Abstract
Background/Aim: We aimed to determine the diagnostic value of the synovial aspiration culture prior to reimplantation in two- (or more) stage exchange of periprosthetic joint infection. Patients and Methods: This was a retrospective study, spanning over ten years including all synovial cultures of patients with two- (or more) stage exchange due to periprosthetic joint infection. Results: A total of 183 patients were included, mean age was 66.6 years (range=12.8-93.4 years). Overall sensitivity of synovial aspiration cultures before reimplantation was 56.6%, specificity 84.6%, negative predictive value (NPV) 63.8%, positive predictive value (PPV) 80.2%, area under the curve (AUC) 70.6%. Sensitivity of the knee in comparison to the hip culture was significantly higher, as well as the NPV and the AUC (p=0.038). In case of complete removal of prosthesis, the sensitivity and AUC were significantly reduced, whereas the specificity was comparable with prosthesis in situ, partial removal or complete removal. Conclusion: Due to the low sensitivity, obtaining several synovial cultures in the prosthesis-free interval to exclude persistence of infection, does not seem reasonable.
Keywords: Arthroplasty, periprosthetic joint infection, synovial culture, diagnostic value, rule-out test, implant removal
Together with aseptic loosening periprosthetic joint infection (PJI) is the second most common reason for implant failure in arthroplasty (1). The incidence ranges from approximately 1% in primary arthroplasty and up to 6% in case of revision arthroplasty (2-5) and the treatment of PJI can be challenging, long and leads to high socio-economic costs (1). In order to perform a purposeful therapy, a sufficient diagnostic work-up with identification of the pathogenic agent is indispensable (6). The synovial culture is one of the essential components in the diagnostic field of PJI (7,8). It is a fast, minimally-invasive and low-cost method and allows not only the diagnosis of PJI but also the identification of the pathogenic agent and its resistogram (3,7,8). Although multiple studies address the diagnostic value of the synovial aspiration cultures for primary diagnostic of PJI, data regarding the value during the treatment process is inconsistent. Questions regarding the value of aspiration culture before reimplantation in two or more stage exchanges, the number of aspirations and the influence of antibiotic treatment on diagnostic performance remain unanswered.
The aim of this study was to determine the diagnostic value of the synovial aspiration culture in the process of the complex PJI treatment, with the main focus on hip and knee arthroplasty and the prosthesis-free interval. Therefore, we conducted this retrospective study over ten years on a Level-I university centre on patients treated for periprosthetic infections of the hip and knee. We hypothesize that synovial aspiration culture prior to reimplantation is not suitable to rule out a persistent infection.
Patients and Methods
Ethical approval. Approval for this study was obtained from our Institution’s Ethical Committee as a waiver. Due to the retrospective nature of the study our hospital’s general consent for utilization of anonymized research data was eligible.
Study design. Retrospective identification of all cases between January 2005 and December 2015 with the diagnosis “infection or inflammatory reaction due to internal joint prosthesis” (T84.5 in ICD-10 classification) in a level I trauma centre and at least one surgical procedure related to this diagnosis. Among these, all patients with periprosthetic joint infection were selected according the criteria defined below.
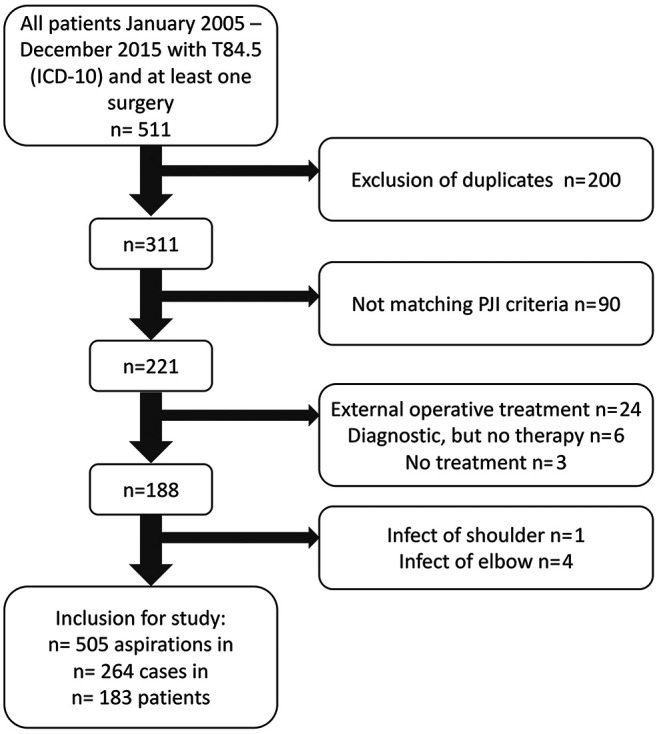
Inclusion was done according to the flow chart (Figure 1). A case was defined as a complete treatment period of the infectious arthroplasty from first diagnosis until infection eradication was achieved. As soon as recurrence of infection occurred, a new case was created. Thus, one patient could account for more than one case.
Figure 1. Flowchart for patient inclusion and exclusion. After exclusion criteria 183 patients could be included, resulting in 264 cases, as some patients had recurrent infection and could be included for more than one case.
Periprosthetic Joint Infection (PJI). PJI was defined according to a modified consensus classification of the Musculoskeletal Society when one of the following parameters was fulfilled (7):
• Sinus tract or open wound in communication with the prosthesis;
• Intraoperative purulence;
• At least two positive tested intraoperative cultures or one positive highly pathogenic agent;
• Histological detection of a periprosthetic membrane type II or III in Krenn and Morawietz classification (9,10).
Definition of prosthetic-infection timing. Early (3 Month), delayed (3-24 month) and late (>24 month) infect were assessed according to Zimmerli et al. (5,11).
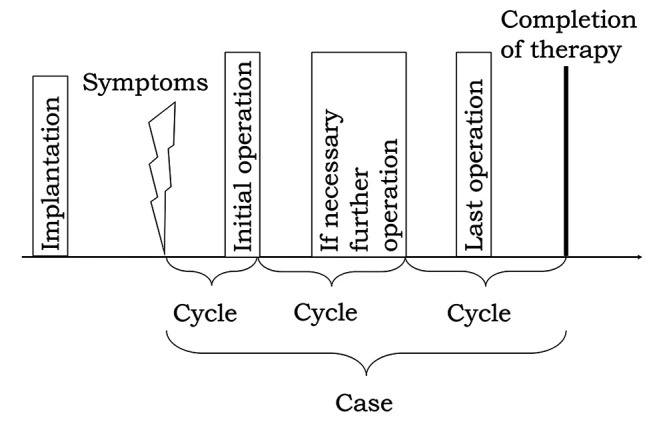
Collection of data. General patient data, medical history and blood levels of infectious parameters (serum C-reactive protein (CRP) and peripheral blood leukocytes) were collected from the patients’ records. As there are multiple synovial aspirations within one case, it was necessary to subdivide every case in defined cycles as shown in Figure 2. The first cycle was from beginning of symptoms to the end of the first operation. Right after that, the next cycle starts and ends with the next operation. The last cycle ends with the termination of the case including the postoperative phase.
Figure 2. Case definition. The first cycle was from beginning of symptoms to the end of the first operation. Then, the next cycle starts and ends with the next operation. The last cycle ends with the termination of the case including the postoperative phase. All cycles together are one case.
Data analysis and statistics. For evaluation of the diagnostic value of the synovial culture every cycle was rated independently for presence of infection according to Renner et al. (7). A PJI was assumed if at least one of the above-mentioned criteria was positive.
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc., La Jolla, USA) and Vassarstats (www.vassarstats.com). Statistical significance was considered for a p-value less than 0.05 (p≤0.05), or if the 95% confidence intervals (CI) had no overlapping. Furthermore, sensitivity, specificity, positive (ppv) and negative predictive value (npv) as well as area under the curve (AUC) was determined as ROC curve with GraphPad Prism.
Results
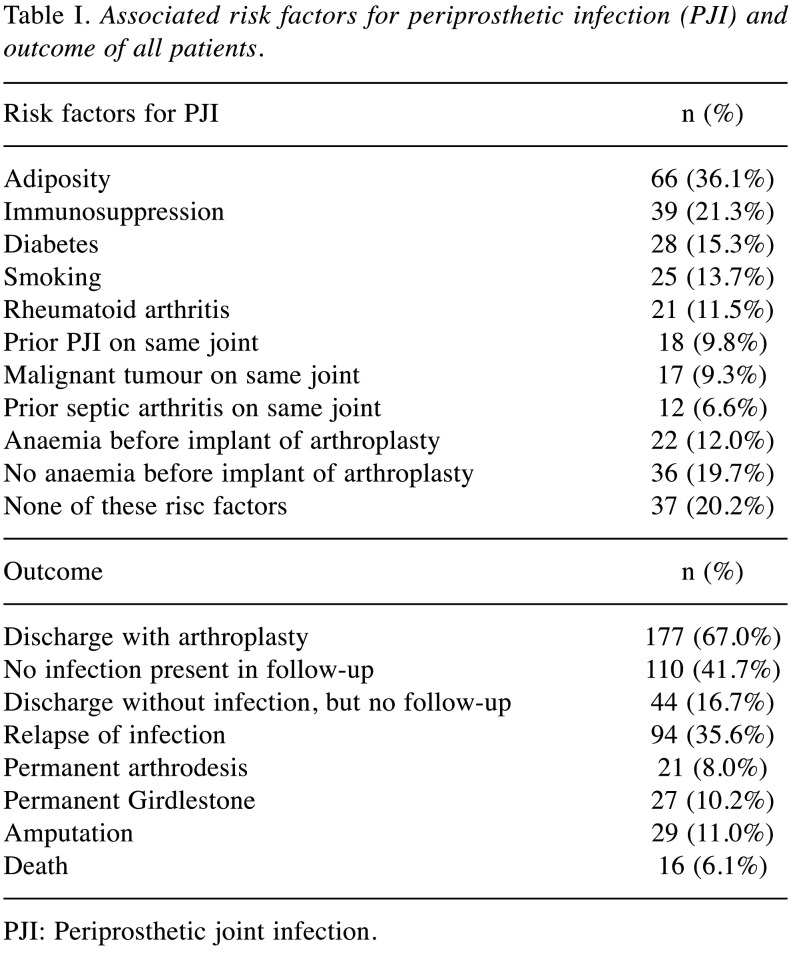
General data. A total of 183 patients were included. Of these, there were 95 (51.9%) female and 88 (48.1%) male patients. In n=108 (59.0%) patients there was an infection of the hip, in n=73 (39.9%) patients an infection of the knee and in n=2 (1.1%) patients an infection of both hip and knee. The mean age was 66.6 (12.8-93.4) years. Fifty patients (27.3%) were included for more than one case, which leads to 1.44 (min/max 1-7) included cases per patient. Table I shows the associated risk factors and outcomes.
Table I. Associated risk factors for periprosthetic infection (PJI) and outcome of all patients.
PJI: Periprosthetic joint infection.
Cycles and cases. Overall, 505 synovial aspiration cultures could be included. Regarding the above-mentioned case definition, this leads to 264 cases. There were 126 (47.7%) female and 138 (52.3%) male patients. In n=165 (62.5%) cases there was an infection of the hip, in n=99 (37.5%) cases an infection of the knee and in n=134 (50.8%) cases there had been a prior revision. The mean number of prior revisions was n=3.58 (min/max 1-28). In n=104 (39.4%) we found an early infection, in n=112 (42.4%) a delayed infection and in n=48 (18.2%) of the cases a late infection.
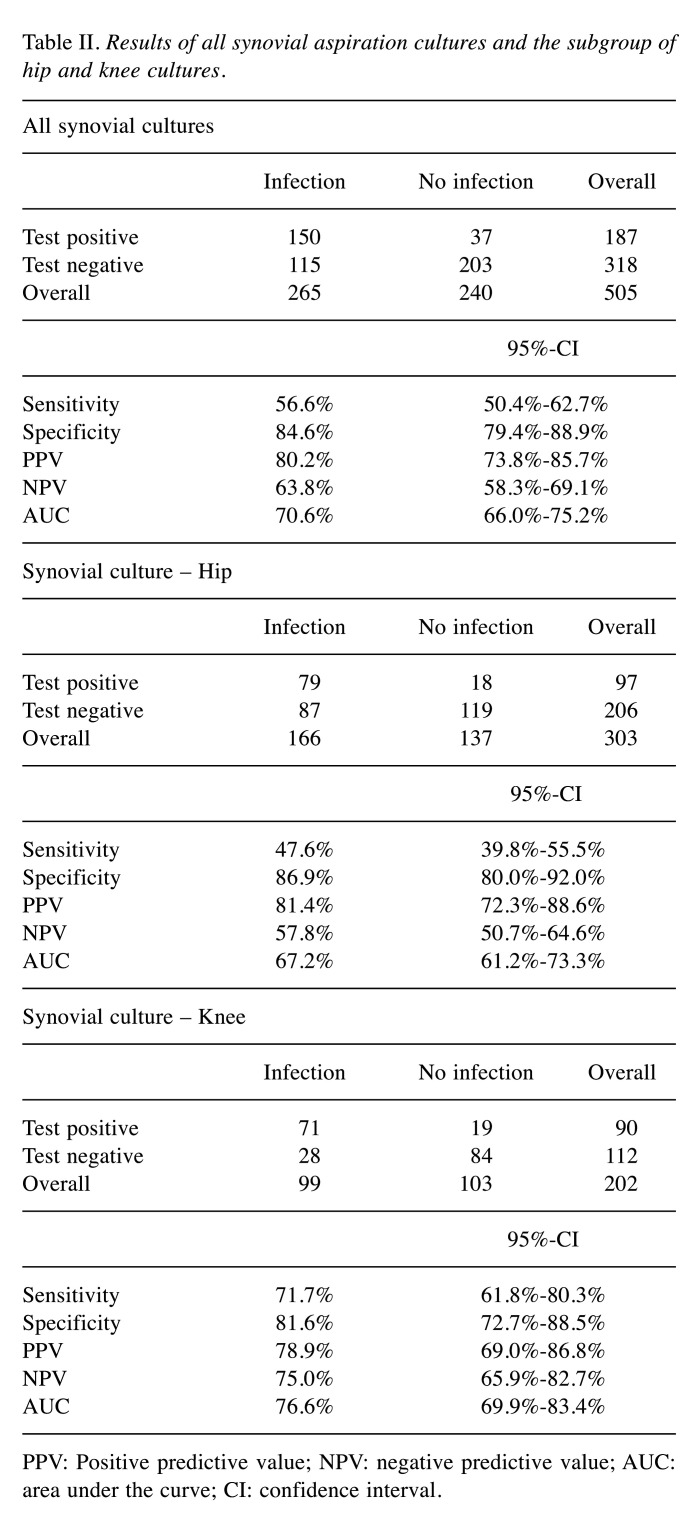
Diagnostic value of synovial culture aspiration. Table II shows the results for synovial cultures overall and itemized for hip and knee. In comparison to the hip culture the sensitivity for the knee culture was significantly higher, as well as the NPV and the AUC (p=0.038).
Table II. Results of all synovial aspiration cultures and the subgroup of hip and knee cultures.
PPV: Positive predictive value; NPV: negative predictive value; AUC: area under the curve; CI: confidence interval.
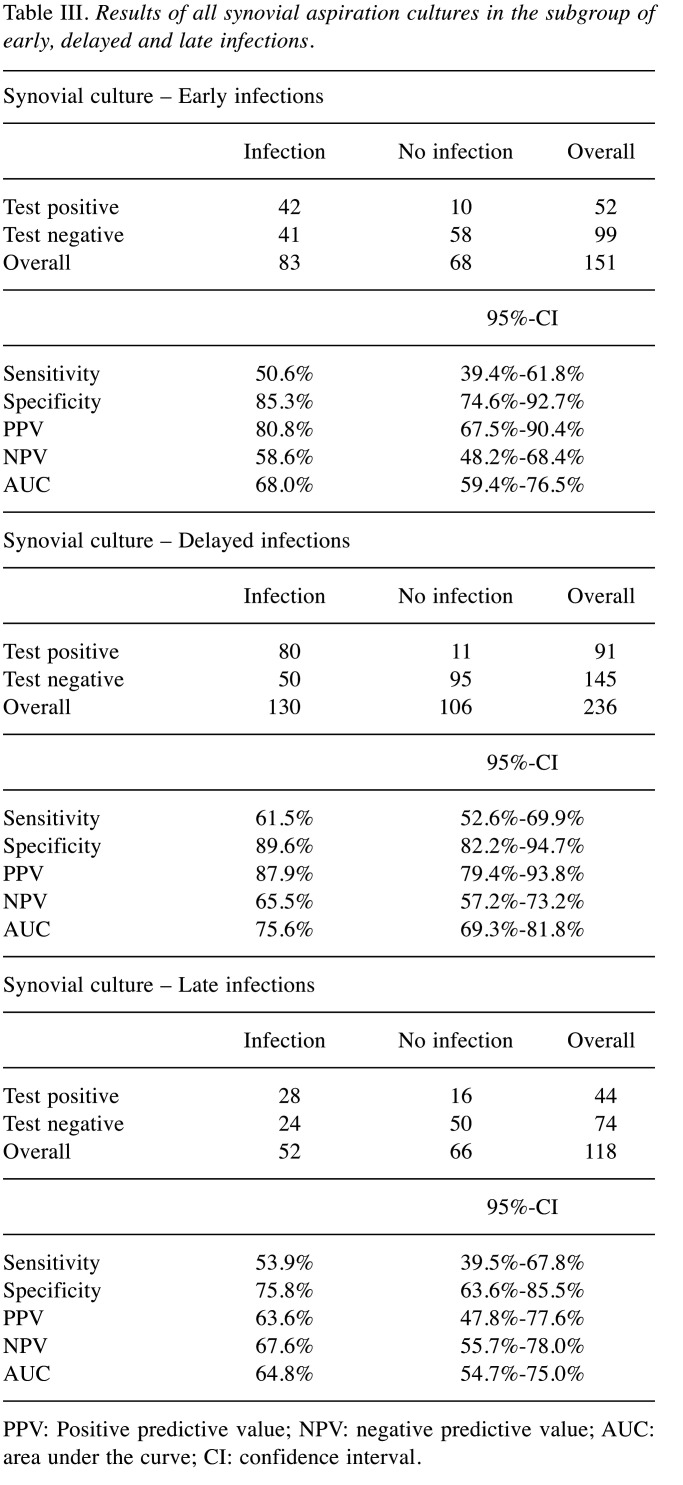
Diagnostic value of synovial culture aspiration in view of time point of infection. In Table III the value of synovial culture for different time points of PJI are presented. We were unable to find any significant differences.
Table III. Results of all synovial aspiration cultures in the subgroup of early, delayed and late infections.
PPV: Positive predictive value; NPV: negative predictive value; AUC: area under the curve; CI: confidence interval.
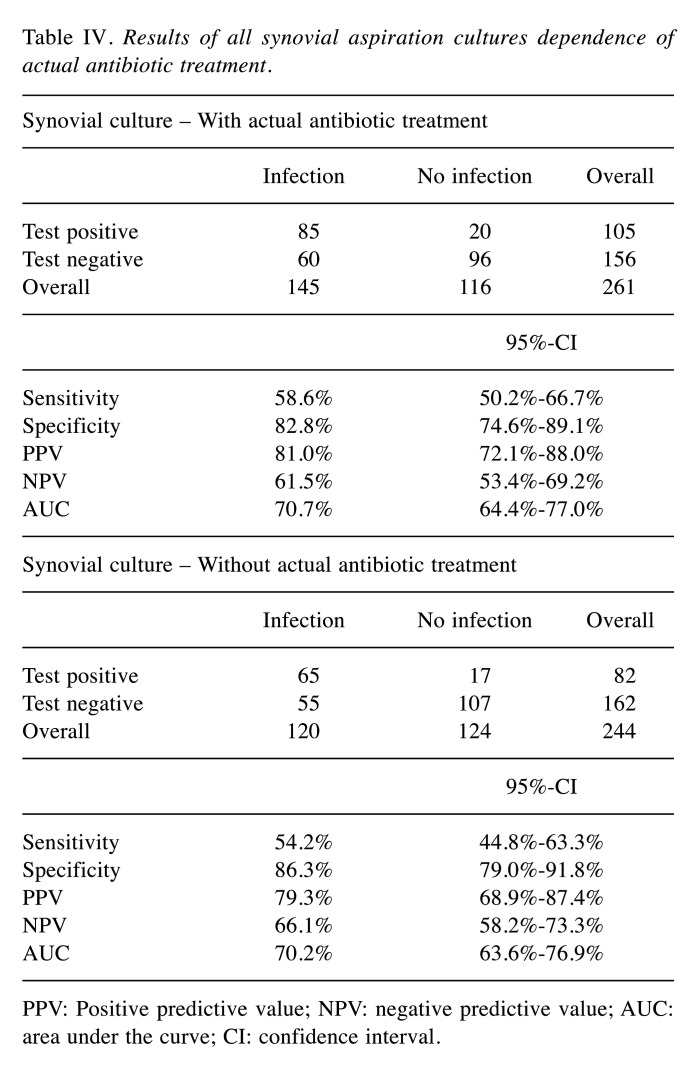
Diagnostic value of synovial culture aspiration in view of antibiotic treatment. There were no significantly different values for synovial culture aspiration with or without (at least two weeks without antibiotics) ongoing antibiotic treatment. Table IV shows the results.
Table IV. Results of all synovial aspiration cultures dependence of actual antibiotic treatment.
PPV: Positive predictive value; NPV: negative predictive value; AUC: area under the curve; CI: confidence interval.
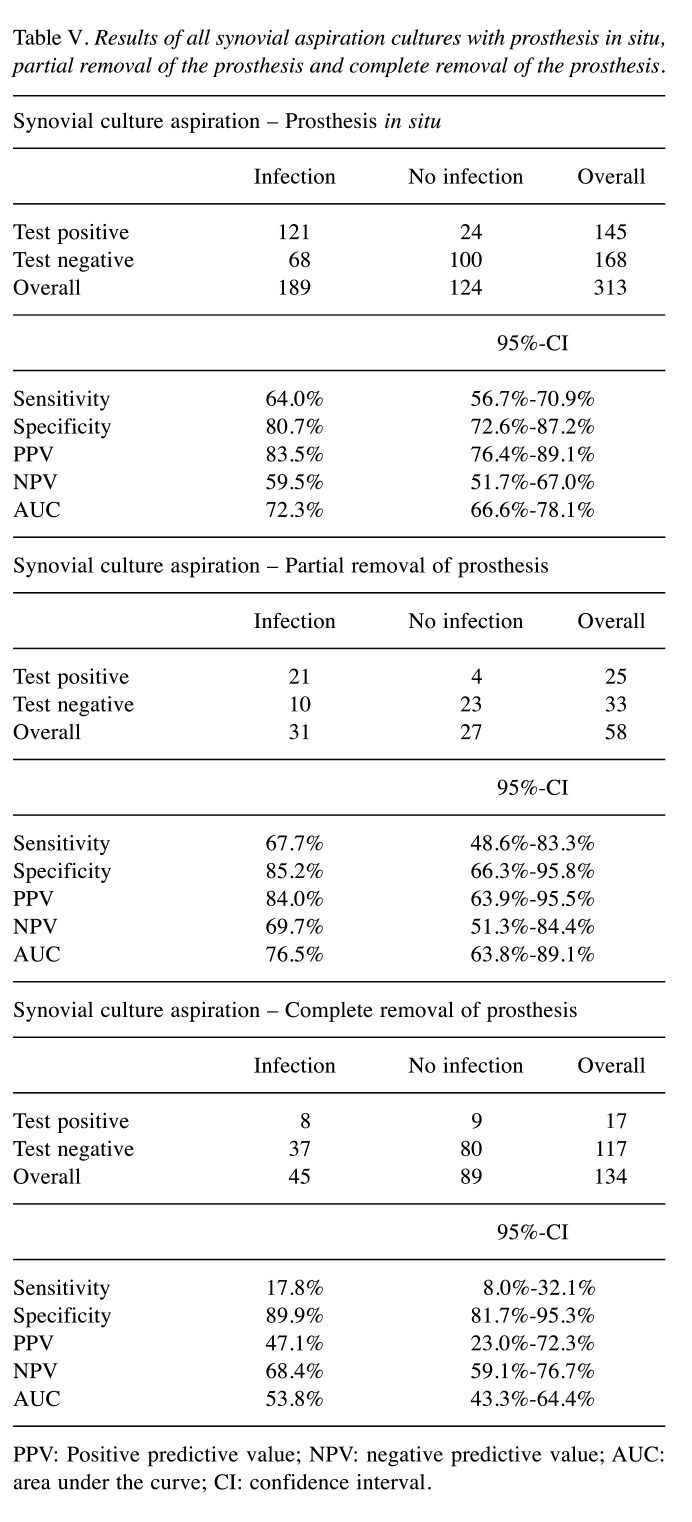
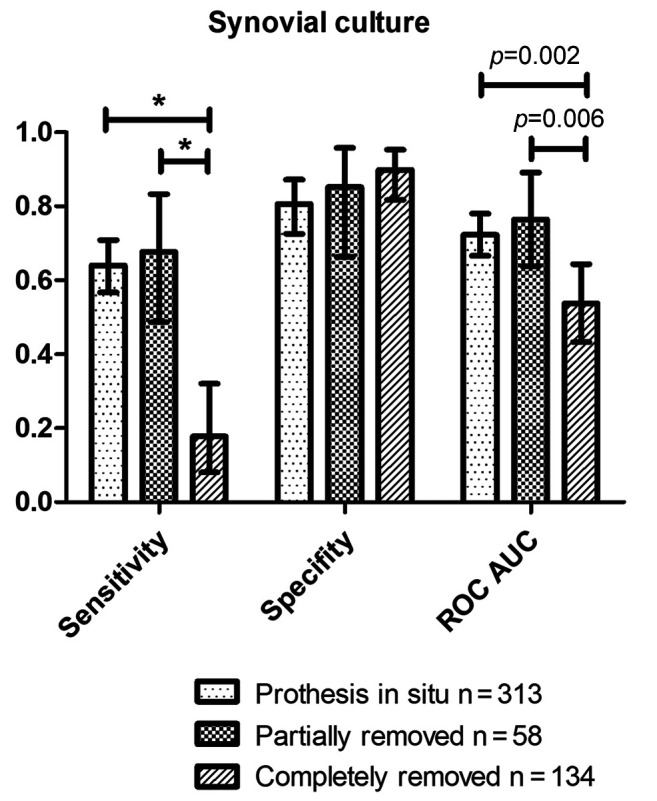
Diagnostic value of synovial culture aspiration in view of prosthesis removal. In case of complete removal of prosthesis, the sensitivity and AUC are significantly reduced, whereas the specificity was comparable in all three different situations with prosthesis in situ, partial removal or complete removal of prosthesis (Table V and Figure 3). The PPV was reduced if the prosthesis was completely removed, however, it was not significant due to a broad range of the CI.
Table V. Results of all synovial aspiration cultures with prosthesis in situ, partial removal of the prosthesis and complete removal of the prosthesis.
PPV: Positive predictive value; NPV: negative predictive value; AUC: area under the curve; CI: confidence interval.
Figure 3. Results for synovial culture aspiration with prosthesis in situ, partial removal and complete removal with presentation of the statistically significant differences (*significant with respect to 95% CI, p-value for ROC in the graphic).
Discussion
We demonstrated, that the diagnostic value of synovial culture varies within the sometimes complex treatment of these infections depending on treatment stage. Furthermore, due to the low sensitivity in the case of complete removal, an aspiration before reimplantation does not seem to make sense in order to reliably exclude infection.
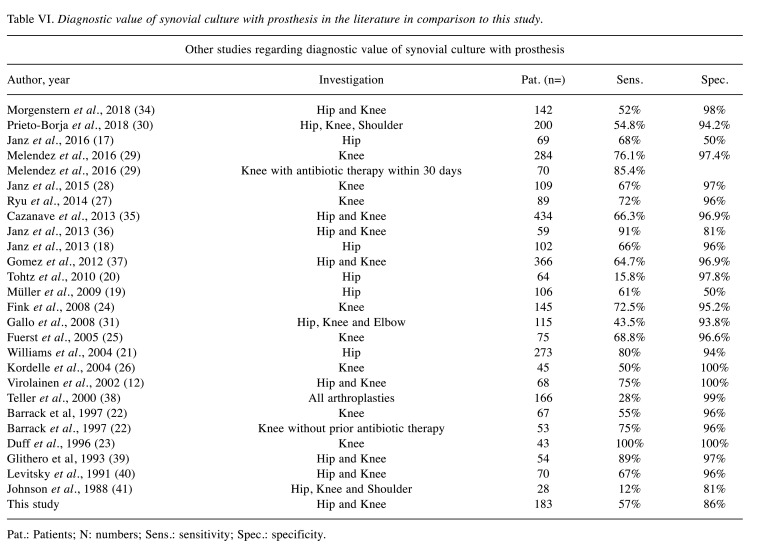
According to the Renner criteria, in addition to the detection of a fistula, the joint puncture aspiration is the essential possibility to detect a periprosthetic joint infection preoperatively (7). Different methods are used to analyse the joint aspiration puncture obtained. Diagnosis usually begins with light microscopy of a gram-stained direct specimen. This method delivers very quick results, is highly specific, but has only moderate sensitivity (12,13). The routine method for examining joint aspiration punctures in the diagnosis of periprosthetic infections is cultural incubation in a nutrient broth. However, this takes a lot of time and is therefore unsuitable for acute diagnosis, but can then be helpful in further therapy by creating an antibiogram (11,14). The sensitivity of the cultural analysis of joint puncture aspiration in the primary diagnosis of periprosthetic infections fluctuates in the literature; a detailed list of the results of other studies can be found in Table VI.
Table VI. Diagnostic value of synovial culture with prosthesis in the literature in comparison to this study.
Pat.: Patients; N: numbers; Sens.: sensitivity; Spec.: specificity.
In comparison to the hip culture the sensitivity for the knee culture was significantly higher, as well as the NPV and the AUC (p=0.038). One possible cause for these findings could be the procedure of joint aspiration. As the knee joint is relatively superficial, the puncture is, in contrast to the hip joint, usually performed without sonography and/or x-ray control. But even with these imaging procedures aspiration of seroma without connection to the hip joint or punctio sicca is not unlikely (15,16).
Regarding our results of the hip culture in comparison with the existing literature sensitivity and specificity are in the range of other studies (sensitivity: 47.6% vs. 58.2%; specificity: 86.9% vs. 78%; see also Table VI for comparison) (17-21). A similar pattern can be seen for the synovial cultures of the knee, at least for sensitivity. Eight studies exclusively examined the value of synovial cultures from knee joint aspiration at the start of therapy (22-29). The mean sensitivity was 70%, the mean specificity 97% (our study: 71.7% and 81.6%) (Table VI). The lower specificity for knee synovial culture of our study is noticeable. Nonetheless, it should be mentioned that in contrast to other studies, samples that were obtained within the sometimes complex therapeutic regimen were also taken into account in our study. This could explain the difference in this issue. We were unable to find any significant differences regarding the timing of the infection, although the general distribution of our data is comparable to the literature (30,31).
Furthermore, there were no significantly different values in our study for synovial culture with or ongoing antibiotic treatment. The number of existing studies for the diagnostic value of synovial cultures in dependence of antibiotic treatment is sparse and contradictory though. Mendelez et al. found a higher sensitivity for synovial culture in patients receiving antibiotic treatment in the last 30 days (29). However, the authors forego a precise analysis of the duration of the antibiotic pause. In contrast, Barrack and colleagues showed a lower sensitivity, if the samples were taken under antibiotic therapy (22). Unfortunately, our study has limited suitability for a differentiated statement of the effect of antibiotic therapy. Due to the retrospective design, it remains in some cases unclear, whether an antibiogram adapted therapy was already carried out and which exact therapy was performed in the ambulatory sector.
In this study, we found that the sensitivity and AUC significantly reduced in case of complete removal of the prosthesis. This underlines the importance of the biofilm on the implant surface for the pathogenesis and diagnosis of periprosthetic infections. On the one hand, the removal of the biofilm is essential for the treatment of a foreign body-associated infection (7). As our data show, however, the diagnostic value of synovial cultures decreases enormously after removal of the implant and consequently the biofilm. These results support that the probability of a successful cultural pathogen detection in the puncture depends crucially on the release of planktonic germs from the biofilm on the implant surface, as already described by other authors (15,32). Moreover, it seems, that it does not make sense to collect synovial samples for cultural analysis in the prosthesis-free interval to exclude a persistent infection.
The specificity was comparable in all three different situations with prosthesis in situ, partial removal or complete removal of prosthesis. However, one has to be careful with interpretation of the high specificity of 89.9% in complete removal, as this means a false positive rate of 10.1% and thus could lead to an unnecessary additional operation. On the other hand, as the specificity of partial removal, for example in megaprosthesis after trauma or tumour is comparable with the complete removal, the synovial culture could be a decision support especially in these critical patients.
The study had certain imitations and strengths. First, it should be pointed out that using a different definition system of periprosthetic infection might have achieved different results. We opted for the consensus classification, but some authors use different definitions, which reduces comparability (33). Further, as this is a retrospective study, a standardization of the puncture/aspiration itself (although these standards did not change in our clinic during the study) cannot be guaranteed with absolute certainty. As mentioned above, in contrast to other studies we included samples within the complex regime of periprosthetic infections as well, which limits the comparability to them. On the other hand, we consider our results to be valid precisely because this comes closer to the clinical course than an isolated observation of the synovial aspiration culture at the beginning of therapy.
Conclusion
It could be shown that the sensitivity decreases significantly when the prosthesis material is completely removed, whereas the results with partially removed prosthesis material are comparable with the results with the prosthesis material left in place. Due to the low sensitivity, obtaining several synovial cultures in the prosthesis-free interval to exclude persistence of infection does not seem reasonable.
Conflicts of Interest
The Authors declare no conflicts of interest.
Author’s Contributions
CM: Study design and realization, analysing and data interpretation, manuscript writing. SL: Study design and realization, data collection, analysing and data interpretation, manuscript reviewing. TG: Data collection, analysis and data interpretation, manuscript reviewing. MOE: Data collection, analysis and data interpretation, manuscript reviewing. TOP: Analysis and data interpretation, manuscript reviewing. TS: analysis and data interpretation, manuscript reviewing. CK: Reviewing of the manuscript. MO: Study design and realization, analysis and data interpretation, manuscript reviewing.
Acknowledgements
The data of this study are part of the doctoral thesis of SL.
References
- 1.Abad CL, Haleem A. Prosthetic joint infections: an update. Curr Infect Dis Rep. 2018;20(7):15. doi: 10.1007/s11908-018-0622-0. [DOI] [PubMed] [Google Scholar]
- 2.Otto-lambertz C, Yagdiran A, Wallscheid F, Eysel P, Jung N. Periprosthetic infection in joint replacement. Dtsch Arztebl Int. 2017;114(20):347–353. doi: 10.3238/arztebl.2017.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renz N, Müller M, Perka C, Trampuz A. [Implant-associated infections - Diagnostics] Chirurg. 2016;87(10):813–821. doi: 10.1007/s00104-016-0234-x. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87(8):1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 6.Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. 2008;10(5):394–403. doi: 10.1007/s11908-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 7.Renner L, Perka C, Trampuz A, Renz N. [Treatment of periprosthetic infections] Chirurg. 2016;87(10):831–838. doi: 10.1007/s00104-016-0255-5. [DOI] [PubMed] [Google Scholar]
- 8.Frommelt L. [Aspiration of joint fluid for detection of the pathogen in periprosthetic infection] Orthopade. 2008;37(10):1027–34. doi: 10.1007/s00132-008-1345-y. quiz 1035-6. [DOI] [PubMed] [Google Scholar]
- 9.Morawietz L, Gehrke T, Classen RA, Barden B, Otto M, Hansen T, Aigner T, Stiehl P, Neidel J, Schröder JH, Frommelt L, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader ChP, Kirschner S, Lintner F, Rüther W, Skwara A, Bos I, Kriegsmann J, Krenn V. [Proposal for the classification of the periprosthetic membrane from loosened hip and knee endoprostheses] Pathologe. 2004;25(5):375–384. doi: 10.1007/s00292-004-0710-9. [DOI] [PubMed] [Google Scholar]
- 10.Morawietz L, Classen RA, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59(6):591–597. doi: 10.1136/jcp.2005.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerli W. Infection and musculoskeletal conditions: Prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol. 2006;20(6):1045–1063. doi: 10.1016/j.berh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Virolainen P, Lähteenmäki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg. 2002;91(2):178–181. doi: 10.1177/145749690209100208. [DOI] [PubMed] [Google Scholar]
- 13.Wouthuyzen-Bakker M, Shohat N, Sebillotte M, Arvieux C, Parvizi J, Soriano A. Is Gram staining still useful in prosthetic joint infections. J Bone Jt Infect. 2019;4(2):56–59. doi: 10.7150/jbji.31312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes HC, Newnham R, Athanasou N, Atkins BL, Bejon P, Bowler IC. Microbiological diagnosis of prosthetic joint infections: a prospective evaluation of four bacterial culture media in the routine laboratory. Clin Microbiol Infect. 2011;17(10):1528–1530. doi: 10.1111/j.1469-0691.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 15.Winkler T, Trampuz A, Hardt S, Janz V, Kleber C, Perka C. [Periprosthetic infection after hip arthroplasty] Orthopade. 2014;43(1):70–78. doi: 10.1007/s00132-013-2132-y. [DOI] [PubMed] [Google Scholar]
- 16.Perka C, Haas N. [Periprosthetic infection] Chirurg. 2011;82(3):218–226. doi: 10.1007/s00104-010-2014-3. [DOI] [PubMed] [Google Scholar]
- 17.Janz V, Bartek B, Wassilew GI, Stuhlert M, Perka CF, Winkler T. Validation of synovial aspiration in girdlestone hips for detection of infection persistence in patients undergoing 2-stage revision total hip arthroplasty. J Arthroplasty. 2016;31(3):684–687. doi: 10.1016/j.arth.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 18.Janz V, Wassilew GI, Hasart O, Tohtz S, Perka C. Improvement in the detection rate of PJI in total hip arthroplasty through multiple sonicate fluid cultures. J Orthop Res. 2013;31(12):2021–2024. doi: 10.1002/jor.22451. [DOI] [PubMed] [Google Scholar]
- 19.Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. [Histopathological diagnosis of periprosthetic joint infection following total hip arthroplasty : use of a standardized classification system of the periprosthetic interface membrane] Orthopade. 2009;38(11):1087–1096. doi: 10.1007/s00132-009-1471-1. [DOI] [PubMed] [Google Scholar]
- 20.Tohtz SW, Müller M, Morawietz L, Winkler T, Perka C. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Relat Res. 2010;468(3):762–768. doi: 10.1007/s11999-009-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J Arthroplasty. 2004;19(5):582–586. doi: 10.1016/j.arth.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Barrack RL, Jennings RW, Wolfe MW, Bertot AJ. The Coventry Award. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res. 1997;(345):8–16. [PubMed] [Google Scholar]
- 23.Duff GP, Lachiewicz PF, Kelley SS. Aspiration of the knee joint before revision arthroplasty. Clin Orthop Relat Res. 1996;(331):132–139. doi: 10.1097/00003086-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Fink B, Makowiak C, Fuerst M, Berger I, Schäfer P, Frommelt L. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br. 2008;90(7):874–878. doi: 10.1302/0301-620X.90B7.20417. [DOI] [PubMed] [Google Scholar]
- 25.Fuerst M, Fink B, Rüther W. [The value of preoperative knee aspiration and arthroscopic biopsy in revision total knee arthroplasty] Z Orthop Ihre Grenzgeb. 2005;143(1):36–41. doi: 10.1055/s-2004-836252. [DOI] [PubMed] [Google Scholar]
- 26.Kordelle J, Klett R, Stahl U, Hossain H, Schleicher I, Haas H. [Infection diagnosis after knee-TEP-implantation] Z Orthop Ihre Grenzgeb. 2004;142(3):337–343. doi: 10.1055/s-2004-818772. [DOI] [PubMed] [Google Scholar]
- 27.Ryu SY, Greenwood-Quaintance KE, Hanssen AD, Mandrekar JN, Patel R. Low sensitivity of periprosthetic tissue PCR for prosthetic knee infection diagnosis. Diagn Microbiol Infect Dis. 2014;79(4):448–453. doi: 10.1016/j.diagmicrobio.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Janz V, Wassilew GI, Kribus M, Trampuz A, Perka C. Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch Orthop Trauma Surg. 2015;135(10):1453–1457. doi: 10.1007/s00402-015-2317-4. [DOI] [PubMed] [Google Scholar]
- 29.Melendez DP, Greenwood-Quaintance KE, Berbari EF, Osmon DR, Mandrekar JN, Hanssen AD, Patel R. Evaluation of a Genus- and Group-Specific Rapid PCR assay panel on synovial fluid for diagnosis of prosthetic knee infection. J Clin Microbiol. 2016;54(1):120–126. doi: 10.1128/JCM.02302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prieto-Borja L, Auñón Á, Blanco A, Fernández-Roblas R, Gadea I, García-Cañete J, Parrón R, Esteban J. Evaluation of the use of sonication of retrieved implants for the diagnosis of prosthetic joint infection in a routine setting. Eur J Clin Microbiol Infect Dis. 2018;37(4):715–722. doi: 10.1007/s10096-017-3164-8. [DOI] [PubMed] [Google Scholar]
- 31.Gallo J, Kolar M, Dendis M, Loveckova Y, Sauer P, Zapletalova J, Koukalova D. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97–104. [PubMed] [Google Scholar]
- 32.Mariconda M, Ascione T, Balato G, Rotondo R, Smeraglia F, Costa GG, Conte M. Sonication of antibiotic-loaded cement spacers in a two-stage revision protocol for infected joint arthroplasty. BMC Musculoskelet Disord. 2013;14:193. doi: 10.1186/1471-2474-14-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parvizi J, Jacovides C, Zmistowski B, Jung KA. Definition of periprosthetic joint infection: is there a consensus. Clin Orthop Relat Res. 2011;469(11):3022–3030. doi: 10.1007/s11999-011-1971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgenstern C, Cabric S, Perka C, Trampuz A, Renz N. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018;90(2):115–119. doi: 10.1016/j.diagmicrobio.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Karau MJ, Schmidt SM, Gomez Urena EO, Mandrekar JN, Osmon DR, Lough LE, Pritt BS, Steckelberg JM, Patel R. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J Clin Microbiol. 2013;51(7):2280–2287. doi: 10.1128/JCM.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C. Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop. 2013;37(5):931–936. doi: 10.1007/s00264-013-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50(11):3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teller RE, Christie MJ, Martin W, Nance EP, Haas DW. Sequential indium-labeled leukocyte and bone scans to diagnose prosthetic joint infection. Clin Orthop Relat Res. 2000;(373):241–247. doi: 10.1097/00003086-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 39.Glithero PR, Grigoris P, Harding LK, Hesslewood SR, McMinn DJ. White cell scans and infected joint replacements. Failure to detect chronic infection. J Bone Joint Surg Br. 1993;75(3):371–374. doi: 10.1302/0301-620X.75B3.8496202. [DOI] [PubMed] [Google Scholar]
- 40.Levitsky KA, Hozack WJ, Balderston RA, Rothman RH, Gluckman SJ, Maslack MM, Booth RE Jr. Evaluation of the painful prosthetic joint. Relative value of bone scan, sedimentation rate, and joint aspiration. J Arthroplasty. 1991;6(3):237–244. doi: 10.1016/s0883-5403(06)80170-1. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JA, Christie MJ, Sandler MP, Parks PF Jr, Homra L, Kaye JJ. Detection of occult infection following total joint arthroplasty using sequential technetium-99m HDP bone scintigraphy and indium-111 WBC imaging. J Nucl Med. 1988;29(8):1347–1353. [PubMed] [Google Scholar]