Abstract
Background/Aim: Advanced prostate cancer is a recalcitrant disease with very limited treatment options. Our laboratory discovered methionine addiction, presumably a characteristic of all cancer types, including prostate cancer, which can be targeted by methionine restriction (MR), through treatment with oral recombinant methioninase (o-rMETase). Patients and Methods: o-rMETase was produced by fermentation of recombinant E. coli containing the Pseudomonas putida methioninase gene, and purified by column chromatography. An advanced prostate cancer patient received o-rMETase as a supplement, 500 units per day, divided into two oral doses of 250 units each. Results: Before treatment, the patient had a rapid rise in PSA levels, from 39 to 56 ng/ml, within 6 weeks. At the 15th week of o-rMETase administration, the PSA levels stabilized at 62 ng/ml. No overt side effects were observed. Conclusion: o-rMETase single treatment can be beneficial for advanced prostate cancer patients.
Keywords: Methioninase, oral, methionine addiction, advanced prostate-cancer patient, PSA
Prostate cancer has extremely limited treatment options and remains a leading cause of death of males in developed countries (1-3). In 1941, Dr. Huggins discovered that surgical castration of prostate cancer patients could slow the disease (4). In the 1970’s, Dr. Shally found that a gonadotropin–releasing hormone analog could substitute for surgical castration to control prostate cancer (5). Both, Dr. Huggins and Dr. Schally, won Nobel Prizes for their studies. The problem is that prostate cancer very often becomes resistant to androgen-deprivation therapy (ADT), eventually killing the patient. It was not until 2015 that the chemotherapeutic drug docetaxel was added to ADT, which extended survival of advanced prostate cancer patients (1-3). However, docetaxel displays serious toxicity (1-3).
Methionine addiction is a general and fundamental hallmark of cancer. Methionine addiction is due to much higher requirements for methionine by cancer cells compared to normal cells (6-13). The methionine addiction of cancer cells is due to excess transmethylation reactions in cancer cells. Methionine addiction depletes the cellular pools from free methionine, and S-adenosylmethionine when cancer cells are put under methionine restriction (12,13). Methionine addiction of cancer is known as the “Hoffman Effect” (14-16). The Hoffman effect is stronger than the Warburg effect for glucose as seen on PET imaging in the clinic (17). The elevated transmethylation in cancer is due in part to the over-methylation of histone H-3 lysine marks, which can at least partially explain methionine addiction (18,19).
Methionine addiction can be targeted with recombinant methioninase (rMETase). The rMETase gene was cloned from P. putida, expressed in Escherichia coli (6,20), and found to be effective against all major cancer types, as shown for example in patient-derived orthotopic xenograft (PDOX) mouse models (21-44).
Intravenous (iv) injection of PEGylated rMETase depleted plasma methionine to <5 μmol/l, with transient anemia being the only side effect in macaque monkeys; iv-injected PEGylated rMETase did not cause anaphylaxis after repeated treatment (45). In contract repeated iv injection of non-PEGylated methioninase caused anaphylaxis (45). The rMETase co-factor pyrodoxal 5-phosphate (PLP) was rapidly lost after iv injection resulting in loss of enzymatic activity (45). A pilot Phase I clinical trial of iv-rMETase showed rapid methionine depletion to 0.1 μM in advanced-stage cancer patients (46,47).
Successful oral administration of rMETase (o-rMETase) in mouse models of cancer (21-26,29,31,38,44), overcame the problem of anaphylaxis and the dis-association of PLP from rMETase. o-rMETase has been shown to be highly effective against sarcoma, pancreatic cancer, colon cancer, and melanoma in patient-derived orthotopic xenograft (PDOX) mouse models (21-26,29,31,38,44). Recently, o-rMETase was tested as a supplement in advanced prostate cancer patients and shown to both stabilize and lower PSA levels (48,49).
Patients and Methods
o-rMETase was produced by fermentation of recombinant E. coli containing the Pseudomonas putida methioninase gene, and was purified by column chromatography. An advanced prostate cancer patient was administered o-rMETase as a supplement, 500 units per day, divided into two oral doses of 250 units each, after breakfast and after dinner. PSA was measured by standard protocols (6,20).
Results and Discussion
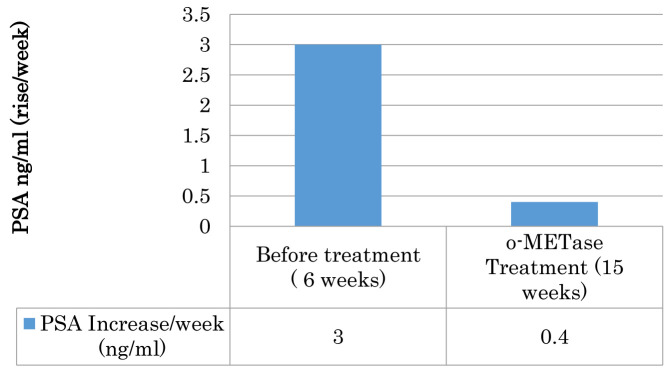
During the six weeks prior to starting the o-rMETase treatment, the patient’s PSA levels increased 3 ng/ml each week from 38 to 56 ng/ml. Following 15 weeks of o-rMETase treatment the patient’s PSA levels increased by only 0.4 ng/ml per week (Figure 1).
Figure 1. Rate of increase in the PSA levels of an advanced prostate-cancer patient before and after o-rMETase treatment. o-rMETase was administered at a dose of 5 mg/250 units, twice daily.
The treatment for prostate cancer has not been substantially improved since the discoveries of Huggins (4) and Schally (5) on the hormonal control of the disease, which is called androgen-depletion therapy (ADT) (1-3). Although docetaxel has shown survival benefit when combined with ADT, it is associated with significant toxicity (1-3). The present report and our two previous reports (48,49) indicate that methionine-deprivation therapy (MDT) is a potential new therapeutic approach for prostate cancer.
o-rMETase has been previously shown to cause a 70% drop in the PSA levels of a bone-metastatic prostate cancer patient who was treated with o-rMETase as a supplement for 3 months, with a starting PSA value of over 2,000 ng/ml (48). o-rMETase has been shown to lower and stabilize PSA levels in 2 other advanced prostate cancer patients (49).
o-rMETase is much safer than iv injected rMETase (45). o-rMETase is being initially developed as a supplement for cancer patients. o-rMETase originated in P. putida, where it can survive and proliferate in a wide range of pH and temperatures. Therefore, o-rMETase could have been evolved into an enzyme that can survive in the low pH of the stomach (31) which is a great advantage over a human engineered methioninase which must be injected (50).
Currently, cancer patients are administered 5 mg (250 units) of o-rMETase twice per day (48,49). The dose and schedule will be optimized in the future, depending on clinical results. It is possible that a low-methionine diet will enhance the efficacy of o-rMETase for prostate and other cancers (51). Since methionine restriction is synergistic with chemotherapy (11,52-54), o-rMETase can be combined with chemotherapy in future studies. A promising combination is that of o-rMETase, an inhibitor of S-adenosylmethionine synthase, and an inhibitor of DNA methylation, which can block the methionine-methylation axis (41,55).
We have also shown that the addition of docetaxel or paclitaxel to o-rMETase is beneficial (56,57) and that patients on ADT and docetaxel may also benefit from the addition of a taxane to o-rMETase.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Qinghong Han produced and purified recombinant methioninase; Robert M. Hoffman and Qinghong Han wrote and revised the manuscript.
Acknowledgements
This study was funded in part by the Robert M. Hoffman Foundation for Cancer Research. This article is dedicated to the memory of AR Moossa, M.D., Sun Lee, M.D. Professor Li Jiaxi and Masaki Kitajima, M.D.
References
- 1.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645–657. doi: 10.1056/NEJMra1701695. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK, STAMPEDE investigators Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43(6):1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman RM. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: a 40-year odyssey. Expert Opin Biol Ther. 2015;15(1):21–31. doi: 10.1517/14712598.2015.963050. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman RM, Erbe RW. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci USA. 1976;73(5):1523–1527. doi: 10.1073/pnas.73.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc Natl Acad Sci USA. 1980;77(12):7306–7310. doi: 10.1073/pnas.77.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H, Lishko VK, Herrera H, Groce A, Kubota T, Hoffman RM. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res. 1993;53(23):5676–5679. [PubMed] [Google Scholar]
- 10.Yano S, Li S, Han Q, Tan Y, Bouvet M, Fujiwara T, Hoffman RM. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget. 2014;5(18):8729–8736. doi: 10.18632/oncotarget.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern PH, Hoffman RM. Enhanced in vitro selective toxicity of chemotherapeutic agents for human cancer cells based on a metabolic defect. J Natl Cancer Inst. 1986;76(4):629–639. doi: 10.1093/jnci/76.4.629. [DOI] [PubMed] [Google Scholar]
- 12.Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro. 1984;20(8):663–670. doi: 10.1007/BF02619617. [DOI] [PubMed] [Google Scholar]
- 13.Coalson DW, Mecham JO, Stern PH, Hoffman RM. Reduced availability of endogenously synthesized methionine for S-adenosylmethionine formation in methionine-dependent cancer cells. Proc Natl Acad Sci USA. 1982;79(14):4248–4251. doi: 10.1073/pnas.79.14.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser P. Methionine dependence of cancer. Biomolecules. 2020;10(4):568. doi: 10.3390/biom10040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauinger L, Kaiser P. Sensing and signaling of methionine metabolism. Metabolites. 2021;11(2):83. doi: 10.3390/metabo11020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrego SL, Fahrmann J, Hou J, Lin DW, Tromberg BJ, Fiehn O, Kaiser P. Lipid remodeling in response to methionine stress in MDA-MBA-468 triple-negative breast cancer cells. J Lipid Res. 2021;62:100056. doi: 10.1016/j.jlr.2021.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirotte B, Goldman S, Massager N, David P, Wikler D, Vandesteene A, Salmon I, Brotchi J, Levivier M. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med. 2004;45(8):1293–1298. [PubMed] [Google Scholar]
- 18.Yamamoto J, Inubushi S, Han Q, Tashiro Y, Sun Y, Sugisawa N, Hamada K, Nishino H, Aoki Y, Miyake K, Matsuyama R, Bouvet M, Endo I, Hoffman R. Cancer-specific overmethylation of histone H3 lysines is linked with methionine addiction and malignancy. bioRxiv. 2021 doi: 10.1101/2020.12.04.412437. [DOI] [Google Scholar]
- 19.Yamamoto J, Han Q, Inubushi S, Sugisawa N, Hamada K, Nishino H, Miyake K, Kumamoto T, Matsuyama R, Bouvet M, Endo I, Hoffman RM. Histone methylation status of H3K4me3 and H3K9me3 under methionine restriction is unstable in methionine-addicted cancer cells, but stable in normal cells. Biochem Biophys Res Commun. 2020;533(4):1034–1038. doi: 10.1016/j.bbrc.2020.09.108. [DOI] [PubMed] [Google Scholar]
- 20.Tan Y, Xu M, Tan X, Tan X, Wang X, Saikawa Y, Nagahama T, Sun X, Lenz M, Hoffman RM. Overexpression and large-scale production of recombinant L-methionine-alpha-deamino-gamma-mercaptomethane-lyase for novel anticancer therapy. Protein Expr Purif. 1997;9(2):233–245. doi: 10.1006/prep.1996.0700. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi K, Kawaguchi K, Kiyuna T, Miyake K, Murakami T, Yamamoto N, Hayashi K, Kimura H, Miwa S, Tsuchiya H, Hoffman RM. Effective metabolic targeting of human osteosarcoma cells in vitro and in orthotopic nude-mouse models with recombinant methioninase. Anticancer Res. 2017;37(9):4807–4812. doi: 10.21873/anticanres.11887. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi T, Han Q, Miyake K, Oshiro H, Sugisawa N, Tan Y, Yamamoto N, Hayashi K, Kimura H, Miwa S, Igarashi K, Bouvet M, Singh SR, Tsuchiya H, Hoffman RM. Combination of oral recombinant methioninase and decitabine arrests a chemotherapy-resistant undifferentiated soft-tissue sarcoma patient-derived orthotopic xenograft mouse model. Biochem Biophys Res Commun. 2020;523(1):135–139. doi: 10.1016/j.bbrc.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi T, Sugisawa N, Yamamoto J, Oshiro H, Han Q, Yamamoto N, Hayashi K, Kimura H, Miwa S, Igarashi K, Tan Y, Kuchipudi S, Bouvet M, Singh SR, Tsuchiya H, Hoffman RM. The combination of oral-recombinant methioninase and azacitidine arrests achemotherapy-resistant osteosarcoma patient-derived orthotopic xenograft mouse model. Cancer Chemother Pharmacol. 2020;85(2):285–291. doi: 10.1007/s00280-019-03986-0. [DOI] [PubMed] [Google Scholar]
- 24.Oshiro H, Tome Y, Kiyuna T, Yoon SN, Lwin TM, Han Q, Tan Y, Miyake K, Higuchi T, Sugisawa N, Katsuya Y, Park JH, Zang Z, Razmjooei S, Bouvet M, Clary B, Singh SR, Kanaya F, Nishida K, Hoffman RM. Oral recombinant methioninase overcomes colorectal-cancer liver metastasis resistance to the combination of 5-fluorouracil and oxaliplatinum in a patient-derived orthotopic xenograft mouse model. Anticancer Res. 2019;39(9):4667–4671. doi: 10.21873/anticanres.13648. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi T, Oshiro H, Miyake K, Sugisawa N, Han Q, Tan Y, Park J, Zhang Z, Razmjooei S, Yamamoto N, Hayashi K, Kimura H, Miwa S, Igarashi K, Bouvet M, Chawla SP, Singh SR, Tsuchiya H, Hoffman RM. Oral recombinant methioninase, combined with oral caffeine and injected cisplatinum, overcome cisplatinum-resistance and regresses patient-derived orthotopic xenograft model of osteosarcoma. Anticancer Res. 2019;39(9):4653–4657. doi: 10.21873/anticanres.13646. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi K, Han Q, Li S, Tan Y, Igarashi K, Murakami T, Unno M, Hoffman RM. Efficacy of recombinant methioninase (rMETase) on recalcitrant cancer patient-derived orthotopic xenograft (PDOX) mouse models: A review. Cells. 2019;8(5):410. doi: 10.3390/cells8050410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyake K, Kiyuna T, Li S, Han Q, Tan Y, Zhao M, Oshiro H, Kawaguchi K, Higuchi T, Zhang Z, Razmjooei S, Barangi M, Wangsiricharoen S, Murakami T, Singh AS, Li Y, Nelson SD, Eilber FC, Bouvet M, Hiroshima Y, Chishima T, Matsuyama R, Singh SR, Endo I, Hoffman RM. Combining Tumor-Selective Bacterial Therapy with Salmonella typhimurium A1-R and Cancer Metabolism targeting with oral recombinant methioninase regressed an Ewing’s sarcoma in a patient-derived orthotopic xenograft model. Chemotherapy. 2018;63(5):278–283. doi: 10.1159/000495574. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi K, Kawaguchi K, Kiyuna T, Miyake K, Miyaki M, Yamamoto N, Hayashi K, Kimura H, Miwa S, Higuchi T, Singh AS, Chmielowski B, Nelson SD, Russell TA, Eckardt MA, Dry SM, Li Y, Singh SR, Chawla SP, Eilber FC, Tsuchiya H, Hoffman RM. Metabolic targeting with recombinant methioninase combined with palbociclib regresses a doxorubicin-resistant dedifferentiated liposarcoma. Biochem Biophys Res Commun. 2018;506(4):912–917. doi: 10.1016/j.bbrc.2018.10.119. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi T, Kawaguchi K, Miyake K, Han Q, Tan Y, Oshiro H, Sugisawa N, Zhang Z, Razmjooei S, Yamamoto N, Hayashi K, Kimura H, Miwa S, Igarashi K, Chawla SP, Singh AS, Eilber FC, Singh SR, Tsuchiya H, Hoffman RM. Oral recombinant methioninase combined with caffeine and doxorubicin induced regression of a doxorubicin-resistant synovial sarcoma in a PDOX mouse model. Anticancer Res. 2018;38(10):5639–5644. doi: 10.21873/anticanres.12899. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi K, Higuchi T, Li S, Han Q, Tan Y, Igarashi K, Zhao M, Miyake K, Kiyuna T, Miyake M, Ohshiro H, Sugisawa N, Zhang Z, Razmjooei S, Wangsiricharoen S, Chmielowski B, Nelson SD, Russell TA, Dry SM, Li Y, Eckardt MA, Singh AS, Singh SR, Eilber FC, Unno M, Hoffman RM. Combination therapy of tumor-targeting Salmonella typhimurium A1-R and oral recombinant methioninase regresses a BRAF-V600E-negative melanoma. Biochem Biophys Res Commun. 2018;503(4):3086–3092. doi: 10.1016/j.bbrc.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi K, Miyake K, Han Q, Li S, Tan Y, Igarashi K, Kiyuna T, Miyake M, Higuchi T, Oshiro H, Zhang Z, Razmjooei S, Wangsiricharoen S, Bouvet M, Singh SR, Unno M, Hoffman RM. Oral recombinant methioninase (o-rMETase) is superior to injectable rMETase and overcomes acquired gemcitabine resistance in pancreatic cancer. Cancer Lett. 2018;432:251–259. doi: 10.1016/j.canlet.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi K, Miyake K, Han Q, Li S, Tan Y, Igarashi K, Lwin TM, Higuchi T, Kiyuna T, Miyake M, Oshiro H, Bouvet M, Unno M, Hoffman RM. Targeting altered cancer methionine metabolism with recombinant methioninase (rMETase) overcomes partial gemcitabine-resistance and regresses a patient-derived orthotopic xenograft (PDOX) nude mouse model of pancreatic cancer. Cell Cycle. 2018;17(7):868–873. doi: 10.1080/15384101.2018.1445907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi K, Han Q, Li S, Tan Y, Igarashi K, Miyake K, Kiyuna T, Miyake M, Chemielwski B, Nelson SD, Russell TA, Dry SM, Li Y, Singh AS, Eckardt MA, Unno M, Eilber FC, Hoffman RM. Intra-tumor L-methionine level highly correlates with tumor size in both pancreatic cancer and melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse models. Oncotarget. 2018;9(13):11119–11125. doi: 10.18632/oncotarget.24264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi K, Igarashi K, Li S, Han Q, Tan Y, Kiyuna T, Miyake K, Murakami T, Chmielowski B, Nelson SD, Russell TA, Dry SM, Li Y, Unno M, Eilber FC, Hoffman RM. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2017;8(49):85516–85525. doi: 10.18632/oncotarget.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Igarashi K, Kawaguchi K, Kiyuna T, Miyake K, Miyake M, Li S, Han Q, Tan Y, Zhao M, Li Y, Nelson SD, Dry SM, Singh AS, Elliott IA, Russell TA, Eckardt MA, Yamamoto N, Hayashi K, Kimura H, Miwa S, Tsuchiya H, Eilber FC, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle. 2018;17(6):801–809. doi: 10.1080/15384101.2018.1431596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igarashi K, Kawaguchi K, Li S, Han Q, Tan Y, Murakami T, Kiyuna T, Miyake K, Miyake M, Singh AS, Eckardt MA, Nelson SD, Russell TA, Dry SM, Li Y, Yamamoto N, Hayashi K, Kimura H, Miwa S, Tsuchiya H, Singh SR, Eilber FC, Hoffman RM. Recombinant methioninase in combination with doxorubicin (DOX) overcomes first-line DOX resistance in a patient-derived orthotopic xenograft nude-mouse model of undifferentiated spindle-cell sarcoma. Cancer Lett. 2018;417:168–173. doi: 10.1016/j.canlet.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi K, Han Q, Li S, Tan Y, Igarashi K, Kiyuna T, Miyake K, Miyake M, Chmielowski B, Nelson SD, Russell TA, Dry SM, Li Y, Singh AS, Eckardt MA, Unno M, Eilber FC, Hoffman RM. Targeting methionine with oral recombinant methioninase (o-rMETase) arrests a patient-derived orthotopic xenograft (PDOX) model of BRAF-V600E mutant melanoma: implications for chronic clinical cancer therapy and prevention. Cell Cycle. 2018;17(3):356–361. doi: 10.1080/15384101.2017.1405195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JH, Han Q, Zhao M, Tan Y, Higuchi T, Yoon SN, Sugisawa N, Yamamoto J, Bouvet M, Clary B, Singh SR, Hoffman RM. Oral recombinant methioninase combined with oxaliplatinum and 5-fluorouracil regressed a colon cancer growing on the peritoneal surface in a patient-derived orthotopic xenograft mouse model. Tissue Cell. 2019;61:109–114. doi: 10.1016/j.tice.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Igarashi K, Li S, Han Q, Tan Y, Kawaguchi K, Murakami T, Kiyuna T, Miyake K, Li Y, Nelson SD, Dry SM, Singh AS, Elliott IA, Russell TA, Eckardt MA, Yamamoto N, Hayashi K, Kimura H, Miwa S, Tsuchiya H, Eilber FC, Hoffman RM. Growth of doxorubicin-resistant undifferentiated spindle-cell sarcoma PDOX is arrested by metabolic targeting with recombinant methioninase. J Cell Biochem. 2018;119(4):3537–3544. doi: 10.1002/jcb.26527. [DOI] [PubMed] [Google Scholar]
- 40.Murakami T, Li S, Han Q, Tan Y, Kiyuna T, Igarashi K, Kawaguchi K, Hwang HK, Miyake K, Singh AS, Nelson SD, Dry SM, Li Y, Hiroshima Y, Lwin TM, DeLong JC, Chishima T, Tanaka K, Bouvet M, Endo I, Eilber FC, Hoffman RM. Recombinant methioninase effectively targets a Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8(22):35630–35638. doi: 10.18632/oncotarget.15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugisawa N, Yamamoto J, Han Q, Tan Y, Tashiro Y, Nishino H, Inubushi S, Hamada K, Kawaguchi K, Unno M, Bouvet M, Hoffman RM. Triple-methyl blockade with recombinant methioninase, cycloleucine, and azacitidine arrests a pancreatic cancer patient-derived orthotopic xenograft model. Pancreas. 2021;50(1):93–98. doi: 10.1097/MPA.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi K, Kawaguchi K, Li S, Han Q, Tan Y, Gainor E, Kiyuna T, Miyake K, Miyake M, Higuchi T, Oshiro H, Singh AS, Eckardt MA, Nelson SD, Russell TA, Dry SM, Li Y, Yamamoto N, Hayashi K, Kimura H, Miwa S, Tsuchiya H, Eilber FC, Hoffman RM. Recombinant methioninase combined with doxorubicin (DOX) regresses a DOX-resistant synovial sarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2018;9(27):19263–19272. doi: 10.18632/oncotarget.24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawaguchi K, Igarashi K, Li S, Han Q, Tan Y, Miyake K, Kiyuna T, Miyake M, Murakami T, Chmielowski B, Nelson SD, Russell TA, Dry SM, Li Y, Unno M, Eilber FC, Hoffman RM. Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as -positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget. 2017;9(1):915–923. doi: 10.18632/oncotarget.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JH, Zhao M, Han Q, Sun Y, Higuchi T, Sugisawa N, Yamamoto J, Singh SR, Clary B, Bouvet M, Hoffman RM. Efficacy of oral recombinant methioninase combined with oxaliplatinum and 5-fluorouracil on primary colon cancer in a patient-derived orthotopic xenograft mouse model. Biochem Biophys Res Commun. 2019;518(2):306–310. doi: 10.1016/j.bbrc.2019.08.051. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Wang J, Lu Q, Xu J, Kobayashi Y, Takakura T, Takimoto A, Yoshioka T, Lian C, Chen C, Zhang D, Zhang Y, Li S, Sun X, Tan Y, Yagi S, Frenkel EP, Hoffman RM. PEGylation confers greatly extended half-life and attenuated immunogenicity to recombinant methioninase in primates. Cancer Res. 2004;64(18):6673–6678. doi: 10.1158/0008-5472.CAN-04-1822. [DOI] [PubMed] [Google Scholar]
- 46.Tan Y, Zavala J Sr, Han Q, Xu M, Sun X, Tan X, Tan X, Magana R, Geller J, Hoffman RM. Recombinant methioninase infusion reduces the biochemical endpoint of serum methionine with minimal toxicity in high-stage cancer patients. Anticancer Res. 1997;17(5B):3857–3860. [PubMed] [Google Scholar]
- 47.Tan Y, Zavala J Sr, Xu M, Zavala J Jr, Hoffman RM. Serum methionine depletion without side effects by methioninase in metastatic breast cancer patients. Anticancer Res. 1996;16(6C):3937–3942. [PubMed] [Google Scholar]
- 48.Han Q, Tan Y, Hoffman RM. Oral dosing of recombinant methioninase is associated with a 70% drop in PSA in a patient with bone-metastatic prostate cancer and 50% reduction in circulating methionine in a high-stage ovarian cancer patient. Anticancer Res. 2020;40(5):2813–2819. doi: 10.21873/anticanres.14254. [DOI] [PubMed] [Google Scholar]
- 49.Han Q, Hoffman RM. Lowering and stabilizing PSA levels in advanced-prostate cancer patients with oral methioninase. Anticancer Res. 2021;41(4):1921–1926. doi: 10.21873/anticanres.14958. [DOI] [PubMed] [Google Scholar]
- 50.Lu WC, Saha A, Yan W, Garrison K, Lamb C, Pandey R, Irani S, Lodi A, Lu X, Tiziani S, Zhang YJ, Georgiou G, DiGiovanni J, Stone E. Enzyme-mediated depletion of serum l-Met abrogates prostate cancer growth via multiple mechanisms without evidence of systemic toxicity. Proc Natl Acad Sci USA. 2020;117(23):13000–13011. doi: 10.1073/pnas.1917362117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP Jr, Ciccarella A, Calcagnotto A, Mikhael PG, Mentch SJ, Liu J, Ables G, Kirsch DG, Hsu DS, Nichenametla SN, Locasale JW. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572(7769):397–401. doi: 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshioka T, Wada T, Uchida N, Maki H, Yoshida H, Ide N, Kasai H, Hojo K, Shono K, Maekawa R, Yagi S, Hoffman RM, Sugita K. Anticancer efficacy in vivo and in vitro, synergy with 5-fluorouracil, and safety of recombinant methioninase. Cancer Res. 1998;58(12):2583–2587. [PubMed] [Google Scholar]
- 53.Kokkinakis DM, Hoffman RM, Frenkel EP, Wick JB, Han Q, Xu M, Tan Y, Schold SC. Synergy between methionine stress and chemotherapy in the treatment of brain tumor xenografts in athymic mice. Cancer Res. 2001;61(10):4017–4023. [PubMed] [Google Scholar]
- 54.Tan Y, Sun X, Xu M, Tan X, Sasson A, Rashidi B, Han Q, Tan X, Wang X, An Z, Sun FX, Hoffman RM. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clin Cancer Res. 1999;5(8):2157–2163. [PubMed] [Google Scholar]
- 55.Sugisawa N, Yamamoto J, Han Q, Tan Y, Tashiro Y, Nishino H, Inubushi S, Hamada K, Kawaguchi K, Unno M, Bouvet M, Hoffman RM. Triple-methyl blockade with recombinant methioninase, cycloleucine, and azacitidine arrests a pancreatic cancer patient-derived orthotopic xenograft model. Pancreas. 2021;50(1):93–98. doi: 10.1097/MPA.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 56.Aoki Y, Tome Y, Wu NF, Yamamoto J, Hamada K, Han Q, Bouvet M, Nishida K, Hoffman RM. Oral-recombinant methioninase converts an osteosarcoma from docetaxel-resistant to -sensitive in a clinically-relevant patient-derived orthotopic-xenograft (PDOX) mouse model. Anticancer Res. 2021;41(4):1745–1751. doi: 10.21873/anticanres.14939. [DOI] [PubMed] [Google Scholar]
- 57.Sugisawa N, Higuchi T, Han Q, Hozumi C, Yamamoto J, Tashiro Y, Nishino H, Kawaguchi K, Bouvet M, Murata T, Unno M, Hoffman RM. Oral recombinant methioninase combined with paclitaxel arrests recalcitrant ovarian clear cell carcinoma growth in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Cancer Chemother Pharmacol. 2021;88(1):61–67. doi: 10.1007/s00280-021-04261-x. [DOI] [PubMed] [Google Scholar]