Abstract
Background/Aim: The molecular mechanisms underlying the association between cell cycle and asthma are poorly understood, and cyclin D1 (CCND1) is found to be upregulated in asthma airway smooth muscle. We investigated whether the most frequently examined functional variants in CCND1 determine asthma susceptibility. Materials and Methods: We genotyped 651 participants for single-nucleotide polymorphisms (SNPs) at rs9344 and rs678653 on CCND1 and assessed the association of these SNPs with asthma risk. Results: Significant differences were found in the distributions of genotypic (p=0.0064) and allelic (p=0.0021) frequencies of CCND1 rs9344. In addition, AG or GG carriers had 0.63- or 0.48-fold adjusted odds ratios for asthma risk (95%confidence intervals=0.48-0.92 and 0.22-0.78, respectively) than those who carried the AA wild-type. Conclusion: Our results suggest that cell cycle regulation may play a role in asthma initiation and development, and the CCND1 rs9344 genotype may serve as an early detection marker for asthma.
Keywords: Asthma, CCND1, cyclin D1, genotype, polymorphism, Taiwan
Asthma is a complex airway disease characterized by eosinophilic infiltration, airflow obstruction, mucus hypersecretion, goblet cell hyperplasia, and airway hyper-responsiveness and remodeling. According to a report by the World Allergy Organization, up to 300 million people suffer from asthma, and its prevalence continues to increase. Thus, asthma is a worldwide health problem (1). Clinically, asthmatic patients usually present with wheezing, cough, and dyspnea. Asthma has an estimated heritability higher than 60% (2,3), but due to high immunological and phenotypical variations among asthmatic patients, asthma is considerably heterogeneous (4). Studies conducted in an animal disease model have suggested that approximately 200 genes are closely related to the etiology of asthma (5). Moreover, a multitude of environmental risk factors, including indoor and outdoor allergens, pollutants, smog, and air particles, may all contribute to higher risk of asthma. To date, a rather substantial body of evidence has documented typical structural alterations in the airways and lung parenchyma among asthmatic patients. These lines of evidence show that abnormal hypertrophy and hyperplasia of airway cells, such as goblet cells, smooth muscle cells, fibroblasts, and epithelial cells, take part in the processes of airway remodeling in asthma (6-10). At the molecular level, the proliferation of these human airway cells is controlled by cell cycle regulatory genes. It is possible that the genotypes of cell cycle regulatory genes may determine the personal susceptibility to asthma and serve as predictive biomarkers. However, related reports are extremely few.
It is well known that one of the characteristics of cancer cells is cell cycle deregulation, while cyclin D1 (encoded by the CCND1 gene) may play an important role in the initiation of tumorigenesis and cancer progression (11). Teaming up with other cyclins and their dependent kinases, cyclin D1 is the gatekeeper protein critically responsible for regulating the transition from the G1 phase to the S phase of the cell cycle (12). In other words, cyclin D1 is the major determinant of cell fate among cell proliferation, cell cycle arrest, and cell death (13-16). Variations at the genomic level, including variant gene amplification, posttranscriptional or posttranslational modifications, gene rearrangements, and polymorphic genotypes of CCND1, may result in abnormal expression levels and/or dysfunction of cyclin D1, leading to higher risk of cancer (17-21).
In 2010, Du and colleagues transfected an antisense CCND1 and abolished the phorbol myristate acetate-induced transition from G1 to S phase and the proliferation of human airway smooth muscle cells (22). It has also been demonstrated that the genotypes of the rs9433 polymorphism in CCND1, combined with particulate matter (PM10) air pollution, modify the degree to which improved air quality may benefit respiratory function in adults in a Swiss population (23).
To date, there has only been one study investigating the association of CCND1 genotype with asthma risk (24). The authors found that the CCND1 rs9344 GG genotype is a protective factor, and obesity status may increase the influence of CCND1 on asthma susceptibility (24). Based on their positive findings, we became interested in revealing the genotypic distribution of CCND1 rs9344 and rs678653 among asthmatic and non-asthmatic Taiwanese and to evaluate their potential to serve as predictive markers for asthma risk determination in Taiwan.
Materials and Methods
Recruitment of participants into asthmatic and non-asthmatic control groups. A total of 198 asthmatic patients were identified at the China Medical University Hospital in central Taiwan. Briefly, their medical histories and clinical data were collected and added to the asthmatic database. In the same period, 453 healthy individuals, age-matched (±5 years) with asthmatic patients admitted to the same hospital for routine health examinations (confirmed to reside in similar residential areas and of Taiwanese nationality), without any diagnosis of neoplastic disease or any malignancy, were manually enrolled into our age- and gender-matched control group. All the participants enrolled in this study were asked to provide informed consent to the use of their tissue and data, while all the experimental protocols were approved and supervised by Human Research Committees of China Medical University hospital (CMUH106-REC1-004). After individual interview, 5-ml blood samples were collected for further DNA extraction, CCND1 genotyping, and statistical analysis.
CCND1 rs9344 and rs678653 genotyping procedures. Genomic DNA was extracted from each person’s peripheral blood leukocytes within 24 h after collection, carefully quantitated, diluted, and stored in a −80˚C freezer as previously described (25-27). The genotypic patterns at CCND1 rs9344 were determined for all subjects by typical polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis as in our previous publications (28,29).
The primer sequences for CCND1 rs9344 were: forward 5’-GTG AAG TTC ATT TCC AAT CCG C-3’ and reverse 5’-GGG ACATCA CCC TCA CTTAC-3’; those for CCND1 rs678653 were: forward 5’-CTC TTG GTT ACA GTA GCG TAG C-3’ and reverse 5’-ATC GTA GGA GTG GGA CAG GT-3’. The PCR reaction conditions were: 1) an initial denaturation at 94˚C for 5 min; 2) 40 cycles of 94˚C for 30 s, 55˚C for 30 s, and 72˚C for 30 s; and 3) a final extension at 72˚C for 10 min that was repeated twice. After PCR, the resultant 167-bp PCR products of the CCND1 rs9344 were mixed with 2 U Nci I and incubated for 3 h at 37˚C. The G-allele PCR products can be further digested, while the products of the A allele cannot. Two fragments, 145 and 22 bp, are present if the product contained the G allele. As for CCND1 rs678653, the resultant 159-bp PCR products were mixed with 2 U Hae III and incubated for 3 h at 37˚C. On digestion with Hae III, the PCR product arising from the G allele was digested into fragments of 111, 26, and 22 bp, whereas the C allele was digested into fragments of 137 and 22 bp. Then, 10 μl of the products from each subject were separated by 3% agarose gel electrophoresis. All genotyping procedures were conducted at least in duplicate by a minimum of two different researchers listed in the Acknowledgments, independently and blindly, and the results were in 100% agreement with each other.
CCND1 rs9344 and rs678653 genotype analysis methods. An unpaired Student’s t-test was applied for the comparison of age index between the asthmatic and non-asthmatic groups. Pearson’s Chi-square was applied to compare the distributions of values among subgroups. The associations between CCND1 genotypes and asthma were estimated via odds ratios (ORs) and 95% confidence intervals (CIs). Any difference was taken as significant when p-value <0.05.
Results
The frequency distributions of selected demographic indexes, such as age and gender, for the 198 asthmatic patients and 453 non-asthmatic controls are compared and presented in Table I. Since we matched all controls and cases by age and gender, there was no difference in age and gender distribution between the case and control groups (p=0.2972 and 0.9956, respectively) (Table I).
Table I. Bone mineral density and bone metabolism-related markers of 47 patients.
aBased on chi-square without Yate’s correction test.
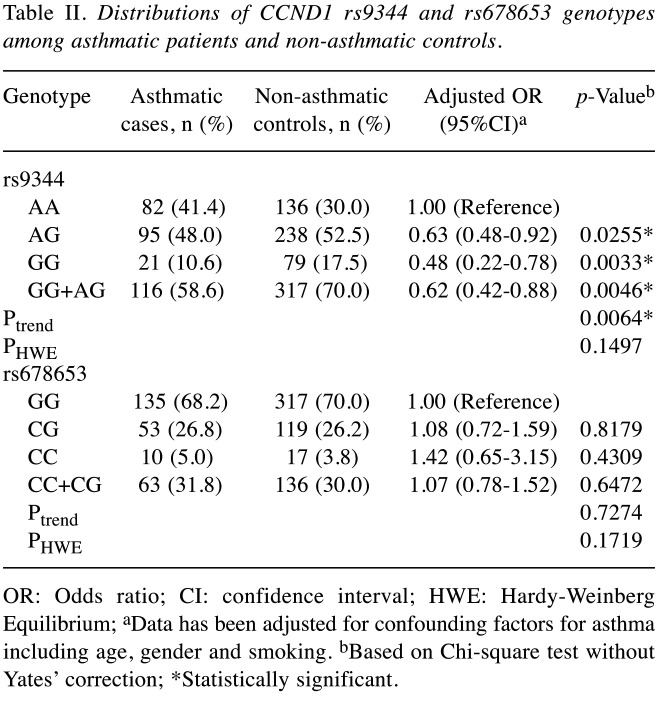
In Table II, the distributions of the genetic frequencies of the CCND1 rs9344 and rs678653 among asthmatic patients and non-asthmatic healthy controls are shown. Interestingly, the results showed that the genetic frequencies of CCND1 rs9344 were differentially distributed between the case and control groups (p=0.0064). The adjusted ORs of the variant AG and GG were 0.63 (95%CI=0.48-0.92) and 0.48 (95%CI=0.22-0.78), respectively, compared with the AA genotype. The results obtained by comparing the combined AG+GG with the AA genotype (adjusted OR=0.62, 95%CI=0.42-0.88) also suggested that people with the AG or GG genotypes were at lower risk than those with the AA genotype (p=0.0046) (Table II, top part). In contrast, there was no such differential distribution of the CCND1 rs678653 genotypes (Table II, bottom part).
Table II. Distributions of CCND1 rs9344 and rs678653 genotypes among asthmatic patients and non-asthmatic controls.
OR: Odds ratio; CI: confidence interval; HWE: Hardy-Weinberg Equilibrium; aData has been adjusted for confounding factors for asthma including age, gender and smoking. bBased on Chi-square test without Yates’ correction; *Statistically significant.
In Table III, the allelic frequencies of the CCND1 rs9344 and rs678653 polymorphisms among asthmatic patients and non-asthmatic healthy controls are shown. The allelic frequencies of CCND1 rs9344 were differentially distributed between the asthmatic and control groups (p=0.0021). In detail, people with the G allele at CCND1 rs9344 had a lower asthma risk than those with the A allele after adjustment for age, gender, and smoking status (adjusted OR=0.70, 95%CI=0.52-0.78). In contrast, neither the genetic nor the allelic frequency of CCND1 rs678653 were differentially distributed between the two groups (Table III).
Table III. Distributions of CCND1 rs9344 and rs678653 genotypes among asthmatic patients and non-asthmatic controls.
OR: Odds ratio; CI: confidence interval. aData has been adjusted for confounding factors for asthma including age, gender and smoking. bBased on Chi-square test without Yates’ correction; *Statistically significant.
Discussion
Asthma is a chronic inflammatory disorder of the airways. Potential inflammatory markers for its early detection and prediction in asthmatic adult patients in Taiwan have been revealed, including interleukin-10 rs3021097 (30) and interleukin-12 rs568408 (31), whereas some others are not (30-32). However, little is known about the contribution of individual genotypes of cell cycle regulation genes to asthma risk. In the current study, we investigated the association of CCND1 rs9344 and rs678653 genotypes and asthma risk in a representative Taiwanese population including 198 asthmatic cases and 453 non-asthmatic controls. The main study findings include 1) subjects carrying the AG and GG genotypes (or allele G), were at of lower risk of asthma compared with those carrying the wild-type AA genotype (or A allele) on CCND1 rs9344 (Tables II and III); 2) as for CCND1 rs678653, no significantly differential distribution of genotypic or allelic frequencies was found (Tables II and III).
The single-nucleotide polymorphism in CCND1 rs9344 has been functionally well examined and found to be associated with the risk of many cancer types (33-37). It has also been associated with the risk of colorectal cancer (38), triple-negative breast cancer (39), lung cancer (40), and oral cancer (41), but not gastric cancer (42), in Taiwan. Cyclin D1 is found over-expressed in several types of epithelial cancers, such as colorectal carcinomas, and an increase in cyclin D1 not only contributes to elevated mismatch repair errors and microsatellite instability but also to tumor progression (43,44).
The current study is the second to reveal that the CCND1 rs9344 genotype is associated with asthma risk. These results are consistent with previous findings of the only study focused on the association of CCND1 rs9344 genotype with asthma risk, which proposed that the A allele of the CCND1 rs9344 polymorphism may serve as a risk factor for asthma (24). In that article, the authors went further to propose two important issues: 1) cell cycle genes such as CCND1 may interact with obesity to determine asthma susceptibility and severity; 2) the CCND1 rs9344 genotype is associated with physician-diagnosed asthma but not with current asthma (24). The actual mechanisms through which the A allele of CCND1 rs9344 acts to determine personal asthma susceptibility remains largely unknown. Since CCND1 rs9344 is an intronic polymorphism, it has been postulated that the polymorphic variants result in differences in alternative splicing. There is evidence showing that the A allele of CCND1 rs9344 may be related to higher expression levels of mRNA than the G allele and responsible for encoding a protein with a truncated C-terminal domain (45,46). The resulting cyclin D1 with an altered C-terminal domain is longer than the gene product of G allele, which may alter the accuracy of the G1/S cell cycle check point, lead to dysfunctional cell proliferation, and cause the overall airway remodeling (47,48). In the future, more investigations about the clinical features, asthma severity, and genetic-environmental interactions in asthma are urgently needed.
In summary, the CCND1 rs9344 genotype may determine personal susceptibility to asthma in Taiwan. The results provide evidence supporting the notion that the genotypes of cell cycle regulators such as those of CCND1 rs9344 examined here may be involved in the asthma development. Most interestingly, the A allele of CCND1 rs9344 may serve as a predictive marker for higher risk of asthma in Taiwan.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Research design was performed by LCH, CKL and HTC. Patient and questionnaire summaries were provided by LCH, STC and HTC. Experimental work was conducted by YCC, CWS, and TCW. Statistical analysis was performed by CLH, MMC, and TCW. LCH and BDT wrote the manuscript, whereas BDT reviewed it and all Authors are responsible for the revision.
Acknowledgements
This study was supported by research grants from Taichung Tzu Chi Hospital (TTCRD110-01) and China Medical University Hospital and Asia University (CMU109-ASIA-05). The assistance from Drs. in the Chest Department in sample and questionnaire collection, and the genotyping and analyzing of the work of Yu-Hsin Lin, Yi-Ru Huang, Yu-Ting Chin and Tai-Lin Huang are highly appreciated by all the Authors.
References
- 1.Mattiuzzi C, Lippi G. Worldwide asthma epidemiology: insights from the Global Health Data Exchange database. Int Forum Allergy Rhinol. 2020;10(1):75–80. doi: 10.1002/alr.22464. [DOI] [PubMed] [Google Scholar]
- 2.Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis. 1990;142(6 Pt 1):1351–1358. doi: 10.1164/ajrccm/142.6_Pt_1.1351. [DOI] [PubMed] [Google Scholar]
- 3.Edfors-Lubs ML. Allergy in 7000 twin pairs. Acta Allergol. 1971;26(4):249–285. doi: 10.1111/j.1398-9995.1971.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 4.Strina A, Barreto ML, Cooper PJ, Rodrigues LC. Risk factors for non-atopic asthma/wheeze in children and adolescents: a systematic review. Emerg Themes Epidemiol. 2014;11:5. doi: 10.1186/1742-7622-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temesi G, Virág V, Hadadi E, Ungvári I, Fodor LE, Bikov A, Nagy A, Gálffy G, Tamási L, Horváth I, Kiss A, Hullám G, Gézsi A, Sárközy P, Antal P, Buzás E, Szalai C. Novel genes in human asthma based on a mouse model of allergic airway inflammation and human investigations. Allergy Asthma Immunol Res. 2014;6(6):496–503. doi: 10.4168/aair.2014.6.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101(4):916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 7.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993;147(2):405–410. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164(3):474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 9.Kraft M, Lewis C, Pham D, Chu HW. IL-4, IL-13, and dexamethasone augment fibroblast proliferation in asthma. J Allergy Clin Immunol. 2001;107(4):602–606. doi: 10.1067/mai.2001.113760. [DOI] [PubMed] [Google Scholar]
- 10.Cohen L, E X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, Castro M, NHLBI Severe Asthma Research Program (SARP) Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med. 2007;176(2):138–145. doi: 10.1164/rccm.200607-1062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motokura T, Bloom T, Kim HG, Jüppner H, Ruderman JV, Kronenberg HM, Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 12.Walker JL, Assoian RK. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 2005;24(3):383–393. doi: 10.1007/s10555-005-5130-7. [DOI] [PubMed] [Google Scholar]
- 13.Hunter T, Pines J. Cyclins and cancer II: Cyclin D and CDK inhibitors come of age. Cell. 2020;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 14.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1(3):222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 15.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602(1):73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 16.Cook SJ, Balmanno K, Garner A, Millar T, Taverner C, Todd D. Regulation of cell cycle re-entry by growth, survival and stress signalling pathways. Biochem Soc Trans. 2000;28(2):233–240. doi: 10.1042/bst0280233. [DOI] [PubMed] [Google Scholar]
- 17.Jayasurya R, Sathyan KM, Lakshminarayanan K, Abraham T, Nalinakumari KR, Abraham EK, Nair MK, Kannan S. Phenotypic alterations in Rb pathway have more prognostic influence than p53 pathway proteins in oral carcinoma. Mod Pathol. 2005;18(8):1056–1066. doi: 10.1038/modpathol.3800387. [DOI] [PubMed] [Google Scholar]
- 18.Bova RJ, Quinn DI, Nankervis JS, Cole IE, Sheridan BF, Jensen MJ, Morgan GJ, Hughes CJ, Sutherland RL. Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin Cancer Res. 1999;5(10):2810–2819. [PubMed] [Google Scholar]
- 19.Michalides R, van Veelen N, Hart A, Loftus B, Wientjens E, Balm A. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995;55(5):975–978. [PubMed] [Google Scholar]
- 20.Wu Y, Fu H, Zhang H, Huang H, Chen M, Zhang L, Yang H, Qin D. Cyclin D1 (CCND1) G870A polymorphisms and cervical cancer susceptibility: a meta-analysis based on ten case-control studies. Tumour Biol. 2014;35(7):6913–6918. doi: 10.1007/s13277-014-1929-6. [DOI] [PubMed] [Google Scholar]
- 21.Vízkeleti L, Ecsedi S, Rákosy Z, Orosz A, Lázár V, Emri G, Koroknai V, Kiss T, Ádány R, Balázs M. The role of CCND1 alterations during the progression of cutaneous malignant melanoma. Tumour Biol. 2012;33(6):2189–2199. doi: 10.1007/s13277-012-0480-6. [DOI] [PubMed] [Google Scholar]
- 22.Du CL, Xu YJ, Liu XS, Xie JG, Xie M, Zhang ZX, Zhang J, Qiao LF. Up-regulation of cyclin D1 expression in asthma serum-sensitized human airway smooth muscle promotes proliferation via protein kinase C alpha. Exp Lung Res. 2010;36(4):201–210. doi: 10.3109/01902140903290022. [DOI] [PubMed] [Google Scholar]
- 23.Imboden M, Schwartz J, Schindler C, Curjuric I, Berger W, Liu SL, Russi EW, Ackermann-Liebrich U, Rochat T, Probst-Hensch NM, SAPALDIA Team Decreased PM10 exposure attenuates age-related lung function decline: genetic variants in p53, p21, and CCND1 modify this effect. Environ Health Perspect. 2009;117(9):1420–1427. doi: 10.1289/ehp.0800430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thun GA, Imboden M, Berger W, Rochat T, Probst-Hensch NM. The association of a variant in the cell cycle control gene CCND1 and obesity on the development of asthma in the Swiss SAPALDIA study. J Asthma. 2013;50(2):147–154. doi: 10.3109/02770903.2012.757776. [DOI] [PubMed] [Google Scholar]
- 25.Chen GL, Wang SC, Shen TC, Chang WS, Lin C, Hsia TC, Bau DT, Tsai CW. Significant association of chitinase 3-like 1 genotypes to asthma risk in Taiwan. In Vivo. 2021;35(2):799–803. doi: 10.21873/invivo.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen LH, Shen TC, Li CH, Chiu KL, Hsiau YC, Wang YC, Gong CL, Wang ZH, Chang WS, Tsai CW, Hsia TC, Bau DT. The significant interaction of excision repair cross-complementing group 1 genotypes and smoking to lung cancer risk. Cancer Genomics Proteomics. 2020;17(5):571–577. doi: 10.21873/cgp.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei JS, Chang WS, Hsu PC, Chen CC, Chin YT, Huang TL, Hsu YN, Kuo CC, Wang YC, Tsai CW, Gong CL, Bau DT. Significant association between the MiR146a genotypes and susceptibility to childhood acute lymphoblastic leukemia in Taiwan. Cancer Genomics Proteomics. 2020;17(2):175–180. doi: 10.21873/cgp.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih LC, Tsai CW, Chang WS, Shen TC, Wang YC, Yang JS, Lin ML, Wang ZH, Bau DT. Association of caspase-8 genotypes with the risk for nasopharyngeal carcinoma in Taiwan. Anticancer Res. 2020;40(10):5503–5508. doi: 10.21873/anticanres.14562. [DOI] [PubMed] [Google Scholar]
- 29.Shih LC, Chang WS, Lee HT, Wang YC, Wang ZH, Chao CY, Yu CC, Lin HY, Shen TC, Kuo CC, Tsai CW, Bau DT. Interaction of interleukin-16 genotypes with betel quid chewing behavior on oral cancer in Taiwan. In Vivo. 2020;34(4):1759–1764. doi: 10.21873/invivo.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia TC, Chang WS, Wang S, Shen TC, Hsiao WY, Liu CJ, Liang SJ, Chen WC, Tu CY, Tsai CW, Hsu CM, Bau DT. The contribution of interleukin-10 promoter genotypes to susceptibility to asthma in adults. In Vivo. 2015;29(6):695–699. [PubMed] [Google Scholar]
- 31.Shen TC, Tsai CW, Chang WS, Wang S, Chao CY, Hsiao CL, Chen WC, Hsia TC, Bau DT. Association of interleukin-12A rs568408 with susceptibility to asthma in Taiwan. Sci Rep. 2017;7(1):3199. doi: 10.1038/s41598-017-03523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao WY, Tsai CW, Chang WS, Wang S, Chao CY, Chen WC, Shen TC, Hsia TC, Bau DT. Association of polymorphisms in DNA repair gene XRCC3 with asthma in Taiwan. In Vivo. 2018;32(5):1039–1043. doi: 10.21873/invivo.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Shu X, Shu XO, Bolla MK, Kweon SS, Cai Q, Michailidou K, Wang Q, Dennis J, Park B, Matsuo K, Kwong A, Park SK, Wu AH, Teo SH, Iwasaki M, Choi JY, Li J, Hartman M, Shen CY, Muir K, Lophatananon A, Li B, Wen W, Gao YT, Xiang YB, Aronson KJ, Spinell JJ, Gago-Dominguez M, John EM, Kurian AW, Chang-Claude J, Chen ST, Dörk T, Evans DGR, Schmidt MK, Shin MH, Giles GG, Milne RL, Simard J, Kubo M, Kraft P, Kang D, Easton DF, Zheng W, Long J. Re-evaluating genetic variants identified in candidate gene studies of breast cancer risk using data from nearly 280,000 women of Asian and European ancestry. EBioMedicine. 2019;48:203–211. doi: 10.1016/j.ebiom.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu H, Cheng C, Wang Y, Kang M, Tang W, Chen S, Gu H, Liu C, Chen Y. Investigation of cyclin D1 rs9344 G>A polymorphism in colorectal cancer: a meta-analysis involving 13,642 subjects. Onco Targets Ther. 2016;9:6641–6650. doi: 10.2147/OTT.S116258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabir M, Baig RM, Mahjabeen I, Kayani MA. Significance of cyclin D1 polymorphisms in patients with head and neck cancer. Int J Biol Markers. 2013;28(1):49–55. doi: 10.5301/JBM.2012.9768. [DOI] [PubMed] [Google Scholar]
- 36.Castro FA, Haimila K, Sareneva I, Schmitt M, Lorenzo J, Kunkel N, Kumar R, Försti A, Kjellberg L, Hallmans G, Lehtinen M, Hemminki K, Pawlita M. Association of HLA-DRB1, interleukin-6 and cyclin D1 polymorphisms with cervical cancer in the Swedish population—a candidate gene approach. Int J Cancer. 2009;125(8):1851–1858. doi: 10.1002/ijc.24529. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Qian X, Wang S, Gao H, Wang L, Huo Y, Zhang S. CCND1 rs9344 polymorphisms are associated with the genetic susceptibility to cervical cancer in Chinese population. Mol Carcinog. 2012;51(2):196–205. doi: 10.1002/mc.20801. [DOI] [PubMed] [Google Scholar]
- 38.Huang CY, Tsai CW, Hsu CM, Chang WS, Shui HA, Bau DT. The significant association of CCND1 genotypes with colorectal cancer in Taiwan. Tumour Biol. 2015;36(8):6533–6540. doi: 10.1007/s13277-015-3347-9. [DOI] [PubMed] [Google Scholar]
- 39.Liu LC, Su CH, Wang HC, Chang WS, Tsai CW, Maa MC, Tsai CH, Tsai FJ, Bau DT. Contribution of personalized Cyclin D1 genotype to triple negative breast cancer risk. Biomedicine (Taipei) 2014;4:3. doi: 10.7603/s40681-014-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsia TC, Liu CJ, Lin CH, Chang WS, Chu CC, Hang LW, Lee HZ, Lo WC, Bau DT. Interaction of CCND1 genotype and smoking habit in Taiwan lung cancer patients. Anticancer Res. 2011;31(10):3601–3605. [PubMed] [Google Scholar]
- 41.Tsai MH, Tsai CW, Tsou YA, Hua CH, Hsu CF, Bau DT. Significant association of cyclin D1 single nucleotide polymorphisms with oral cancer in Taiwan. Anticancer Res. 2011;31(1):227–231. [PubMed] [Google Scholar]
- 42.Kuo HW, Huang CY, Fu CK, Liao CH, Hsieh YH, Hsu CM, Tsai CW, Chang WS, Bau DT. The significant association of CCND1 genotypes with gastric cancer in Taiwan. Anticancer Res. 2014;34(9):4963–4968. [PubMed] [Google Scholar]
- 43.Donnellan R, Chetty R. Cyclin D1 and human neoplasia. Mol Pathol. 1998;51(1):1–7. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bala S, Peltomäki P. CYCLIN D1 as a genetic modifier in hereditary nonpolyposis colorectal cancer. Cancer Res. 2001;61(16):6042–6045. [PubMed] [Google Scholar]
- 45.Fan YZ, Fu JY, Zhao ZM, Chen CQ. Inhibitory effect of norcantharidin on the growth of human gallbladder carcinoma GBC-SD cells in vitro. Hepatobiliary Pancreat Dis Int. 2007;6(1):72–80. [PubMed] [Google Scholar]
- 46.Sobti RC, Kaur P, Kaur S, Singh J, Janmeja AK, Jindal SK, Kishan J, Raimondi S. Effects of cyclin D1 (CCND1) polymorphism on susceptibility to lung cancer in a North Indian population. Cancer Genet Cytogenet. 2006;170(2):108–114. doi: 10.1016/j.cancergencyto.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Lu C, Dong J, Ma H, Jin G, Hu Z, Peng Y, Guo X, Wang X, Shen H. CCND1 G870A polymorphism contributes to breast cancer susceptibility: a meta-analysis. Breast Cancer Res Treat. 2009;116(3):571–575. doi: 10.1007/s10549-008-0195-y. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Jiao X, Wang C, Shirley LA, Elsaleh H, Dahl O, Wang M, Soutoglou E, Knudsen ES, Pestell RG. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 2010;70(21):8802–8811. doi: 10.1158/0008-5472.CAN-10-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]