing.14
Direct measurement of CO is essential when performing PLR to assess its effects. The mean cutoff value that achieved best sensitivity and specificity was an increase in CO of 10% or more during PLR.14 Multiple minimally invasive or noninvasive techniques have been used to evaluate PLR-induced changes in CO.9,15–17 However, many patients share eligibility for these two common tests that can reliably predict FR in hemodynamically unstable patients on low tidal volume ventilation strategy, and no study has made a direct comparison between them.
Methods
Study Design
Prospective study.
Study Setting
The study was held at Cairo University hospitals, critical care department, between November 2018 and November 2019.
Study Population
After purposeful sampling technique was used and local ethics committee approval, 55 patients were included with obtaining written informed consent.
Inclusion Criteria
Adult patients with hemodynamic instability who are intubated and mechanically ventilated with volume assist-control ventilation (ACV) and using low tidal volume strategy (6 ml/kg/IBW). All patients were fully sedated and not on vasopressors or receiving stable doses of vasopressors.18
Exclusion Criteria
Contraindications for PPV (spontaneous breathing, cardiac arrhythmias, open chest, right-side heart failure, pulmonary or intra-abdominal hypertension, HR/RR < 3.6) or contraindications for PLR (head trauma, venous compression stockings).
Measurements
PPV measurements were obtained by using the Mindray monitors (iMEC10) through radial arterial lines connected. Cardiac output was obtained through VTI assessment by using echocardiography machine through Doppler study which was performed by the same operator using a standard transthoracic probe (P4-2, Siemens Medical System, Malvern, PA, USA) and a dedicated unit (Acuson ×300, Siemens Medical System, Malvern, PA, USA).19
TVC was done by temporarily elevating tidal volume from 6 ml/kg IBW to 8 ml/kg IBW for 60 seconds. PLR test was done by lifting both lower limbs while straight for 45° with the trunk lowered in the supine position for 90 seconds. All other ventilator parameters like respiratory rate and PEEP were unchanged during the study.
CO Measurement by Transthoracic Echocardiography
Stroke volume was obtained through velocity time integral (VTI) of left ventricular outflow tract (LVOT) blood flow using Doppler tracing envelopes. The product of LVOT area and VTI equals the stroke volume. CO can be obtained by multiplying the SV by heart rate. In our study, CO was obtained from the average of three envelopes of LVOT Doppler tracings in each step.
Study Protocol
We recorded five sets of hemodynamic measurements (HR, MAP, PPV, CO, and RR) at different times as shown in protocol sequence (Fig. 1).
Fig. 1.
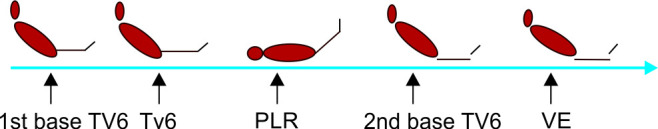
Study protocol and codes.
First group of measurements was obtained in semi-recumbent position (45°) on tidal volume 6 ml/kg/IBW (designated as first base).
Then, TVC was done while the patient is totally sedated, and a second group of measurements was recorded (TVC).
Then, PLR test was done, and a third group of measurements was obtained at the end (PLR).
The patient was then got back to the semi-recumbent position, and a fourth set of measurements was recorded again after 90 seconds (designated as base 2, pre-VE).
Finally, the same measurements were obtained after VE with 4 ml/kg crystalloid solution (ringer lactate or normal saline 0.9%) over 15 minutes (designated as after VE).
Patients were considered responders if cardiac output was increased with volume expansion by more than 15% (from baseline 2 pre-VE). Test was canceled when sudden change in HR occurred, pulmonary edema and severe decrease in blood pressure.
Data Management and Statistical Analysis
Multiple PPV and CO readings were coded as shown in Figure 1. Data were coded and entered using the Statistical Package for the Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY, USA). Comparisons between quantitative variables were done using the nonparametric Mann–Whitney test.20 For comparing categorical data, chi-square (×2) test was performed.21
Results
Study Population
Eligibility process is shown in Flowchart 1. Sixteen patients were responders (Rs), and thirty were nonresponders (NRs). The general characteristics of the responders and nonresponders are reported in Table 1, and none of them was significant. Also, majority of studied patients (40) had septic shock but shock type did not differ significantly between both groups.
Flowchart 1.

Eligibility Process for study population.
Table 1.
Patient characteristics in responders and nonresponders
| Patient characteristics | Responders (n = 16) | Nonresponders (n = 30) | p-value | |
|---|---|---|---|---|
| Gender freq. (%) | Male | 8 (50) | 20 (66.7) | 0.27 |
| Female | 8 (50) | 10 (33.3) | ||
| Age (years) | Mean ± SD | 60.25 ± 13.82 | 59.97 ± 12.98 | 0.954 |
| Ideal body weight (kg) | Mean ± SD | 70.5 ± 7.98 | 66.33 ± 8.57 | 0.13 |
| BMI (kg/m2) | Mean ± SD | 30.31 ± 3.24 | 29.13 ± 3.79 | 0.287 |
| APACHE II score | Mean ± SD | 19.75 ± 6.88 | 19.9 ± 4.99 | 0.711 |
| Shock type | Cardiogenic | 1 (6.3) | 0 (0) | 0.056 |
| Septic | 13 (81.3) | 27 (90) | ||
| Septic and cardiogenic | 0 (0) | 3 (10) | ||
| Septic and hypovolemic | 2 (12.5) | 0 (0) |
Effects of TVC test, PLR, and VE on hemodynamic variables in R and NR are shown in Table 2. Responders had higher PPV0 (at baseline 6 ml TV) with mean value of 16.81 with SD of 7.3 with significant p value of (<0.001) with best cutoff value of 10.5 and area under ROC of 0.78 with a sensitivity and specificity of 87% and 83%, respectively.
Table 2.
Hemodynamic variables and lung compliance between responders and nonresponders
| Parameters | Group | Base 1 | TVC | PLR | Base 2 pre-VE | VE 500 ml |
|---|---|---|---|---|---|---|
| HR (bpm) | R | 101 ± 14 | 100 ± 14 | 102 ± 14 | 104 ± 14 | 98 ± 13 |
| NR | 109 ± 15 | 107 ± 14 | 109 ± 15 | 110 ± 15 | 106 ± 14 | |
| SAP (mm Hg) | R | 95 ± 10 | 90 ± 10 | 97 ± 11 | 97 ± 11 | 104.5 ± 11 |
| NR | 97 ± 12 | 93 ± 12 | 99 ± 12 | 97 ± 12 | 102 ± 13 | |
| MAP (mm Hg) | R | 70 ± 6 | 67 ± 5 | 72 ± 5.5 | 71 ± 6 | 77.5 ± 5 |
| NR | 68 ± 6.5 | 65 ± 6 | 70 ± 6.5 | 68.5 ± 6.5 | 72 ± 6.5 | |
| DAP (mm Hg) | R | 58 ± 6 | 56 ± 6 | 60 ± 5.6 | 60 ± 5.7 | 64 ± 5.5 |
| NR | 54 ± 6 | 51.5 ± 6 | 55 ± 6.5 | 54 ± 6.5 | 57 ± 6.5 | |
| PPV (%) | R | 16.8 ± 7.2 | 22.5 ± 8.8 | 12.3 ± 7.3 | 15 ± 6.8 | 8 ± 1.74 |
| NR | 10.2 ± 5 | 12.2 ± 6 | 8 ± 4 | 9 ± 4.5 | 7 ± 4 | |
| CO (VTI) (ml/m) | R | 4.5 ± 1.20 | 3.92 ± 1.24 | 5.37 ± 1.21 | 4.58 ± 1.15 | 5.54 ± 1.17 |
| NR | 5.08 ± 0.79 | 4.53 ± 0.81 | 5.0 ± 0.78 | 5.0 ± 0.78 | 5.08 ± 0.80 | |
| Compliance (ml/cm H2O) | R | 24.38 ± 3.76 | 29.06 ± 3.71 | – | – | – |
| NR | 29.87 ± 6.95 | 34.73 ± 6.94 | – | – | – |
R: responders; NR: nonresponders; HR: heart rate; SAP: systolic arterial pressure; MAP: mean arterial pressure; DAP: diastolic arterial pressure; PPV: pulse pressure variation; CO: cardiac output; TVC: tidal volume challenge; PLR: passive leg raising; VE: volume expansion
Responders had significant increase in PPV with tidal volume challenge 8 ml (delta PPV1) with best cutoff value of 3.5 with area under the curve of 0.95 with sensitivity of 93.8% and specificity of 93.9%. In our study, we had one case with false-negative test and two cases with false positive test. Also, PPV at 8 ml/kg/IBW predicted responders with p value less than 0.001 (Table 3). At TVC, mean Cstat increased to 29.06 ± 3.71 ml/cm H2O in responders (compared to 24.38 ± 3.76 ml/cm H2O at base one) and 34.73 ± 6.94 ml/cm H2O in nonresponders (compared to 29.87 ± 6.95 ml/cm H2O at base one) (Table 3).
Table 3.
Changes of hemodynamic variables throughout the study between responders and nonresponders
| PPV and CO changes | Responders (n = 16) | Nonresponders (n = 30) | p value |
|---|---|---|---|
| Mean ±SD | Mean ±SD | ||
| PPV base 1 (PPV0) | 16.81 ± 7.3 | 10.23 ± 4.86 | <0.001* |
| CO base 1 (CO0) (l/m) | 4.5 ± 0.21 | 5.09 ± 0.8 | 0.220 |
| PPV with TVC (PPV1) | 22.56 ± 8.8 | 12.27 ± 5.88 | <0.001* |
| TVC PPV (delta PPV1) (PPV1-PPV0) | 5.75 ± 2.35 | 2.03 ± 1.56 | <0.001* |
| CO with TVC (CO1) (l/m) | 3.93 ± 1.25 | 4.54 ± 0.81 | 0.055 |
| PPV with PLR (PPV2) | 12.31 ± 7.35 | 8.07 ± 3.9 | 0.005* |
| PLR-PPV (delta PPV2) (PPV2-PPV0) | 4.5 ± 1.55 | 2.17 ± 1.668 | <0.001* |
| CO with PLR (CO2) (l/m) | 5.23 ± 1.08 | 5.03 ± 0.78 | 0.720 |
| PLR CO (delta CO2) (CO2-CO0) (l/m) | 0.18 ± 0.10 | −0.01±0.1 | <0.001* |
| CO base 2 (pre-VE) (l/m) | 4.58 ± 1.16 | 5.01 ± 0.78 | 0.308 |
| PPV base 2 (pre-VE) | 15.06 ± 6.87 | 9.23 ± 4.48 | <0.001* |
| PPV with VE (PPV3) | 8.13 ± 1.75 | 7.37 ± 4.2 | 0.024* |
| Delta PPV3 (PPV3-PPV pre-VE) | 6.94 ± 5.94 | 1.87 ± 1.11 | <0.001* |
| CO with VE (CO3) (l/m) | 5.54 ± 1.17 | 5.08 ± 0.8 | 0.379 |
| VE-CO (delta CO3) (CO with VE-CO pre-VE) | 0.22 ± 0.07 | 0.02 ± 0.08 | <0.001* |
p-value statistically significant at ?0.05; PPV: pulse pressure variation; CO: cardiac output; TVC: tidal volume challenge; PLR: passive leg raising; VE: volume expansion; SD: standard deviation
During PLR test, there was a significant decrease in PPV (delta PPV2) in responders with 87.5% sensitivity and 80% specificity (best cutoff value 2.5 and area under the curve of 0.877); the mean CO increased significantly with PLR test in responders 0.18 ± 0.10 compared to − 0.01 ± 0.1 in nonresponders; this was highly significant with statistically best cutoff value of 6.5% with 0.94 area under the curve, 93.8% sensitivity, and 86.7% specificity, but sensitivity was 75% with specificity of 86% with cutoff value of 10% (0.099) increase in CO (Table 4).
Table 4.
Diagnostic ability of various variables to predict fluid responsiveness
| 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| Test result variable(s) | Area under the curve | p value | Cutoff | Sensitivity (%) | Specificity (%) | Lower bound | Upper bound |
| PPV (PPV0) | 0.870 | <0.001* | 0.766 | 0.974 | 10.5 | 87.5 | 83.3 |
| TVC-PPV (delta PPV1) | 0.956 | <0.001* | 0.895 | 1.000 | 3.5 | 93.8 | 93.9 |
| PLR-PPV (delta PPV2) | 0.877 | <0.001* | 0.772 | 0.982 | 2.5 | 87.5 | 80 |
| VE-PPV (delta PPV3) | 0.894 | <0.001* | 0.801 | 0.986 | 2.5 | 81.3 | 83.3 |
| PLR-CO (delta CO2) | 0.946 | <0.001* | 0.887 | 1.000 | 0.0659 | 93.8 | 86.7 |
PPV0: (pulse pressure variation at baseline 6 ml/kg/IBW); delta PPV1: (PPV with tidal volume challenge test-PPV 0); delta PPV2: (PPV with PLR-PPV0); delta PPV3: (PPV with VE-PPV pre-VE); delta CO2: (cardiac output with PLR-CO at baseline 6 m/kg IBW)
PPV pre-VE mean value was 15.06 with SD of 6.87, which was significant but did not affect the number of responders and nonresponders; also, CO pre-VE mean value was 4.58 with SD of 1.16 with no significance, which means that the PLR test and tidal volume challenge effects on hemodynamics were rapidly reversible after the end of this test. Changes of PPV test with volume expansion with 500 ml crystalloids (delta PPV 3) showed significant decrease in PPV in responders with sensitivity of 81% and specificity of 83% with area under the curve of 0.89 and best cutoff value of 2.5 (Table 4).
PPV and CO changes with tidal volume challenge, PLR and VE are shown in Table 4. PPV, PPV-TVC, PPV-PLR, CO-PLR, and PPV-VE tests showed statistically significant (p-value ≤0.05) between R and NR as shown in Table 3, which means that they can be used for the discrimination of FR in hemodynamically unstable patients; however, the area under the curve was the highest for PPV-TVC (delta PPV1) 0.95 and CO-PLR (delta CO2) 0.94.
Discussion
Our study demonstrates that FR in hemodynamically unstable patients who are mechanically ventilated using low tidal volume can be efficiently predicted when PPV increases for more than 3.5 with tidal volume challenge and when PLR induces 6.5% increase in CO monitored through VTI method by Doppler echocardiography. And both tests are equally reliable with a comparable AUC.
In our study, responders had a significant increase in PPV with tidal volume challenge from 6 to 8 ml/kg IBW (delta PPV1) with best cutoff value of 3.5 with area under the curve of 0.95 with a sensitivity of 93.8% and a specificity of 93.9%. Myatra et al. found that PPV with TVC strongly predicted FR with AUROC of 0.99 with a cutoff value of 3.5;11 the difference in specificity may be because we had relatively larger number of cases.
In agreement with our study, some studies have confirmed the reliability of delta PPV with tidal volume challenge 8 to 12 ml/kg for the detection of FR in patients with gray zone PPV (9–13) in perioperative settings and when VT was increased from 6 to 10 ml/kg PBW in critically ill patients.22,23 Also in operating room, PPV changes with TVC from 6 to 8 ml/kg/PBW have reliably predicted FR in neurosurgical patients and also during robot-assisted laparoscopic surgery in the Trendelenburg position with lung-protective ventilation.24,25 The reason behind improved reliability of PPV with tidal volume challenge is that low tidal volume might be insufficient to make a considerable change in the intrathoracic pressure.26
PPV can be unreliable in ARDS patients as the transmission of airway pressure is reduced with a lower intrathoracic pressure changes in these patients due to low lung compliance (Cstat).26 PPV reliability is reduced when static lung compliance (Cstat) is less than 30 ml/cm H2O.23,26 In our study, Cstat had increased to greater than 30 ml/cm H2O (32.76 ± 6.56) after the “tidal volume challenge.”
We found that PPV at 6 ml/kg IBW tidal volume predicted FR with sensitivity of 87% and specificity of 83% with cutoff value of 10.5. Myatra et al. found that PPV at low tidal volume of 6 ml/kg PBW did not predict fluid responsiveness with AUROC of 0.69. This difference may be explained by our greater mean of PPV 16.81 ± 7.3 compared to 8 ± 3, which is nearly double their patient's number.11 We did not limit our study to patients in gray zone of PPV (10–13) to detect the actual sensitivity and specificity of each test in hemodynamically unstable patients. Even though PPV alone showed good reliability in our group of patients, reliability has increased with TVC. The ΔPPV with fluid challenge also reliably discriminated FR with sensitivity and specificity of 93% and 86%, respectively; however, Myatra et al. reported 94% sensitivity and 100% specificity with 0.98 AUC.11
In our study, PLR test-induced changes in CO detected by echo VTI method predicted FR with a sensitivity of 93.9% and specificity of 86.7% with statistically best cutoff value of 6.5% increase in CO, with a less practical clinical significance, as it is difficult to detect such changes in CO using echocardiography VTI method and may be time-consuming, also when considering cutoff value of 10% increase in CO; sensitivity decreased to 75%. Thiel et al. reported that SV rises by 15% or more during PLR predicted FR with a sensitivity and specificity of 81% and 93%, respectively.15 Although direct CO measurement with thermodilution methods is reliable and easier than echocardiography, it is not always available.
In our study, we had a significant decrease in PPV with PLR (delta PPV2) in responders with 87.5% sensitivity and 80% specificity (best cutoff value 2.5 at AUC of 0.877). These data are consistent with Lamia et al. study which demonstrated that cardiac output changes or its directly derived parameters such as SV had a higher diagnostic significance than pulse pressure (PP) changes with PLR.17 Also during sepsis, pulse pressure is poorly correlated with stroke volume because of high total arterial compliance.27,28
Strengths of the Study
Ventilating patients on low tidal volume is a common practice today, and many of these patients share eligibility for PLR test and TVC-PPV. This is probably the first study with a direct comparison between these two common and reliable tests.
Study Limitations
PPV is unreliable in patients with cardiac arrhythmias or spontaneous breathing, and the use of tidal volume challenge cannot bypass these limitations. Echocardiography cannot be done in patients with poor window and does not give a direct measure of CO and needs some experience. Also, patients with PPV in a gray zone (9–13) need further study to confirm our findings.
Conclusion
Fluid responsiveness in hemodynamically unstable patients ventilated with low tidal volume strategy can be efficiently predicted when PPV increases more than 3.5 with tidal volume challenge and when PLR induces 6.5% increase in CO monitored through VTI method by Doppler echocardiography, and both tests are equally reliable. Being simple, easily interpreted, and does not need CO monitoring, we recommend the use of TVC-induced changes in PPV.
Acknowledgments
The research team especially thanks each and everyone in the critical care department at Kasralainy hospitals for their utmost help and welcome that was priceless for the completion of the practical part of this research.
Orcid
Ahmed I Elsayed https://orcid.org/0000-0001-7495-3494
Khaled AW Selim https://orcid.org/0000-0003-3679-3586
Hanan E Zaghla https://orcid.org/0000-0001-8840-498X
Hossam E Mowafy https://orcid.org/0000-0003-3333-0754
Mohammed A Fakher https://orcid.org/0000-0002-7200-0806
Footnotes
Source of support: Nil
Conflict of interest: None
Ethics approval and consent for participation: Applied.
References
- 1.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17(5):R246. doi: 10.1186/cc13072. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients-a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–1870. doi: 10.1097/CCM.0000000000004617. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med. 2013;41(2):472–480. doi: 10.1097/CCM.0b013e31826ab377. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Michard F, Teboul J-L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–2008. doi: 10.1378/chest.121.6.2000. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642–2647. doi: 10.1097/CCM.0b013e3181a590da. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016;6(1):1–11. doi: 10.1186/s13613-016-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasanin A. Fluid responsiveness in acute circulatory failure. J Intensive Care. 2015;3(1):50. doi: 10.1186/s40560-015-0117-0. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent J-L. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517–523. doi: 10.1007/s00134-005-2586-4. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Myatra SN, Monnet X, Teboul JL. Use of ‘tidal volume challenge’ to improve the reliability of pulse pressure variation. Crit Care. 2017;21(1):60. doi: 10.1186/s13054-017-1637-x. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarado-Sánchez JI. The passive leg raising test (PLR). Colomb J Anesthesiol. 2015;43(3):214–218. doi: 10.1016/j.rcae.2015.03.005. DOI: [DOI] [Google Scholar]
- 13.Cherpanath TGV, Hirsch A, Geerts BF, Lagrand WK, Leeflang MM, Schultz MJ, et al. Predicting fluid responsiveness by passive leg raising: a systematic review and meta-analysis of 23 clinical trials. Crit Care Med. 2016;44(5):981–991. doi: 10.1097/CCM.0000000000001556. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Monnet X, Marik P, Teboul J-L. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1935–1947. doi: 10.1007/s00134-015-4134-1. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Thiel SW, Kollef MH, Isakow W. Non-invasive stroke volume measurement and passive leg raising predict volume responsiveness in medical ICU patients: an observational cohort study. Crit Care. 2009;13(4):R111. doi: 10.1186/cc7955. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafanechère A, Pène F, Goulenok C, Delahaye A, Mallet V, Choukroun G, et al. Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care. 2006;10(5):1–8. doi: 10.1186/cc5044. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul J-L. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125–1132. doi: 10.1007/s00134-007-0646-7. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Vincent J-L, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Tan C, Rubenson D, Srivastava A, Mohan R, Smith MR, Billick K, et al. Left ventricular outflow tract velocity time integral outperforms ejection fraction and Doppler-derived cardiac output for predicting outcomes in a select advanced heart failure cohort. Cardiovasc Ultrasound. 2017;15(1):18. doi: 10.1186/s12947-017-0109-4. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFarland,, Thomas W., Jan M. Yates. “Mann–whitney u test.”. https://doi.org/10.1007/978-3-319-30634-6 Introduction to nonparametric statistics for the biological sciences using R. Springer, Cham, 2016:103–132. DOI: [Google Scholar]
- 21.Bewick V, Cheek L, Ball J. Statistics review 8: Qualitativedata – tests of association. http://dx.doi.org/10.1186/cc2428. Crit Care. 2004;8:46–53. doi: 10.1186/cc2428. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min JJ, Gil NS, Lee JH, Ryu DK, Kim CS, Lee SM. Predictor of fluid responsiveness in the ‘grey zone’: augmented pulse pressure variation through a temporary increase in tidal volume. Br J Anaesth. 2017;119(1):50–56. doi: 10.1093/bja/aex074. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Charron C, Fessenmeyer C, Cosson C, Mazoit J-X, Hebert J-L, Benhamou D, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511–1517. doi: 10.1213/01.ane.0000209015.21418.f4. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Messina A, Montagnini C, Cammarota G, De Rosa S, Giuliani F, Muratore L, et al. Tidal volume challenge to predict fluid responsiveness in the operating room. Eur J Anaesthesiol. 2019;36(8):583–591. doi: 10.1097/EJA.0000000000000998. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Jun, J-H, Chung RK, Baik HJ, Chung MH, Hyeon J-S, Lee Y-G, et al. The tidal volume challenge improves the reliability of dynamic preload indices during robot-assisted laparoscopic surgery in the Trendelenburg position with lung-protective ventilation. BMC Anesthesiol. 2019;19(1):142. doi: 10.1186/s12871-019-0807-6. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teboul, J-L., Monnet X. Annual Update in Intensive Care and Emergency Medicine 2011. Springer; Berlin, Heidelberg: 2011. “Meaning of pulse pressure variation during ARDS.”; pp. 322–331. DOI: [Google Scholar]
- 27.García MIM. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015;41(7):1247–1255. doi: 10.1007/s00134-015-3898-7. DOI: [DOI] [PubMed] [Google Scholar]
- 28.Cherpanath TGV, Smeding L, Lagrand WK, Hirsch A, Schultz MJ, Groeneveld JAB. Pulse pressure variation does not reflect stroke volume variation in mechanically ventilated rats with lipopolysaccharide-induced pneumonia. Clin Exp Pharmacol Physiol. 2014;41(1):98–104. doi: 10.1111/1440-1681.12187. DOI: [DOI] [PubMed] [Google Scholar]


