Abstract
Background and objective
SARS-CoV-2 infection has bimodal distribution in Europe with a first wave in March to June 2020 and a second in September 2020 to February 2021. We compared the frequency, clinical characteristics and outcomes of adults with acute lymphoblastic leukemia (ALL) and infection in the first vs. second pandemic waves in Spain.
Patients and Methods
In this prospective study the characteristics of ALL and COVID-19 infection, comorbidities, treatment and outcome in the two periods were compared. The study ended when vaccination against SARS-CoV-2 was implemented in Spain.
Results
Twenty eight patients were collected in the first wave and 24 in the second. The median age was 46.5 years (range 20–83). Patients from the first wave had a trend to more severe ALL (higher frequency of patients under induction or submitted to transplantation or under immunosuppressive therapy). No significant differences were observed in need for oxygen support, intensive care unit (ICU) requirement, days in ICU and time to COVID-19 infection recovery. Seventeen patients (33%) died, with death attributed to COVID infection in 15 (29%), without significant differences in the 100 day overall survival (OS) probabilities in the two waves (68% ± 17% vs. 56% ± 30%). The only prognostic factor for OS identified by was the presence of comorbidities at COVID-19 infection (HR: 5.358 [95% CI: 1.875- 15.313]).
Conclusion
The frequency and mortality of COVID-19 infection were high in adults with ALL, without changes over time, providing evidence in favor of vaccination priority for these patients.
Keywords: Acute lymphoblastic leukemia, Adults, Covid-19 infection, Outcome
Microabstract
The characteristics and outcome of ALL in adults with COVID-19 infection in the first two waves of the pandemic in Spain were compared. The frequency and mortality of COVID-19 infection were high in adults with ALL, without changes over time. Comorbidities at COVID-19 infection was the only prognostic factor for survival.
INTRODUCTION
SARS-CoV-2 (COVID-19) infection has a negative impact on the outcomes of patients with cancer, with mortality rates over 20%.1, 2, 3 This is especially important in adults with hematologic neoplasias and in those submitted to allogeneic hematopoietic stem cell transplant (HSCT), in whom the death rate is over 30%4, 5, 6, 7, 8 compared to 4% in children.4 Patients with active disease and those receiving intensive chemotherapy or immunotherapy are especially vulnerable. Apart from the cancer itself other factors such as advanced age, poor general status and neutropenia contribute to this high mortality rate. There are striking differences in the death rate among the different hematologic neoplasias, with acute myeloblastic leukemia and lymphoproliferative diseases showing the highest rate.6, 7, 8
Information of the incidence and outcome of COVID-19 infection in patients with acute lymphoblastic leukemia (ALL) is scarce. Given the low frequency of ALL in adults, reports usually combine patients with other hematologic cancers. Among studies focused on ALL, a report from Italy performed in the first peak of the pandemic described a low incidence of COVID-19 infection among patients with Philadelphia chromosome-positive (pH+) ALL and suggested that tyrosine kinase inhibitors (TKI) may play a role in protecting patients from the infection.9
The first wave of COVID-19 had dramatic consequences in all countries, whose health care systems were not adequately prepared for such a pandemic and for the clinical consequences of a previously unknown disease in human beings. The explosive rise in SARS-CoV-2 infection, the severity of multisystem involvement in some patients and the lack of effective therapies explain the high lethality rate.10 Several Western countries are currently suffering a second or third wave of the infection. Improvements in health care systems, the development of guidelines for the management of COVID-19 in cancer patients11 and the vaccination programs will decrease the incidence and lethality of this infection.
Comparative studies of the impact of COVID-19 infection in specific hematologic neoplasias in the first vs. second infection waves are scarce and to our knowledge have not been done in ALL. Our objective was to compare the frequency, clinical characteristics and outcomes of adults with ALL and COVID-19 infection in the first vs. second SARS-CoV-2 infection waves in Spain.
PATIENTS and METHODS
Patients recruitment
Between March 1, 2020 to May 31, 2020 (the period of the first wave of COVID-19 infection in Spain) two registries from the PETHEMA (Programa Español de Tratamientos en Hematología) and GETH (Grupo Español de Trasplante Hematopoyético y Terapia Celular) groups were activated to recruit adult patients (age over 18 years) with ALL and COVID-19 infection confirmed by polymerase chain reaction. The PETHEMA registry was based on the registry developed by the American Society of Hematology (ASH) for hematologic diseases (www.ashresearchcollaborative.org/covid-19-registry), and the GETH registry was specifically developed for hematological diseases and COVID-19 infection in Spain.6 Both registries were merged for this study, resulting in a unique registry and database, which was sent to the Hematology Departments of 82 Spanish hospitals. The same strategy was performed during the second COVID-19 wave (between September 12, 2020 and January 12, 2021). The study was closed at that date, when the vaccination for COVID-19 was initiated at a population level in Spain. The study was approved by a reference Institutional Review Board (reference code 2020–113–1) and was registered by the Spanish Agency of Medicines and Health Products (reference code GETCLO-2020–01) and by the European Medicines Agency (reference code EUPAS34365EBMT). Specific recommendations for the management of ALL patients with COVID-19 were implemented by the PETHEMA Group in the beginning of the first wave of COVID-19 infection (available at www.sehh.es/covid-19/recomendaciones/123986).
Data analyzed
The following data were collected and analyzed: demographics and clinical and biologic features of ALL, patient comorbidities, COVID-19 infection (symptoms, SARS-COV-2-related hospital admission, oxygen requirement, intensive care unit (ICU) admission, and COVID-19 therapy), baseline laboratory variables (absolute lymphocyte and neutrophil counts, C-reactive protein [CRP] and d-dimer levels) at the time or within 3 days after SARS-CoV-2 detection, ALL therapy and patient outcomes. Weekly reminders were sent to centers for patient inclusion during the two periods of the study. The first part of the study was closed for follow-up on July 10, 2020 and the second part was closed on January 12, 2021. The results of the first part of the study were published in abstract form.
Statistical analysis
The primary objective was the comparison of survival of ALL patients in the two COVID-19 infection waves. Secondary objectives were the comparison of the frequency and clinical and biological features of ALL, the characteristics of COVID-19 infection and patient management in the two waves, as well as the prognostic factors for overall survival (OS). The main clinical and hematological variables were compared by median test (continuous variables) and the Pearson or Fisher exact tests (categorical variables). OS curves were plotted by the Kaplan-Meier method and compared by the log-rank test. Multivariable analysis for OS was performed using the Cox proportional hazards regression model. Data collection and statistical analyses were performed at the PETHEMA Data Center for ALL using SPSS software (v.24). Two-sided P-values <0.05 were considered statistically significant.
RESULTS
Patients and all characteristics
Fifty six patients were included, 4 of whom were excluded for COVID-19 infection occurring more than 3 years after the end of ALL therapy [n = 3] and 1 for having Burkitt lymphoma. Twenty eight patients were recruited in the first wave and 24 in the second. The median time from ALL diagnosis to COVID-19 infection was 6.12 (range, 0 – 96.43) months. Table 1 shows and compares the main clinical and biological characteristics of the patients included in the first and the second COVID-19 infection waves. The median age (range) of the series was 46.5 (20–83) years, with 34 patients (65%) being older than 40 years. Comorbidities were present in 18 patients (35%), being arterial hypertension the most frequent. ALL was of B-cell precursors in 38 patients (73%, pH+ in 8). At COVID-19 infection diagnosis, 31 patients (60%) were under frontline treatment; 16 (31%) were receiving salvage therapy, 1 (2%) was under palliative therapy and 4 (7%) had recently finished ALL therapy. Eight patients were submitted to allogeneic hematopoietic stem cell transplantation (HSCT) (5 at COVID-19 infection diagnosis), CAR T (n = 1, 2 years prior to COVID-19 infection) or received immunotherapy (inotuzumab, [n = 6, 2 at COVID-19 infection], blinatumomab, [n = 1, prior to COVID-19 infection]). Eleven patients were under immunosuppressive therapy at the time of COVID-19 infection (fludarabine [n = 6], tacrolimus [n = 3], antithymocyte globulin [n = 1] and rituximab [n = 1]). Patients from the first wave showed a trend to having more severe ALL than those from the second wave (more patients receiving ≥2 lines of therapy, submitted to HSCT or under immunosuppressive therapy) (Table 1).
Table 1.
Main Acute Lymphoblastic Leukemia Characteristics and Treatment of Patients Recruited During the First and the Second COVID-19 Infection Waves
| Overall (n = 52) | First COVID-19 wave (n = 28) | Second COVID-19 wave (n = 24) | P value | |
|---|---|---|---|---|
| Age, yr, median (range) | 46.5 (20–83) | 46.5 (20–78) | 47.5 (24–83) | 1.000 |
| Age range, n (%)AYA, 19–39 yr.Adult, 40–59 yr.Elderly, 60–89 yr. | 18 (35)23 (44)11 (21) | 9 (32)13 (47)6 (21) | 9 (38)10 (42)5 (20) | 0.916 |
| Gender, male/female | 26/26 | 15/13 | 11/13 | 0.578 |
| Ethnicity, n (%)CaucasianAsianUnspecified | 41 (79)2 (4)9 (17) | 23 (82)2 (7)3 (11) | 18 (75)06 (25) | 0.190 |
| Active or ex-smoker, n (%) | 13/50 (26) | 3/26 (12) | 10 (42) | 0.015 |
| Comorbidities, n (%)DiabetesCoronary artery diseaseHeart failureHypertensionChronic liver diseasePrior cancerAutoimmune diseasePsychiatric disorderOther | 18 (35)311732124a | 9 (32)111521001 | 9 (37)200211123 | 0.686 |
| General status (ECOG scale), n (%)0123 | 21/51 (41)22/51 (43)3/51 (6)5/51 (10) | 11 (39)13 (47)2 (7)2 (7) | 10/23 (44)9/23 (39)1/23 (4)3/23 (13) | 0.843 |
| WBC count at ALL diagnosis, x109/L, median (range) | 12.6 (0.8–297) | 12.6 (1.2–297) | 12.5 (0.8–168) | 0.768 |
| ALL phenotype, n (%)Early pre-BCommonPre-BB, unspecifiedPro-T/pre-TCortical TMature TT, unspecifiedMixed phenotype | 5 (9.5)25 (48)b5 (9.5)3 (6)5 (9.5)5 (9.5)b2 (4)b1 (2)b1 (2) | 1 (4)10 (35)4 (14)3 (11)5 (18)4 (14)1 (4)00 | 4 (17)15 (63)1 (4)001 (4)1 (4)1 (4)1 (4) | 0.065c |
| Philadelphia chromosome status, n (%)NegativePositive | 44 (85)8 (15) | 22 (79)6 (21) | 22 (92)2 (8) | 0.262 |
| ALL treatment at COVID infection, n (%)InductionConsolidationMaintenanceSalvage therapyPalliative therapyEnd of therapy | 14 (27)15 (29)2 (4)16 (31)d1 (2)4 (7) | 10 (36)3 (11)1 (3.5)11 (39)1 (3.5)1 (3.5) | 4 (17)11 (46)1 (4)5 (21)03 (12) | 0.021 |
| Number of lines of treatment, n (%)1≥2 | 34 (65)18 (35) | 15 (54)7 (46) | 19 (79)5 (21) | 0.053 |
| Prior allogeneic HSCT, n (%) | 8 (15) | 8 (29) | 0 | NA |
| Interval HSCT-COVID-19 infection, months, median (range) | 3.3 (0.2–46.9) | 3.3 (0.2–46.9) | – | NA |
| Immunosuppressive treatment (excluding corticosteroids) at COVID-19 infection, n (%) | 11/46 (24)e | 9/26 (35) | 2/20 (10) | 0.036 |
ECOG = Eastern Cooperative Oncology Group; WBC = white blood cell; ALL = Acute lymphoblastic leukemia; HSCT = hematopoietic stem cell transplant.
Extensive intestinal resection (n = 1), hypothyroidism (n = 2), ischemic duodenal necrosis and resection (n = 1)
Lymphoblastic lymphoma in 1 case
B-cell precursor vs. T-ALL: 18 vs. 20, 10 vs. 3.
Rescue chemotherapy (n = 8), allogeneic HCST (n = 4), inotuzumab ozoganicin (n = 2), blinatumomab (n = 1), unknown (n = 1).
Fludarabine (n = 6), tacrolimus (n = 3), rituximab (n = 1) and antithymocyte globulin (n = 1).
Data of COVID-19 infection
Table 2 shows the COVID-19 infection symptoms, the main laboratory parameters, and the treatments implemented in the first and the second waves. No differences were observed in COVID-19 symptoms. Twelve patients (23%) showed neutropenia <0.5 × 109/L and 24 (47%) lymphocytopenia <0.5 × 109/L at COVID-19 infection, with a higher frequency of severe neutropenia and lymphocytopenia in the first vs. the second wave. More patients received antiviral therapy in the first wave. The therapy for COVID-19 was different in the two periods, with significantly higher use of hydroxychloroquine, remdesivir and lopinavir-ritonavir in the first wave and corticosteroids combined or not with remdesivir in the second wave. No significant differences were observed in the need for oxygen support (12 patients vs. 8), ICU requirement (7 patients vs. 4), days in the ICU (medians 16 vs. 21) and time to COVID-19 infection recovery (medians 17 vs. 13 days).
Table 2.
COVID-19 Infection Symptoms, Main Laboratory Parameters and Treatment in the First and the Second COVID-19 Infection Waves
| Overall (n = 52) | First COVID-19 wave (n = 28) | Second COVID-19 wave (n = 24) | P value | |
|---|---|---|---|---|
| COVID-19 infection symptoms, n (%)FeverCoughFatigueDyspneaDiarrheaRhinorrheaMusculoskeletal painAnosmiaDiaphoresisNausea/vomitingHeadacheAbsence of symptoms | 39 (75)29 (74)24 (62)13 (33)14 (36)9 (23)6 (15)10 (26)3 (8)3 (8)1 (3)3 (8)13 (25) | 23 (82)18 (78)16 (70)11 (48)9 (39)7 (30)4 (17)4 (17)2 (9)2 (9)1 (4)05 (18) | 16 (67)11 (69)8 (50)2 (12)5 (31)2 (12)2 (12)6 (37)1 (6)1 (16)03 (19)8 (33) | 0.199 |
| Neutrophil count, x109/L, median(range)≥0.5 × 199/L<0.5 × 109/L | 1.7 (0–18)39/51 (77)12/51 (23) | 1.4 (0–6.5)17 (61)11 (39) | 2.9 (0–18)22/23 (96)1/23 (4) | 0.1250.003 |
| Lymphocyte count, x109/L, median (range)≥0.5 × 199/L<0.5 × 109/L | 0.5 (0–5.9)27/51 (53)24/51 (47) | 0.3 (0–1.5)10 (36)18 (64) | 1.2 (0–5.9)17/23 (74)6/23 (26) | 0.0080.007 |
| C-reactive protein, mg/L, median (range) | 22.5 (0.04–311.7) | 28.7 (0.9–311.7) | 13 (0.04–194) | 0.227 |
| D-dimer, ng/mL, median (range)a | 690 (120–31,200) | 690 (120–31,200) | 857.5 (210–10,381) | 1.000 |
| Treatment of COVID-19 infection, n (%)Hydroxychloroquine/chloroquineAzithromycinLopinavir-ritonavirRemdesivirTocilizumabSiltuximabConvalescent serumRibavirinCorticosteroids | 34 (65)24 (71)11 (32)14 (41)4 (12)11 (32)1 (3)3 (9)1 (3)12 (35) | 25 (89)24 (96)11 (44)14 (56)2 (8)8 (32)1 (4)1 (4)05 (20) | 9 (37)0002 (22)3 (33)02 (22)1 (11)7 (78) | <0.001 |
| Oxygen support, n (%) | 20 (38) | 12 (43) | 8 (33) | 0.482 |
| Intensive care unit (ICU) support, n (%) | 11 (21) | 7 (25)b | 4 (17) | 0.463 |
| Days in ICU, median (range) | 16 (1–47) | 16 (1–47) | 21 (11–30) | 1.000 |
ICU = intensive care unit.
n = 34.
In 1 additional patient ICU support was not provided due to advanced age and poor general status.
Patients outcome
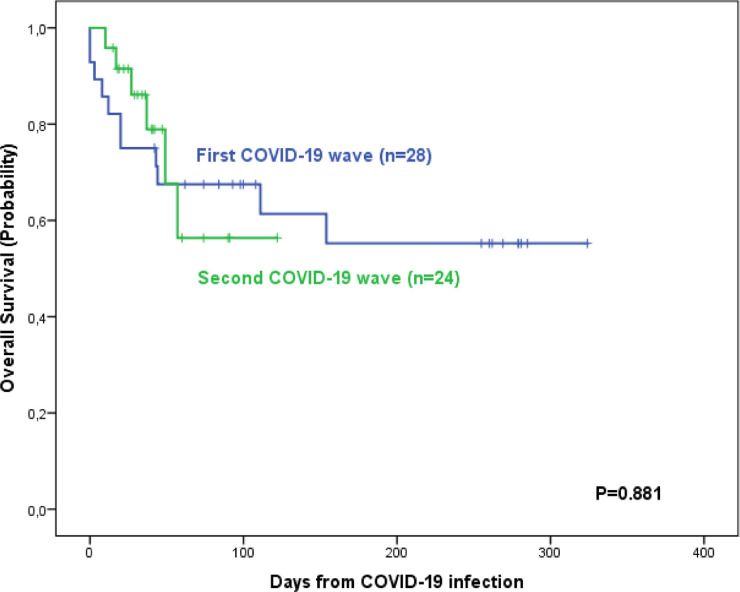
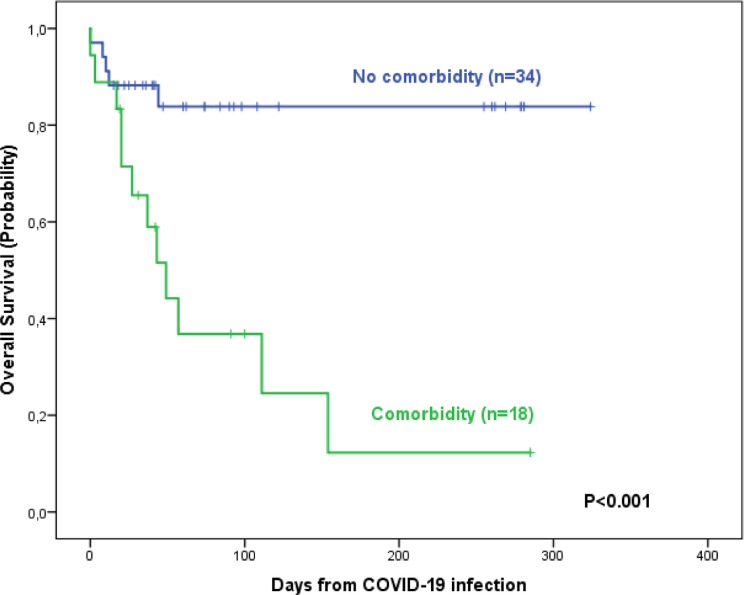
Table 3 shows and compares the patient outcomes according to the wave of COVID-19 infection. Seventeen patients (33%) died (11 vs. 6). No differences in the frequency of deaths were observed in pH+ vs. pH-negative patients (3/8 [38%] vs. 14/44 [32%]). The main causes of death were COVID-19 infection (n = 10), Pseudomonas sepsis during COVID-19 infection (n = 3), ALL relapse (n = 3, 2 during COVID-19 infection) and ALL therapy-related (n = 1). The death rate in patients transferred to the ICU was 73% (8 out of 11). No differences were observed on comparison of the 100 day survival probabilities (68% [51%−85%] vs. 56% [26%−86%]) (Figure 1 ). In the univariable analysis, advanced age, poor general status, the presence of comorbidities, severe neutropenia and lymphocytopenia, and the number of treatment lines for ALL were associated with a poorer OS. Only the presence of comorbidities at COVID-19 infection has an unfavorable impact on OS in the multivariable analysis (Table 4 and Figure 2 ).
Table 3.
Patient Outcomes According to the Wave of COVID-19 Infection
| Overall (n = 52) | First COVID-19 wave (n = 28) | Second COVID-19 wave (n = 24) | P value | |
|---|---|---|---|---|
| COVID-19 infection resolution | 36 (69) | 18 (64) | 18 (75) | 0.404 |
| Infection onset-clinical recovery interval, days, median (range) | 14 (2–47) | 17 (2–47) | 12.5 (5–39) | 0.095 |
| Alive patients at close of follow-upa | 35 (67) | 17 (61) | 18 (75) | 0.274 |
| Causes of death (n = 17)COVID-19 infectionPseudomonas sepsis & COVID-19 infectionLeukemia progression & COVID-19 infectionLeukemia progressionALL treatment-related mortality | 103211 | 62210 | 41001 | 0.467 |
| Infection onset-death interval, days, median (range) | 20 (0–154) | 20 (0–154) | 32 (10–57) | 0.335 |
ALL = acute lymphoblastic leukemia.
Median follow-up: 74 (15–324) days.
Figure 1.
Survival probability of ALL patients according to the wave of COVID-19 infection.
Table 4.
Univariable and Multivariable Analyses of Prognostic Factors for Overall Survival
| Prognostic factor | N | OS univariable HR (95%CI) | P value | OS multivariable HR (95%CI) | P value |
|---|---|---|---|---|---|
| Age<60 yr.≥60 yr. | 4111 | Reference2.957 (1.123; 7.783) | 0.028 | NS (0.305) | |
| GenderMaleFemale | 2626 | Reference1.428 (0.547; 3.726) | 0.467 | – | |
| ECOG score0–1≥2 | 438 | Reference3.213 (1.022; 10.101) | 0.046 | NS (0.340) | |
| SmokerNeverActive or ex-smoker | 3713 | Reference5.625 (0.745; 42.455) | 0.094 | – | |
| ComorbiditiesNoYes | 3418 | Reference5.548 (1.944; 15.834) | 0.001 | Reference5.358 (1.875; 15.313) | 0.002 |
| Neutrophil count≥0.5 × 199/L<0.5 × 199/L | 3912 | Reference2.577 (0.980; 6.778) | 0.055 | – | |
| Lymphocyte count≥0.5 × 199/L<0.5 × 199/L | 2724 | Reference3.618 (1.179; 11.102) | 0.025 | NS (0.286) | |
| C-reactive protein<22.46 mg/L≥22.46 mg/L | 2323 | Reference2.294 (0.796; 6.610) | 0.124 | – | |
| D-dimer<690 ng/mL≥690 ng/mL | 1717 | Reference1.747 (0.570; 5.352) | 0.329 | – | |
| Number of lines of ALL treatment1≥2 | 3418 | Reference2.692 (1.018; 7.116) | 0.046 | NS (0.125) | |
| Stage of treatmentInduction Consolidation/Maintenance | 1417 | 1.543 (0.344; 6.915)Reference | 0.571 | – | |
| Leukemia statusCRActive disease | 2527 | Reference3.452 (1.122; 10.615) | 0.031 | NS(0.167) | |
| Prior transplantNoYes | 448 | 1.073 (0.307; 3.757)Reference | 0.912 | – | |
| Ongoing steroid treatment at time of infectionNoYes | 1927 | 1.010 (0.384; 2.657)Reference | 0.983 | – | |
| Ongoing immunosuppression treatment at time of infectionNoYes | 3511 | 1.442 (0.464; 4.477)Reference | 0.527 | – | |
| COVID-19 treatment with steroidsNoYes | 2212 | Reference2.576 (0.858; 7.740) | 0.092 | – |
ALL = acute lymphoblastic leukemia; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; OS = overall survival.
Figure 2.
Survival probability of patients with acute lymphoblastic leukemia and COVID-19 infection according to the presence of comorbidities.
DISCUSSION
This study shows that the frequency and characteristics of COVID-19 infection were similar in patients with ALL during the 2 waves of the pandemic. Despite some differences in ALL severity and the pharmacologic management of COVID-19 infection, the patient outcomes were similar, with a 3 month survival probability of around 60%, confirming the high lethality of this infection among ALL patients.
Up to March 4, 2021, 115,302,421 confirmed cases and 2561,992 deaths (2.2%) by COVID-19 infection had been recorded worldwide.13 The first wave of the pandemic occurred in many Western countries, including Spain, in March 2020. Given the magnitude of the pandemic and the lack of effective therapies against the SARS CoV-2 virus, a population lockdown was made in most countries. Many countries in Europe are facing second or third waves of COVID-19 infection. Several studies performed during the first pandemic wave showed that patients with cancer had a high mortality rate of over 20%.1, 2, 3 This rate was even higher in adult patients with malignant hematologic diseases, ranging from 30% to 40%.4, 5, 6, 7, 8 Hematologic neoplasms have generally been grouped in these studies, and there are scarce studies in patients with specific hematologic cancers.14, 15, 16, 17 The evidence in ALL is even scarcer and publications are mainly focused on children, in whom the estimated death rate was 4%.4 , 18 , 19 A study from Italy showed a low incidence of COVID-19 infection among adult patients with pH+ ALL,9 suggesting that TKI may play a role in protecting patients from the infection, as was also suggested for chronic myeloid leukemia.20
To our knowledge this is the first comparative study of the characteristics and outcomes of adult ALL patients in the first vs. the second wave of COVID-19 infection. The clinical and biologic ALL features were similar in both waves and the frequency of pH+ ALL was not different to that observed in the general population. There was a trend to more severe ALL in patients from the first wave. During the second wave most patients were receiving first-line therapy and showed a lower frequency of severe neutropenia and lymphocytopenia at the time of COVID-19 infection. The clinical manifestations of COVID-19 infection were similar in both periods, and more patients were asymptomatic during the second wave, although without statistical significance. The treatment of the COVID-19 infection was different in the two infection waves, reflecting the changes in its management over time. Less antiviral therapy was given during the second wave due to the perception that most drugs empirically given during the first wave were ineffective. Despite these changes in COVID-19 infection therapy, the severity was similar over time in our study, with no significant differences among groups in need of oxygen support, ICU requirement and duration of ICU stay. This contrasts with mortality in the general Spanish population in whom the rate of death seemed to be lower in the second wave.21
The main objective of this study was to know if there were changes in the survival probability of ALL patients in the two infection waves. One would expect that the increase of the resources of health care systems, the use of many different approaches to treat the COVID-19 pandemic, the higher availability of diagnostic tests and the early development of recommendations for the management of ALL patients with COVID-19 infection11 , 22 would improve the prognosis of these patients. However, despite small improvements23 no significant advances have occurred to date in the treatment of COVID-19 infection24 which is probably the main explanation for the lack of improvement in survival on comparison of the two waves. It is of note that this study ended at the time of onset of vaccination programs in Spain, and there is no doubt that vaccination is the more effective approach for eradication of COVID-19 infection. The only prognostic factor for OS identified by multivariable analysis was the presence of comorbidities at COVID-19 infection, in accordance with several studies involving patients with hematological malignancies.6 , 25
Some limitations in this study should be pointed out. First, it was based on voluntary declaration by the physicians of the participating Spanish hospitals, given the lack of an epidemiologic registry of COVID-19 infection in cancer patients in Spain. Although a weekly reminder to centers for patient inclusion was given during the two periods of the study, underreporting cannot be ruled out. Second, the study was closed before the end of the second pandemic wave (at the time of onset of vaccination, to avoid its interference in the frequency and severity of the infection) and this may have been a possible limitation for the inclusion of patients in the second group. Third, the limited number of ALL patients identified in each infection wave might not have allowed detecting differences in patient characteristics or outcomes. In fact, ALL is a rare disease in adults and consequently, large series of COVID-19 infection focused on adult ALL patients are not available to date. Finally, this study, as many published reports of COVID-19 in patients with blood cancer, is heavily enriched with hospitalized patients, thus mortality risk estimates may not be accurate for all patients with ALL.
The development and availability of vaccines against SARS CoV-19 within a short period of time has been one of the hallmarks in the fight against this pandemic and will decisively contribute to a reduction in the frequency and severity of this infection in the near future. However, patients with hematologic malignancies are still among the groups with high mortality rates, as shown in this and many other studies, and for that reason the main hematologic and oncologic societies, including the Spanish Society of Hematology,26 have developed recommendations for vaccination to these patients. In summary, this study shows that the frequency and mortality of COVID-19 infection were high in adult patients with ALL, without changes over time, and provides additional evidence in favor of vaccination priority for adult patients with ALL.
Clinical practice points
What is already known about this subject? SARS-CoV-2 (COVID-19) infection has a negative impact on the outcomes of patients with cancer, with mortality rates over 20%. This outcome is poorer in patient with malignant hematologic diseases, such as acute leukemias.
What are the new findings? This study shows that the frequency and characteristics of COVID-19 infection were similar in adult patients with ALL, a feature not being extensively reported in ALL patients. This poor prognosis did not change during the 2 waves of the pandemic that have occurred in Spain. Despite some differences in ALL severity and in the pharmacologic management of COVID-19 infection during the two waves, the patient outcomes were similar, with a 3 month survival probability of around 60%, confirming the high lethality of this infection among ALL patients.
How might it impact on clinical practice in the foreseeable future? The high frequency and mortality of COVID-19 infection in adults with ALL, without changes over time, provides strong evidence in favor of vaccination priority for these patients.
Funding
Supported in part by 2017 SGR288 (GRC) Generalitat de Catalunya and “La Caixa” Foundation.
Disclosure
None
Permission statement
For original data, please contact jribera@iconcologia.net or mmorgades@iconcologia.net. Deidentified individual participant data are available indefinitely at www.pethema.org. The study protocol is also available at the same website.
Acknowledgments
We thank the physicians from the following Spanish hospitals for their contribution to this registry. Institut Català d'Oncologia-Hospital Germans Trias i Pujol, Badalona. Institut Català d'Oncologia-Hospital Doctor Josep Trueta, Girona. Hospital Universitari Vall d'Hebron, Barcelona. Fundación Jiménez Díaz, Madrid. Hospital Universitari i Politècnic La Fe, Valencia. Hospital Universitario Infanta Leonor, Madrid. Hospital Universitario, Salamanca. Hospital General Universitario Gregorio Marañón, Madrid. Hospital Universitario, Guadalajara. Hospital Universitario, A Coruña. Hospital Ramón y Cajal, Madrid. Hospital de la Santa Creu i Sant Pau, Barcelona. Hospital 12 de Octubre, Madrid. Institut Català d'Oncologia-Hospital Duran i Reynals. IDIBELL, L'Hospitalet de Llobregat. Hospital Clínico Universitario, Valencia. Hospital Arnau de Vilanova, Lleida. Hospital Puerta de Hierro, Madrid. Hospital HM Sanchinarro, Madrid. Hospital General, Alicante. Hospital Universitario, Donostia. Hospital Universitario Virgen del Rocío, Sevilla. Hospital Universitario Central de Asturias, Oviedo. Complejo Asistencial, Ávila. Institut Català d'Oncologia, Hospital Verge de la Cinta, Tortosa. Hospital San Pedro de Alcántara, Cáceres. Hospital Lucus Augusti, Lugo. Complejo Hospitalario de Navarra, Pamplona. Hospital La Princesa, Madrid. Hospital Clínico Universitario, Valladolid. Hospital Universitario Virgen de la Macarena, Sevilla. Hospital Clínico Universitario Virgen de la Arrixaca, Murcia.
ncited Reference
REFERENCES
- 1.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumor subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assaad S., Avrillon V., Fournier M.L., et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijenthira A., Gong I.Y., Fox T.A., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood W.A., Neuberg D.S., Thompson J.C., et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv. 2020;4:5966–5975. doi: 10.1182/bloodadvances.2020003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piñana J.L., Martino R., García-García I., et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Suárez J., de la Cruz J., Cedillo Á., et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;8:133. doi: 10.1186/s13045-020-00970-7. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A., Bhatt N.S., St Martin A., et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. 30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foà R., Bonifacio M., Chiaretti S., et al. Philadelphia-positive acute lymphoblastic leukaemia (ALL) in Italy during the COVID-19 pandemic: a Campus ALL study. Br J Haematol. 2020;190:e3–e5. doi: 10.1111/bjh.16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilinski A., Emanuel E.J. COVID-19 and Excess All-Cause Mortality in the US and 18 Comparison Countries. JAMA. 2020;324:2100–2102. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S., Rausch C.R., Jain N., et al. Treating Leukemia in the Time of COVID-19. Acta Haematol. 2020;144:132–145. doi: 10.1159/000508199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribera J.M., Morgades M., Coll R., et al. 62nd Annual Meeting of the Blood. 136 (suppl 1) American Society of Hematology; 2020. Frequency, clinical characteristics and outcome of adult patients with acute lymphoblastic leukemia (ALL) and COVID-19 in Spain: results of a survey from PETHEMA and GETH Groups. Abstract 3726 (PB) [Google Scholar]
- 13.https://coronavirus.jhu.edu/map.html. Accessed in February 17, 2021.
- 14.Martínez-López J., Mateos M.V., Encinas C., et al. Multiple myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic factors of inpatient mortality. Blood Cancer J. 2020;10:103. doi: 10.1038/s41408-020-00372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Cruz-Benito B., Lázaro-Del Campo P., Ramírez-López A., et al. Managing the front-line treatment for diffuse large B cell lymphoma and high-grade B cell lymphoma during the COVID-19 outbreak. Br J Haematol. 2020;191:386–389. doi: 10.1111/bjh.17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fürstenau M., Langerbeins P., De Silva N., et al. COVID-19 among fit patients with CLL treated with venetoclax-based combinations. Leukemia. 2020;34:2225–2229. doi: 10.1038/s41375-020-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara F., Zappasodi P., Roncoroni E., Borlenghi E., Rossi G. Impact of Covid-19 on the treatment of acute myeloid leukemia. Leukemia. 2020;34:2254–2256. doi: 10.1038/s41375-020-0925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmiegelow K. Have COVID-19 affected ALL epidemiology? Acta Paediatr. 2021;110:387–388. doi: 10.1111/apa.15631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub J.W., Ge Y., Xavier A.C. COVID-19 and childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2020;67:e28400. doi: 10.1002/pbc.28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galimberti S., Petrini M., Baratè C., et al. Tyrosine Kinase Inhibitors Play an Antiviral Action in Patients Affected by Chronic Myeloid Leukemia: a Possible Model Supporting Their Use in the Fight Against SARS-CoV-2. Front Oncol. 2020;10:1428. doi: 10.3389/fonc.2020.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_52_COVID-19.pdf. Accessed on March 1, 2021.
- 22.https://www.sehh.es/covid-19/recomendaciones/123986-recomendaciones-para-el-tratamiento-de-la-leucemia-aguda-linfoblastica-linfoma-linfoblastico-y-leucemia-de-Burkitt.2021
- 23.The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borah P., Mirgh S., Sharma S.K., et al. Effect of age, comorbidity and remission status on outcome of COVID-19 in patients with hematological malignancies. Blood Cells Mol Dis. 2021;87 doi: 10.1016/j.bcmd.2020.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://www.sehh.es/publicaciones/guias-recomendaciones 2021