Abstract
Background/Aim: Dilatation of the main pulmonary artery (mPA) is a common incidental finding in chest imaging and often leads to consultation. The aim of this study was to determine the prevalence of mPA dilatation in a coronary artery CT angiography (CCTA) population. Patients and Methods: The study investigated 985 consecutive patients scheduled for diagnostic CCTA. The transverse axial diameter of the mPA was measured. The prevalence of mPA dilatation was estimated using different reference values (Framingham Heart Study: 28.9 mm for males and 26.9 mm for females, Bozlar: 29.5 mm for both genders and Karazincir: 32.6 mm for males and 31.9 mm for females). Results: The patient mean age was 53.0±9.7 years (66.5% were women). Body surface area (BSA) correlated moderately with the mPA diameter (r=0.423, p<0.001). The prevalence of mPA dilatation varied from 5.9% (Karazincir) to 33.7% (Framingham Heart Study) in the overall study population. Conclusion: The prevalence of mPA dilatation is high in a CCTA patient population when using a cut-off value from the Framingham Heart Study.
Keywords: Dilatation, CT-angiography, pulmonary artery, prevalence
Pulmonary hypertension, caused either by primary pulmonary artery hypertension or by secondary reasons, such as chronic thromboembolic disease, is the most common cause behind main pulmonary artery (mPA) dilatation (1). Dilatation of the mPA is a common incidental finding in chest imaging and often leads to medical consultation (1-3). Significant mPA dilatation may result in life-threatening complications, such as rupture or dissection of the mPA, which can lead to a critical cardiac tamponade (4). In addition, an aneurysmal dilatation may compress intrathoracic structures such as the left main coronary artery, pulmonary veins, the main bronchi, or recurrent laryngeal nerves. Regardless of the possibly fatal complications, standardized treatment options are not well established for the mPA aneurysms, pseudo-aneurysms and dissection (4).
The relationship between the mPA diameters and body surface area (BSA) has been demonstrated in several studies (5-7). The association between the mPA and ascending aortic (AA) diameters has been examined for the prediction of pulmonary arterial (PA) pressure (8,9). Ng et al. reported that a ratio of mPA to AA diameter greater than 1 is associated with a mean PA pressure of 20 mmHg or higher, with a sensitivity of 70%, a specificity of 92%, and a positive predictive value of 96% (10). Case reports of patients with both mPA and AA dilatation have been published (11,12).
Nonetheless, studies of the prevalence of mPA dilatation are scarce. Our main purpose was to evaluate the prevalence of mPA dilatation in a coronary artery CT angiography (CCTA) population where mPA dilatation is a common incidental finding, especially when the diagnostics of coronary artery disease (CAD) has lately shifted towards CCTA according to ESC 2019 guidelines (13). The other purposes were to compare the published reference values for mPA dilatation in our population and to evaluate the associations between mPA and AA dilatation and identify predisposing risk factors for mPA dilatation.
Patients and Methods
The study was approved by the Ethics Committee of the Hospital District of Northern Savo and it follows the rules of Declaration of Helsinki. CCTA imaging was performed on the basis of clinical indications; thus, the patients were not exposed to additional radiation and patients’ clinical treatment was unaffected by this retrospective study. The population of the present study has also been evaluated in prior publications (14-16).
Study population. This retrospective study examined 1065 consecutive patients with a low-to-moderate pretest probability for CAD who had been imaged with CCTA between January 2012 and March 2018 in Kuopio University Hospital. Seventy-nine patients were excluded due to motion artefacts or inadequate visibility of mPA or AA in CCTA and one patient due to age under 16 years. Thus, the final study population was 985 patients.
Risk factors for cardiovascular diseases as well as other characteristics were collected from the medical records for all patients. Details of the definition of the cardiovascular risk factors have been described in a prior study (15). The CCTA interpretation had been done by imaging cardiologists or cardiac radiologists with over 6 years of experience in cardiac imaging.
Pulmonary diseases (asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis and acute or chronic pulmonary embolism) were collected from the medical records for 100 patients having the largest mPA diameters. In addition, further diagnostic tests and other clinical consequences after CCTA were registered for these patients.
Height and weight at the time of CCTA were available for 732 patients. Obesity was determined when BMI was greater than or equal to 30 kg/m2 and overweight when BMI was greater than or equal to 25 kg/m2. To obtain body size adjusted values for mPA diameter, an mPA size index (mPASI), determined as the ratio of mPA diameter and BSA, was calculated for further analysis.
CCTA data acquisition. CCTA imaging was performed during mid-diastole according to routine clinical practice using four different CT scanners capable of ECG-gated fast coronary imaging (Somatom Definition AS 64; Somatom Definition AS+ 128; Definition Edge; Definition Flash, Siemens Medical Solutions, Erlangen, Germany). The patients were scanned in the supine position with their hands above their head to avoid artefacts. The tube voltage, varying between 80 and 120 kV, was adjusted according to the patient’s size. The image area extended from the tracheal bifurcation to the inferior cardiac apex. The in-plane resolution was 512 x 512 pixels, with z-axis coverage including the area from the bifurcation to the diaphragm. The heart rate was optimized to be below 65 beats/minute by administering 5-20 mg metoprolol succinate (Seloken 1 mg/ml, Genexi, Fontenay sous Bois, France) intravenously. The detailed imaging procedure has been presented in a previous study (14).
Measurements of pulmonary artery and ascending aortic diameters. One experienced observer (SPK) retrospectively analyzed the CCTA images on an IDS7 diagnostic workstation (version 17.3.6; Sectra Imtec, Linköping, Sweden). The slice thickness was 0.6 mm. The transverse axial diameter of the mPA at the level of the bifurcation of the right PA was measured (Figure 1A). The diameter of AA was assessed at the same level as the mPA perpendicular to the centerline of aorta and it was named as AAp. Additionally, the diameter of the aortic root (AAr) was measured at the maximal sinus valsalva plane (Figure 1B-D) (17).
Figure 1. (A) The transverse axial diameter (1) of the mPA was measured at the level of the bifurcation of the right pulmonary artery. (B) The diameter of aortic root was measured at sinus valsalva plane (2) and the diameter of AA was measured from the outer wall to the outer wall at the same level, designated here as the mPA measurement (3) by using multiplanar reconstruction (C-D).

Reference values for mPA and aortic diameters. The published reference values for mPA diameters have been compiled in Table I according to the different imaging modalities.
Table I. Literature review of the different reference values of main pulmonary artery (mPA).
COPD, Chronic obstructive pulmonary disease; CT, computed tomography; ECG, electrocardiography; MRI, magnetic resonance imaging
Truong et al. investigated 706 healthy subjects without pulmonary or cardiovascular diseases from the Framingham Heart Study who had been imaged with non-contrast ECG gated eight-slice cardiac multi-detector CT (5). The 90th percentile cut-off value for normal mPA diameter was defined to be 28.9 mm for males and 26.9 mm for females (5).
Previously, Bozlar et al. had utilized contrast-enhanced multidetector CT to evaluate 126 healthy adults with normal thoracic CT findings and normal PA pressure. Gender was not significantly associated with mPA diameters (p=0.08); thus, the 95th percentile cut-off value for both genders was set to 29.5 mm (18).
Karazincir et al. investigated 112 patients without pulmonary pathology with contrast-enhanced chest CT. On the basis of their results, the 95% cut-off value for mPA diameter was set to 32.6 mm for males and 31.9 mm for females (6).
The reference values for normal AA, in terms, according to the European Society of Cardiology (ESC) guidelines for aortic diseases (2014) have been set to be less than 40 mm at the AAr and AAp levels (17).
Statistical analysis. The normality of the mPA diameter data was analyzed by using the Kolmogorov-Smirnov test. Since parameters with a skewed distribution (association between mPA diameter and cardiovascular risk factors) were tested with the Mann-Whitney U-test and the results are presented as median values with interquartile range (IQR).
Correlations between the mPA diameters and continuous scaled parameters were tested by the Spearman correlation test. Multivariate logistic regression analysis was used to test the relationship between mPA diameter and cardiovascular risk factors. Statistical significance was set to p<0.05 and high statistical significance to p<0.001. All statistical analyses were performed by using SPSS Statistics 27 (IBM, Chicago, USA).
Results
Study population. The mean age of the patients (n=985) was 53.0±9.7 years and the majority of patients were women (n=655, 66.5%). Most patients (87.5%) had at least one cardiovascular risk factor. Detailed characteristics of the study population as well as mean values of mPA and AA diameters and PA/AA-ratio are shown in Table II. Illustrative cases of mPA and AA dilatation are presented in Figure 2.
Table II. Detailed characteristics of the study population.
AAp, Ascending aorta at level of main pulmonary artery; AAr, ascending aortic root; BMI, body mass index; BSA, body surface area; CAD, coronary artery disease; mPA, main pulmonary artery. Smoking determined as patients who still smoked regularly or had stopped <30 years in the past.
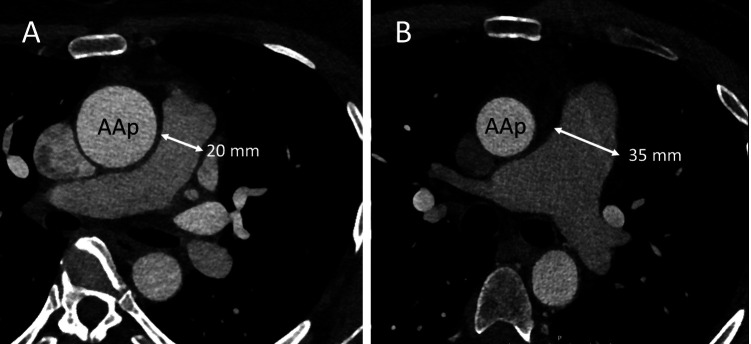
Figure 2. Illustrative images of two patient cases. Aortic diameters were measured with multiplanar reconstruction perpendicular to the centerline of the vessel. (A) A 47-year-old female. The diameter of the ascending aorta at the level of pulmonary artery (AAp) is larger (37 mm) than the diameter of the main pulmonary artery (mPA, 20 mm). At the aortic root (AAr), the diameter is 34 mm. (B) A 52-year-old male. The diameter of the mPA (35 mm) clearly exceeds that of the AAp (30 mm) and can be considered as dilated according to the reference values (5). The diameter of the AAr was 39 mm.
Prevalence of mPA dilatation. According to the cut-off values published by Truong et al. (Framingham Heart Study), the overall prevalence of mPA dilatation was as high as 33.7% (34.8% in males and 33.1% in females). On the other hand, if one applies the cut-off values published by Bozlar et al., then our overall prevalence of mPA dilatation would be lower; 18.1% (29.1% in males and 12.5% in females). When using the cut-off values published by Karazincir et al., the overall prevalence of mPA dilatation was as low as 5.9%. (9.4% in males and 4.1% in females). The prevalence of the mPA dilatation according to these different reference values are presented in Figure 3. The overall prevalence of AA dilatation was 22.5%, being as high as 52.7% in males but only 7.8% in females according to ESC guidelines for aortic diseases.
Figure 3. Prevalence of the main pulmonary artery (mPA) dilatation according to different reference values published in the literature.
Factors correlating to mPA diameter. Of the 100 patients having the largest mPA diameters (range=31.2 mm-44.1 mm), 14 (14%) had asthma, 3 (3%) had suffered prior pulmonary embolism, 1 (1%) had COPD and 1 (1%) had pulmonary fibrosis. In the present study, the presence of pulmonary diseases did not associate significantly (p>0.05) with larger mPA diameters.
The diameter of mPA correlated significantly with BSA (r=0.423, p<0.001). Significant correlations were also found with weight (r=0.418, p<0.001), height (r=0.286, p<0.001), and BMI (r=0.325, p<0.001). The age of the patient did not correlate with the mPA diameter (r=-0.020, p=0.537) but correlated rather weakly with body size-adjusted mPASI values (r=0.245, p<0.001).
In Table III, the factors which independently associated with the mPA diameter, mPASI and PA/AA-ratio, are presented. When testing these factors with multivariate regression analysis, only CAD (B=0.6 mm, p=0.016), male gender (B=1.9 mm, p<0.001) and obesity (B=1.7 mm, p<0.001) associated with the larger mPA diameters whereas positive family history for CAD (B=-0.8 mm, p=0.004) associated with the smaller diameter of the mPA.
Table III. Risk factors for increased diameter of the main pulmonary artery (mPA). The main pulmonary artery size index (mPASI) was determined as the ratio of mPA diameter to body surface area. The PA/AA-ratio was determined as the ratio of mPA diameter and ascending aortic diameter.
AA, Ascending aorta; BAV, bicuspid aortic valve; CAD, coronary artery disease.
mPA diameters in a ’healthy’ subpopulation. From the total study population (n = 985), we selected for further analyses a ‘healthy’ subgroup with (1) no CAD (no signs of over 50% stenosis or coronary calcification) in CCTA, (2) non-smokers, and (3) not overweight. The size of this ‘healthy’ subgroup was 103 patients and the mean age was 52.1±9.9 years with the majority being females (n=83, 78.3%). The mean mPA diameter was 24.6±3.6 mm, being 26.8±4.7 mm in males and 24.0±3.1 mm in females. In this ‘healthy’ subpopulation, the overall prevalence of mPA dilatation was 18.9% when using the cut-off values devised by Truong et al., i.e., almost three times higher than when using the cut-off values by Bozlar et al. (6.6%), and almost ten times higher than when using the cut-off values supplied by Karazincir et al. (1.9%).
The relationship between mPA and AA diameters. The diameter of mPA correlated moderately with the AAp diameter (r=0.461, p<0.001) and with the AAr diameter (r=0.486, p<0.001). The body size adjusted mPA diameter, mPASI, correlated only weakly with the AA diameter (r=0.178, p<0.001). Patients with dilated AA according to the ESC guidelines had also larger mPA diameters (mean 28.6 mm, IQR 26.5-31.5 mm) than the patients with normal AA (mean 25.6 mm, IQR 23.5-27.9 mm, p<0.001). However, AA dilatation was not associated with greater mPASI values when adjusted for the body size (p>0.05).
Clinical consequences of the diagnosis of mPA dilatation. Among the 100 patients having the largest mPA diameters, the diagnosis of mPA dilatation did not lead to any clinical consequences in 94 patients (94%). However, mPA dilatation led to additional imaging in 4 patients (cardiac MRI for 3 patients and pulmonary high-resolution CT for one patient). As a result of these subsequent diagnostic imaging, one ventricular septum defect closure and one percutaneous coiling of pulmonary arteriovenous malformation were performed.
Discussion
The key finding of the present study was that pulmonary artery dilatation is a frequent incidental finding when examining a consecutive population undergoing CCTA. A relatively high number of our patients had one or more risk factors for cardiovascular diseases, therefore, the study population could not be considered as a healthy population. However, the patients were relatively healthy with regard to pulmonary diseases. In general, this population represents well a real-life patient population in which the diagnosis of mPA dilatation is made incidentally. Since the diagnostics of CAD has lately shifted towards CCTA, this study provides valuable information for the daily clinical work.
The published mPA reference values can be divided according to the imaging modality that has been used (Table I). Truong et al. described reference values for mPA dilatation in the so far largest study population consisting of 700 middle-aged Americans (Framingham Heart Study) (5) who were healthy with respect to pulmonary and cardiovascular diseases. In the report of Gallego et al., the prevalence of mPA dilatation was studied with CT in patients with congenital heart disease and the prevalence of mPA dilatation in that population was 18% when using an upper limit of 29 mm for both genders (19). The number of reference values obtained with CT imaging is highest in the published data (5,6,18,20) including both contrast enhanced (6,18) and non-enhanced (5,20) CT studies. In addition, reference values obtained with MRI (7) and ultrasound imaging (21) have been published. We selected the reference values based on CT imaging studies so that they would be comparable with our own CT based data.
According to the best of our knowledge, the prevalence of mPA dilatation has not been widely studied. According to the reference values adapted from the Framingham Heart Study, the prevalence of mPA dilatation was high, present in approximately every third patient in our study population. One can speculate that there may be several factors explaining this high prevalence. One of the possible factors is the size of the patient. In our study, the diameter of mPA correlated significantly with BMI, height and especially BSA, paralleling the results of several previously published studies (5-7). In the study of Truong et al., the healthy subgroup did not include overweight patients. In our ‘healthy’ subgroup of patients with normal weight, the prevalence of mPA dilatation was only 19%. The prevalence of mPA dilatation was smaller if we applied the reference values published by Bozlar et al. (18) and Karazincir et al. (6) in smaller study populations based on Turkish citizens. It must also be noted that the traditional method to measure the cut-off values is mean±2SD (95%) (22) while the results in the study by Truong et al. are given as 90% percentiles. Obviously, the 95% cutoff value seems to be reasonable if one wishes to avoid over-diagnosis. Additionally, the method of measuring the mPA diameter is crucial for reproducibility. Most typical method is to measure the diameter from the transverse axial slice at the level of the bifurcation of the right PA (5).
The relationship between mPA and aortic diameter has been mainly studied by calculating the PA/AA-ratio for the prediction of pulmonary pressure (8,9). However, certain genetic syndromes such as Marfan syndrome predispose to both aortic and PA dilatation (23). We have previously shown a high prevalence of AA dilatation in this same population (15). The present study detected a positive correlation between the mPA and AA diameters. It is obvious that the larger main vessel diameters were evident in patients with larger BSAs, in other words, both aorta and pulmonary arteries are larger in patients with a greater body size. However, if there is a disease behind the mPA dilatation, this correlation may no longer be valid.
It is well recognized that obesity results in elevated PA pressure (24) and mPA dilatation, thus becoming increasingly recognized as an independent risk factor for mPA dilatation. The significantly increased PA/AA-ratio found here supports this suggestion. Several mechanisms have been postulated to cause pulmonary hypertension in obese patients e.g., obstructive sleep apnea, obesity hypoventilation syndrome and obesity cardiomyopathy (25).
It is known that pulmonary hypertension with different etiologies is the most common cause of mPA dilatation. COPD is a common cause for pulmonary hypertension; this disease is mainly induced by smoking, pulmonary fibrosis and embolism (26). However, in our study only one patient among the 100 patients having the largest mPA diameters had COPD while 14 had asthma. Thus, the present study population was relatively healthy regarding pulmonary diseases. One fourth of our patients were smokers but smoking was not associated with mPA dilatation. It could be speculated that since the patients were rather young, smoking might not yet have progressed to COPD and had not seriously affected in pulmonary pressure.
Our study also investigated the cardiovascular risk factors or possible predictive issues associating with mPA dilatation. Previously, hypertension, diabetes and CAD have been reported to associate with increased mPA diameter (5). However, in the multivariate regression analysis, only CAD, male gender and obesity associated with the increased mPA diameter, which might indicate that after all the patient’s body size is the most affecting factor. Left ventricular systolic and diastolic dysfunction can lead to mPA dilatation. Both of these conditions can be caused by CAD (26). In the present study, approximately 40% of the patients had been diagnosed with CAD. It is well-known that CAD associates with high mortality and morbidity rates and its prevention is crucial (27).
One of the main limitations of this retrospective study was the limited data of patient lung capacity and underlying pulmonary diseases. Since all the factors that influence in ventilation can have an impact on pulmonary circulation, perfusion and vascular resistance, they also can have a high impact on the diameter of the mPA. Another limitation is that the reproducibility of our mPA measurements was not assessed. However, the reproducibility of the method has been reported to be excellent in previous studies (5,6), and the reproducibility of the AA measurements have been shown to be excellent in a previous MRI study (28). A high number of patients had risk factors for cardiovascular diseases, thus the prevalence of mPA dilatation is higher than in the healthy population. However, examined population represents that clinical population where mPA dilatation diagnosis is usually done.
In conclusion, the prevalence of mPA dilatation proved to be high in a consecutive CCTA population. Every third patient had dilated mPA when using the current clinical reference values adopted from the Framingham Heart Study. The diameter of mPA correlated strongly with BSA suggesting that it would be reasonable to use body size-adjusted reference values in clinical practice. In the present study population, the clinical impact of the observed mPA dilatation was low and led rarely to clinical interventions. In the future, the increasing number of patients scheduled for CCTA makes it important to accurately identify those patients with mPA dilatation of clinical significance.
Conflicts of Interest
The Authors have no conflicts of interest.
Authors’ Contributions
SPK: Study conception, data acquisition, data analysis, data interpretation, drafting and writing manuscript. TL: Study conception, data interpretation and manuscript review. MK: Data acquisition. JP: Data acquisition. JV: Data acquisition. RV: Study conception, data interpretation and manuscript review. MH: Study conception, data interpretation and manuscript review and writing.
Acknowledgements
This study has received funding by Instrumentarium Science Foundation (SPK).
References
- 1.Raymond TE, Khabbaza JE, Yadav R, Tonelli AR. Significance of main pulmonary artery dilation on imaging studies. Ann Am Thorac Soc. 2014;11(10):1623–1632. doi: 10.1513/AnnalsATS.201406-253PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J, Kirschner J, Pawa S, Wiener DE, Newman DH, Shah K. Computed tomography use in the adult emergency department of an academic urban hospital from 2001 to 2007. Ann Emerg Med. 2010;56(6):591–596. doi: 10.1016/j.annemergmed.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Wittram C, Meehan MJ, Halpern EF, Shepard JA, McLoud TC, Thrall JH. Trends in thoracic radiology over a decade at a large academic medical center. J Thorac Imaging. 2004;19(3):164–170. doi: 10.1097/01.rti.0000117623.02841.e6. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 5.Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, O’Donnell CJ, Hoffmann U. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging. 2012;5(1):147–154. doi: 10.1161/CIRCIMAGING.111.968610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karazincir S, Balci A, Seyfeli E, Akoğlu S, Babayiğit C, Akgül F, Yalçin F, Eğilmez E. CT assessment of main pulmonary artery diameter. Diagn Interv Radiol. 2008;14(2):72–74. [PubMed] [Google Scholar]
- 7.Burman ED, Keegan J, Kilner PJ. Pulmonary artery diameters, cross sectional areas and area changes measured by cine cardiovascular magnetic resonance in healthy volunteers. J Cardiovasc Magn Reson. 2016;18:12. doi: 10.1186/s12968-016-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol. 1984;19(1):16–22. doi: 10.1097/00004424-198401000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Mahammedi A, Oshmyansky A, Hassoun PM, Thiemann DR, Siegelman SS. Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging. 2013;28(2):96–103. doi: 10.1097/RTI.0b013e318271c2eb. [DOI] [PubMed] [Google Scholar]
- 10.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14(4):270–278. doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Sughimoto K, Nakano K, Gomi A, Nakatani H, Nakamura Y, Sato A. Pulmonary artery aneurysm with ascending aortic aneurysm concomitant with bilateral bicuspid semilunar valves. Ann Thorac Surg. 2006;82(6):2270–2272. doi: 10.1016/j.athoracsur.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 12.Dyamenahalli U, Abraham B, Fontenot E, Prasad V, Imamura M. Pathologic aneurysmal dilation of the ascending aorta and dilation of the main pulmonary artery in patients with Kabuki syndrome: valve-sparing aortic root replacement. Congenit Heart Dis. 2007;2(6):424–428. doi: 10.1111/j.1747-0803.2007.00136.x. [DOI] [PubMed] [Google Scholar]
- 13.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, ESC Scientific Document Group 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 14.Korhonen M, Mustonen P, Hedman M, Vienonen J, Onatsu J, Vanninen R, Taina M. Left atrial appendage morphology and relative contrast agent concentration in patients undergoing coronary artery CTA. Clin Radiol. 2018;73(11):982.e17–982.e26. doi: 10.1016/j.crad.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Kauhanen SP, Saari P, Jaakkola P, Korhonen M, Parkkonen J, Vienonen J, Vanninen R, Liimatainen T, Hedman M. High prevalence of ascending aortic dilatation in a consecutive coronary CT angiography patient population. Eur Radiol. 2020;30(2):1079–1087. doi: 10.1007/s00330-019-06433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kauhanen SP, Liimatainen T, Kariniemi E, Korhonen M, Parkkonen J, Vienonen J, Vanninen R, Hedman M. A smaller heart-aorta-angle associates with ascending aortic dilatation and increases wall shear stress. Eur Radiol. 2020;30(9):5149–5157. doi: 10.1007/s00330-020-06852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ, ESC Committee for Practice Guidelines 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 18.Bozlar U, Ors F, Deniz O, Uzun M, Gumus S, Ugurel MS, Yazar F, Tayfun C. Pulmonary artery diameters measured by multidetector-row computed tomography in healthy adults. Acta Radiol. 2007;48(10):1086–1091. doi: 10.1080/02841850701545755. [DOI] [PubMed] [Google Scholar]
- 19.Gallego P, Rodríguez-Puras MJ, Serrano Gotarredona P, Valverde I, Manso B, González-Calle A, Adsuar A, Cubero JM, Díaz de la Llera L, Ordoñez A, Hosseinpour AR. Prevalence and prognostic significance of pulmonary artery aneurysms in adults with congenital heart disease. Int J Cardiol. 2018;270:120–125. doi: 10.1016/j.ijcard.2018.05.129. [DOI] [PubMed] [Google Scholar]
- 20.Edwards PD, Bull RK, Coulden R. CT measurement of main pulmonary artery diameter. Br J Radiol. 1998;71(850):1018–1020. doi: 10.1259/bjr.71.850.10211060. [DOI] [PubMed] [Google Scholar]
- 21.Sheikhzadeh S, De Backer J, Gorgan NR, Rybczynski M, Hillebrand M, Schüler H, Bernhardt AM, Koschyk D, Bannas P, Keyser B, Mortensen K, Radke RM, Mir TS, Kölbel T, Robinson PN, Schmidtke J, Berger J, Blankenberg S, von Kodolitsch Y. The main pulmonary artery in adults: a controlled multicenter study with assessment of echocardiographic reference values, and the frequency of dilatation and aneurysm in Marfan syndrome. Orphanet J Rare Dis. 2014;9:203. doi: 10.1186/s13023-014-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma B, Jain R. Right choice of a method for determination of cut-off values: A statistical tool for a diagnostic test. Asian Journal of Medical Sciences. 2018;5(3):30–34. doi: 10.3126/ajms.v5i3.9296. [DOI] [Google Scholar]
- 23.Dean JC. Marfan syndrome: clinical diagnosis and management. Eur J Hum Genet. 2007;15(7):724–733. doi: 10.1038/sj.ejhg.5201851. [DOI] [PubMed] [Google Scholar]
- 24.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–210. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman SE, Andrus BW. Obesity and pulmonary hypertension: a review of pathophysiologic mechanisms. J Obes. 2012;2012:505274. doi: 10.1155/2012/505274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeper MM, Ghofrani HA, Grünig E, Klose H, Olschewski H, Rosenkranz S. Pulmonary hypertension. Dtsch Arztebl Int. 2017;114(5):73–84. doi: 10.3238/arztebl.2017.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauhanen SP, Hedman M, Kariniemi E, Jaakkola P, Vanninen R, Saari P, Liimatainen T. Aortic dilatation associates with flow displacement and increased circumferential wall shear stress in patients without aortic stenosis: A prospective clinical study. J Magn Reson Imaging. 2019;50(1):136–145. doi: 10.1002/jmri.26655. [DOI] [PubMed] [Google Scholar]