Abstract
Background
: While a growing body of literature describes antibody dynamics in serum, little is known about breast milk antibody titers in the months following SARS-CoV-2 infection.
Objectives
: We evaluated the dynamics of the humoral immune response to SARS-CoV-2 in two women who were breastfeeding when infected. We assessed paired breast milk and serum samples for six months post-infection for antibodies specific to the SARS-CoV-2 receptor binding domain (RBD) of the spike protein.
Results
: Starting at 10 days after symptom onset, IgA antibody levels were persistent over a 6-month time period in human milk. For both mothers, no detectable IgA was found in the samples collected pre-symptom onset. RBD-specific IgG and IgM antibodies in tandem serum collected from the two donors demonstrated stable IgG levels over the six-month time period post-symptom onset.
Conclusions
: We found that breastfeeding mothers produced a durable IgA response for up to six months following COVID-19 infection, suggesting an important role for breast milk in protection of infants.
1. Introduction
Transfer of maternal SARS-CoV-2 antibodies to infants through breast milk may be important in protecting infants from COVID-19. IgA is the main SARS-CoV-2-reactive immunoglobulin in breast milk [1], [2], [3], making up 90% of the antibodies found in human milk [4]. The long-term persistence of antibodies in breast milk following maternal infection with SARS-CoV-2 is unknown, with four studies reporting longitudinal data on antibody titers in milk only up to 12 weeks [5], [6], [7], [8]. Mapping antibody dynamics post-infection is important for understanding the long-term impact of breastfeeding following SARS-CoV-2 infection.
We report a longitudinal evaluation, spanning six months post-symptom onset, of the antibody response to SARS-CoV-2 infection in breast milk and in serum among two breastfeeding individuals who tested positive for SARS-CoV-2 postpartum.
2. Methods
2.1. Participants
Serum and breast milk samples were collected from five women, including two enrolled in a prospective longitudinal cohort study of adults with COVID-19 and three healthy controls. Participants with PCR-confirmed SARS-CoV-2 infection were enrolled 30–60 days following mild illness that did not require hospitalization; information on the infants was not available. The three healthy controls indicated no prior history of a positive SARS-CoV-2 test or any recent symptoms consistent with COVID-19, and tested negative on a serum antibody test. At the time of enrollment, all participants underwent informed consent.
2.2. Sample collection and processing
Subjects underwent collection of blood samples at the time of enrollment and returned periodically for longitudinal sample collection. Nursing subjects also contributed breast milk self-collected at home. The two SARS-CoV-2 infected participants provided breast milk samples spanning from three weeks prior to symptom onset to six months post-illness onset. Whole blood was centrifuged, and the subsequent serum was aliquoted, frozen at −80 °C, and then heat-inactivated prior to testing. Collected breast milk samples were thawed, centrifuged, aliquoted, and frozen at −80 °C.
2.3. Measurement of SAR-CoV-2-specific antibodies
We adopted a previously described enzyme-linked immunosorbent assay (ELISA) [9] to measure IgA, IgG, and IgM antibody concentrations to the SARS-CoV-2 Receptor Binding Domain (RBD) site to analyze both serum and human milk samples. Ninety-six-well plates were coated overnight with spike protein RBD of SARS-CoV-2/Wuhan/2019 (Immune Tech, New York, NY) diluted in phosphate-buffered saline (PBS) at 4 °C. To correct for background interference for breast milk samples, an additional set of plates were coated only with PBS. After overnight incubation, coated plates were washed three times with PBS containing 0.1% Tween-20 (PBS-T) (Sigma-Aldrich, Saint Louis, MO). Plates were blocked with PBS-T and 3% bovine serum albumin (BSA) (Sigma Aldrich, Saint Louis, MO) for one hour at room temperature. Milk and serum samples were diluted in PBS-T/1% BSA with a starting dilution of 1:5 and 1:25, respectively. Milk samples were serially diluted two-fold and serum samples were diluted three-fold; these sequential dilutions were added in duplicate (100uL/well) to the plate after the blocking solution was removed. After two-hour incubation at room temperature, the plates were washed three times with PBS-T. Anti-human-IgG Fab-specific, IgM Fc5μ fragment-specific, or IgA α-chain-specific (Jackson ImmunoResearch, West Grove, PA) were diluted 1:5000 in PBS-T/1% BSA and added to plates to incubate for one hour. Plates were washed three times and developed with ABTS solution (Sigma Aldrich, Saint Louis, MO) followed by quenching with HCl after 20 min and read at 405 nm.
2.4. Statistical analysis
For serum samples, AUC was calculated as the total peak area under the titration curve using GraphPad Prism software. To account for the heterogeneity of background absorbance arising from non-antibody breast milk components, the antigen-free plate absorbance reading was subtracted from antigen-coated plate absorbance reading. The resulting AUC [adjusted] for breast milk samples was calculated as the area under the titration curve by plotting the adjusted absorbance values with GraphPad Prism software.
2.5. Ethical considerations
The study was approved by the University of Washington Human Subjects Institutional Review Board (Study IDs 00,000,959 and 00,002,929).
3. Results
3.1. COVID-19 infected participant characteristics
Both participants had mild SARS-CoV-2 infections that did not require hospitalization. Participant A developed symptoms 236 days after delivery. She sought care from their primary care provider reporting fever, myalgias, shortness of breath, dyspnea, fatigue, and loss of sense of taste or smell. Participant B developed symptoms 265 days after delivery, and reported rhinorrhea, diarrhea, and loss of sense of taste or smell, and did not require any medical care for her illness.
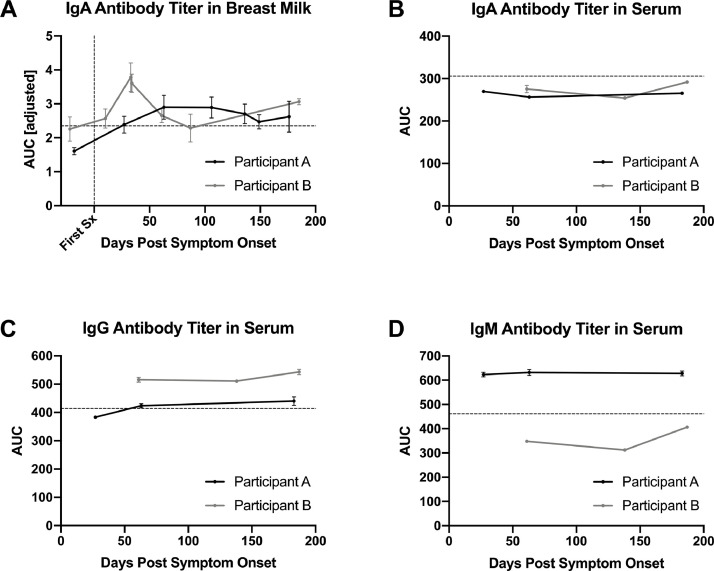
3.2. IgA antibody levels in breast milk persistent over 6 months post-infection
Antibodies specific to the RBD of SARS-CoV-2 were quantified in breast milk samples from two COVID-19 infected mothers and compared to the values of healthy controls. For both mothers, no detectable IgA was found in the sample collected pre-symptom onset. Starting at 10 days post-symptom onset, IgA levels were persistent over a six-month period (Fig. 1 A). The IgA level in Participant A gradually increased over a three-month period before decreasing and plateauing. The IgA antibody titer in Participant B peaked at 33 days post-symptom onset and decreased to a consistent level over the next five months. Although IgA-specific antibodies were detected and quantified at three standard deviations above the average healthy control breast milk samples, IgG and IgM were not above the positive cut-off. The assay was not sensitive enough to distinguish breast milk IgG and IgM levels from the negative controls, even when adjusting for background absorbance readings (data not shown).
Fig. 1.
Antibody binding titers over time in serum and breast milk from two women infected with SARS-CoV-2 while breastfeeding an infant. A) Longitudinal SARS-CoV-2 RBD-specific IgA titer in breast milk pre- and post- symptom onset quantified by area under the curve of ELISA after adjustment for background signal by subtraction of an antigen-free plate (AUC [adjusted]). Horizontal dashed line indicates 3 standard deviations above the average AUC of healthy negative control breast milk samples. The vertical dashed line indicates day of symptom onset. B – D) Longitudinal SARS-CoV-2 RBD-specific antibody titer from the serum as quantified by area under the curve (AUC) of ELISA. Horizontal dashed lines indicate the mean AUC values of 6 negative control samples, (2 collection time points per the 3 healthy control donor). B) IgA antibody titer in serum. C) IgG antibody titer in serum. D) IgM antibody titer in serum. All samples, both breast milk and serum, are implemented in duplicates and all graphs depict the mean ± standard error.
3.3. Serum antibody titers differed from breast milk antibody titers
Serum IgA, IgG, and IgM antibody levels were measured in samples from the same two breastfeeding mothers in the six months following symptom onset. Both participants were found to have IgG antibodies specific to the RBD region of SARS-CoV-2 after illness, while only one had a level of IgM distinguishable from negative controls (Fig. 1C-D). No detectable IgA antibody level was found in serum samples (Fig. 1B). Over the six-month period, the IgG levels remained steady for both participants (Fig. 1C).
4. Discussion
Breastfeeding mothers produced durable anti-RBD IgA antibodies in breast milk beginning ten days after symptom onset and persisting over 6 months. This abundance of IgA is consistent with the unique ability of dimeric IgA to cross the mucosal environment into the mammary gland and the preferential expression of J-chain by mucosal plasma cells [10]. While this study was not designed to evaluate the level of protection that IgA conferred from mother to infant during prolonged lactation periods, patterns of protection found in other respiratory viruses suggest this confers effective passive immunity [9,11].
The RBD-specific antibodies in serum collected from the two SARS-CoV-2 infected mothers demonstrated stable titers over the six-month time period post-symptom onset. While the main antibody isotype quantified in serum was IgG, only Participant A had detectable levels of IgM distinguishable from the SARS-CoV-2 PCR-negative controls. With Participant A's first serum collection occurring 61 days following symptom onset, the undetectable levels of IgM are consistent with known rapid decline following initial peak humoral response [12].
This study has several limitations. First, a larger sample size is needed to definitively characterize SARS-CoV-2 humoral immunity in milk. We also only assessed the immune response to the RBD site due to the critical role of the RBD site in facilitating viral fusion into host cells [13,14], but evaluation of other antigens could expand the understanding of neutralizing capabilities of breast milk antibodies.
Although non-randomized observational studies have found that breastfeeding is associated with protection against respiratory viruses via transfer of immunity between mother and infant [15], protection against SARS-CoV-2 infection specifically has yet to be established. Understanding the dynamics of antibodies in breast milk is crucial information for evaluating the potential impact on infants of vaccinating women who are pregnant or breastfeeding.
Authors’ Contributions
Study concept and design: C.J.D., D.J.M, H.Y.C. Executing experiments and data analysis: C.J.D., K.D.S. Manuscript Preparation: C.J.D., D.J.M., K.D.S, J.K.L., N.M.F., C.R.W., C.J.F., H.Y.C. Administrative, technical and material support: D.J.M., K.D.S, J.K.L., N.M.F., C.R.W., C.J.F., H.Y.C.
Financial support
This study was supported by awards from the Emergent Ventures Prize for Social Leadership from the Mercatus Center at George Mason University and the Bill and Melinda Gates Foundation.
Conflicts of interest
Helen Y. Chu receives research support from Gates Ventures, NIH, CDC, DARPA, Sanofi-Pasteur, and Cepheid and serves on the advisory boards for Merck, Pfizer, Ellume, and the Gates Foundation. The other authors declared no conflicts of interest.
Acknowledgements
We give a heartfelt thanks to the study participants for donating breast milk and blood samples during a stressful time in their lives. We thank Kate Crawford, Lauren Gentles, and Allison Greaney, and Dr. Jesse Bloom for helpful input. We thank Dylan McDonald and Ariana Magedson, for assistance with enrollment of participants and Kristen Huden and Callista Nackviseth, for their work sample processing. We also thank Dr. Alex Greninger and Greg Pepper at UW Virology for assistance with providing serum antibody testing.
References
- 1.Fox A., Marino J., Amanat F., Krammer F., Hahn-Holbrook J., Zolla-Pazner S., Powell R.L. MedRxiv; 2020. Evidence of a Significant Secretory-Iga-Dominant SARS-CoV-2 Immune Response in Human Milk Following Recovery from COVID-19. 2020.05.04.20089995. [DOI] [Google Scholar]
- 2.Lebrão C.W., Cruz M.N., da Silva M.H., Dutra L.V., Cristiani C., Affonso Fonseca F.L., Suano-Souza F.I. Early Identification of IgA Anti-SARSCoV-2 in Milk of Mother With COVID-19 Infection. J. Hum. Lact. 2020;36:609–613. doi: 10.1177/0890334420960433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Keulen B.J., Romijn M., Bondt A., Dingess K.A., Kontopodi E., van der Straten K., den Boer M.A., Bosch B.J., Brouwer P.J.M., de Groot C.J.M., Hoek M., Li W., Pajkrt D., Sanders R.W., Schoonderwoerd A., Tamara S., Timmermans R.A.H., Vidarsson G., Stittelaar K.J., Rispens T.T., Hettinga K.A., van Gils M.J., Heck A.J.R., van Goudoever J.B. MedRxiv; 2020. Breastmilk; a Source of SARS-CoV-2 Specific IgA Antibodies. [DOI] [Google Scholar]
- 4.Hurley W.L., Theil P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y., Chi X., Hai H., Sun L., Zhang M., Xie W.F., Chen W. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg. Microbes Infect. 2020;9:1467–1469. doi: 10.1080/22221751.2020.1780952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace R.M., Williams J.E., Järvinen K.M., Belfort M.B., Pace C.D.W., Lackey K.A., Gogel A.C., Nguyen-Contant P., Kanagaiah P., Fitzgerald T., Ferri R., Young B., Rosen-Carole C., Diaz N., Meehan C.L., Caffé B., Sangster M.Y., Topham D., McGuire M.A., Seppo A., McGuire M.K. Characterization of sars-cov-2 rna, antibodies, and neutralizing capacity in milk produced by women with covid-19. MBio. 2021;12:1–11. doi: 10.1128/mBio.03192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox A., Marino J., Amanat F., Krammer F., Hahn-Holbrook J., Zolla-Pazner S., Powell R. 2020. Evidence of a Significant Secretory-Iga-Dominant SARS-CoV-2 Immune Response in Human Milk Following Recovery from COVID-19. [DOI] [Google Scholar]
- 8.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., Baez A.M., Shook L.L., Cvrk D., James K., De Guzman R.M., Brigida S., Diouf K., Goldfarb I., Bebell L.M., Yonker L.M., Fasano A., Rabi S.A., Elovitz M.A., Alter G., Edlow A.G. MedRxiv; 2021. COVID-19 Vaccine Response in Pregnant and Lactating women: a Cohort Study. 2021.03.07.21253094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazur N.I., Horsley N.M., Englund J.A., Nederend M., Magaret A., Kumar A., Jacobino S.R., De Haan C.A.M., Khatry S.K., Leclerq S.C., Steinhoff M.C., Tielsch J.M., Katz J., Graham B.S., Bont L.J., Leusen J.H.W., Chu H.Y. Breast milk prefusion F immunoglobulin g as a correlate of protection against respiratory syncytial virus acute respiratory illness. J. Infect. Dis. 2019;219:59–67. doi: 10.1093/infdis/jiy477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandtzaeg P. The Mucosal Immune System and Its Integration with the Mammary Glands. J. Pediatr. 2010;156:S8. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Schlaudecker E.P., Steinhoff M.C., Omer S.B., McNeal M.M., Roy E., Arifeen S.E., Dodd C.N., Raqib R., Breiman R.F., Zaman K. IgA and Neutralizing Antibodies to Influenza A Virus in Human Milk: a Randomized Trial of Antenatal Influenza Immunization. PLoS One. 2013;8:6–13. doi: 10.1371/journal.pone.0070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand S.P., Prévost J., Nayrac M., Beaudoin-Bussières G., Benlarbi M., Gasser R., Brassard N., Laumaea A., Gong S.Y., Bourassa C., Brunet-Ratnasingham E., Medjahed H., Gendron-Lepage G., Goyette G., Gokool L., Morrisseau C., Bégin P., Martel-Laferrière V., Tremblay C., Richard J., Bazin R., Duerr R., Kaufmann D.E., Finzi A. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Reports Med. 2021;2 doi: 10.1016/j.xcrm.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 14.Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K.H.D., Dingens A.S., Nargi R.S., Sutton R.E., Suryadevara N., Rothlauf P.W., Liu Z., Whelan S.P.J., Carnahan R.H., Crowe J.E., Bloom J.D. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain that Escape Antibody Recognition. Cell Host Microbe. 2020:1–34. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantry C.J., Howard C.R., Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]