Abstract
Background:
An association between chronic cough and obstructive sleep apnea (OSA) has been reported in prior studies with resolution or improvement in cough after continuous positive airway pressure (CPAP) therapy. Controlled studies of the benefit of CPAP quality of life measures have not been conducted.
Research Question:
Does CPAP therapy for OSA improve cough in patients with chronic unexplained cough?
Study Design and Methods:
Patients with unexplained chronic cough (> 2 months duration of cough) and OSA were randomized to receive either CPAP or sham CPAP therapy for 6 weeks. The primary end point was the change in health status assessed with the Leicester Cough Questionnaire (LCQ) in patients treated with CPAP vs. sham CPAP. Secondary end-points were changes in exhaled breath-condensate markers of airway inflammation (Interleukin-6, Nitrite/nitrates, hydrogen peroxide and 8-isoprostanes).
Results:
A total of 22 patients with chronic unexplained cough and OSA were randomized of whom18 completed 6 weeks of treatments with either CPAP or sham CPAP. The CPAP vs. sham CPAP treated group were comparable in terms of sex distribution, body-mass index and OSA severity. Following CPAP therapy, there was a significantly greater improvement in total LCQ scores as compared to those treated with sham therapy (ANCOVA p value 0.016). No significant differences were noted in LCQ domain scores or exhaled breath condensate marker changes between CPAP-treated vs. sham CPAP-treated groups.
Conclusion:
Treatment of comorbid OSA in patients with chronic cough improved cough-quality of life measures following treatment of OSA with CPAP in this pilot study. Larger studies to understand this association and unravel mechanisms of CPAP benefit in chronic cough need to be undertaken.
Clinical Trial Registration NCT03172130
Prior abstract
Sundar KM. Chronic cough and obstructive sleep apnea. Understanding the relationship between two common disorder. Platform presentation at American Cough Conference, Reston, Virginia, June 7, 2019.
Unexplained chronic cough (UCC) is a frequent problem in both primary care and specialty clinics (1). UCC is a diagnosis rendered after patients fail to improve their cough despite following the 2006 ACCP guidelines for management of chronic cough (defined as more than 2 months in duration) (2). Studies show that up to third of patients seen in specialist cough clinics persist with their cough despite utilizing the empiric integrative approach outlined in the 2006 ACCP cough guidelines (3). A number of studies have shown that obstructive sleep apnea (OSA) is prevalent in such patients with UCC (4–8). Besides the finding of OSA in UCC, improvements in cough have been reported following therapy for OSA with continuous positive airway pressure (CPAP) (4, 5, 7–9).
The effectiveness of CPAP therapy in improving cough in patients with UCC and OSA was assessed in a randomized controlled study.
Methods
Participants
Adult patients with chronic cough seen in Pulmonary clinics at the University of Utah Hospital and Clinics were evaluated for possible enrolment into this study. Following were the inclusion criteria for enrolment into this study.
Cough more than 2 months of duration
Age more than 18 years
Smoking < 5 pack-years and history of tobacco use more than 10 years prior to enrolment.
Evaluation and treatment for suspected gastroesophageal reflux disease (GERD), upper airway cough syndrome (UACS) or cough-variant asthma (CVA) for at least 1 month prior to being screened for enrolment.
Normal chest radiography or computerized tomography scans (patients with up to 2 lung nodules less than 3 mm were allowed if there was no history of malignancy elsewhere).
No evidence of airflow limitation (FEV1/FVC > 0.7) or significant chest restriction (FVC>70% predicted), and predicted diffusion capacity more than 50% predicted on pulmonary function tests.
Diagnosis of OSA based on polysomnography or home sleep apnea study.
Following were the exclusion criteria for the study
Pregnancy
Positive methacholine challenge test (if performed).
Prior history of asthma for which inhaled or systemic corticosteroids have been used. Patients with wheezing on auscultation also were excluded.
Recent pneumonia (less than 6 months)
Congestive heart failure, acute or chronic renal disease, jaundice or chronic liver disease, pulmonary embolism, stroke or neurodegenerative disease, malignancy.
Age more than 70 years
Use of supplemental oxygen or CPAP therapy
Use of opiates and/or benzodiazepines
Alcoholism, drug dependence (including chewing tobacco) or illicit drug use.
Prior Nissen fundoplication, esophageal or laryngeal surgery.
Craniofacial abnormalities that preclude CPAP placement.
Protocol
All patients underwent chest radiography, pulmonary function testing and evaluation for OSA using in-lab attended polysomnography or level III portable home sleep apnea study through Sleep-Wake Center, University of Utah. Evaluation and testing for sleep-disordered breathing was based upon clinician’s discretion and his or her clinical suspicion for OSA. Diagnosis of OSA was established using the International Classification of Sleep Disorders criteria with patients with apnea-hypopnea index ≥5/hour deemed as having OSA (10). OSA was further categorized as mild, moderate and severe based on AHI (apnea-hypopnea index) with patients with AHI of 5–14.9 deemed as mild, AHI of 15–29.9 as moderate and AHI of ≥ 30 as severe OSA (11). Patients were enrolled into the study if they met the above criteria for diagnoses chronic cough and OSA after which they were randomized to sham CPAP or CPAP therapy for 6 weeks using the sham CPAP or CPAP equipment provided by Phillips Respironics Inc. (Figure 1). After 6 weeks, both CPAP and sham CPAP-treated patients were placed on CPAP that was obtained through their insurance-approved CPAP supplier. Study was approved by the University of Utah Institutional Review Board and during consenting procedure, patient were informed of potential risks of being randomized to sham CPAP therapy and risks of not having their underlying OSA being untreated for a period of 6 weeks. This study was done in accordance with the amended Declaration of Helsinki and written informed consent was obtained from all patients.
Figure 1.
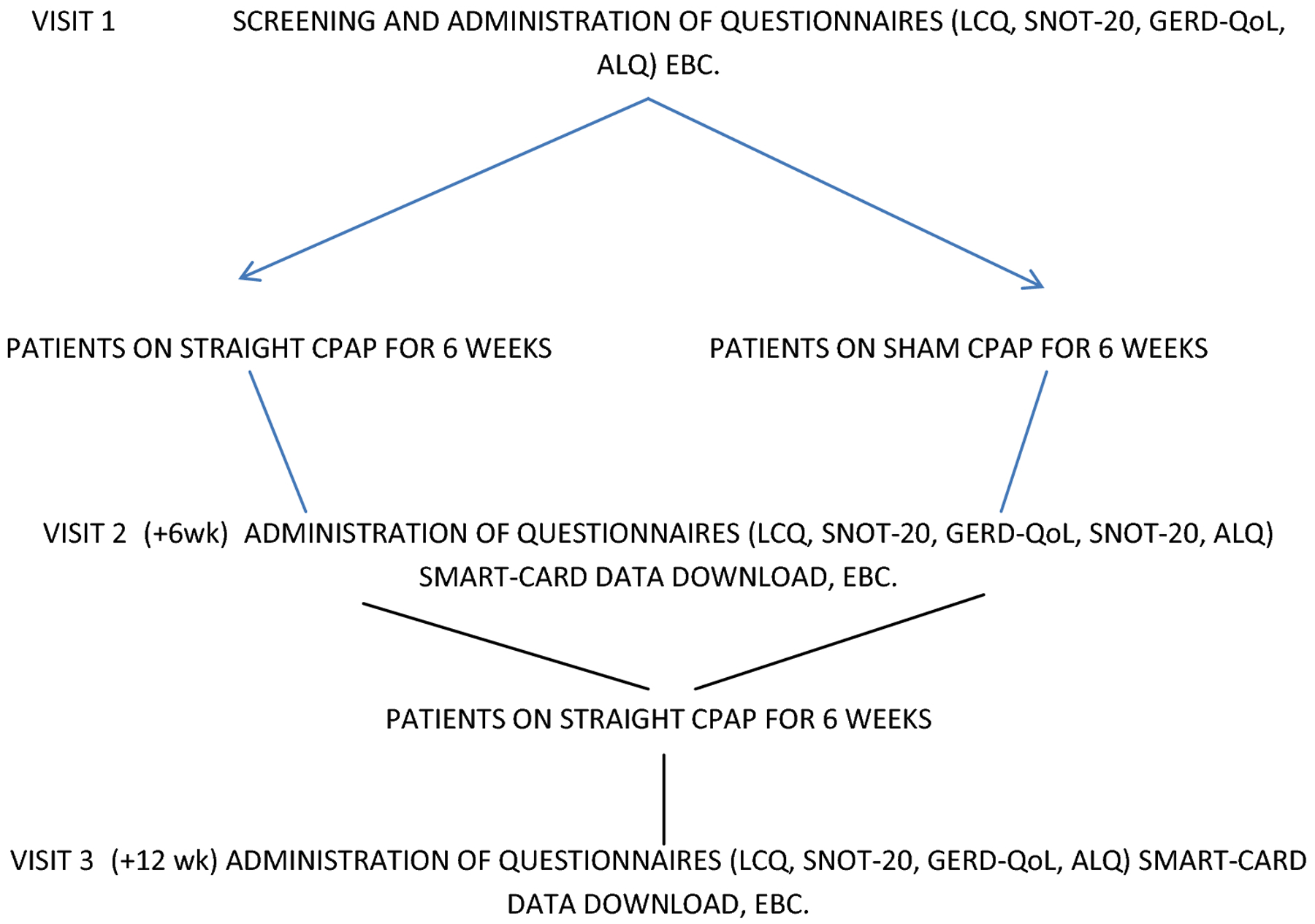
Flowchart showing study protocol and visit procedures. Abbreviations: LCQ – Leicester Cough Questionnaire, SNOT-20 – Sinonasal outcomes-20 questionnaire, GERD-QOL – GERD Health-related Quality of Life questionnaire, ALQ – Asthma Life Questionnaire, LCM – Leicester Cough Monitoring, EBC – Exhaled Breath Condensate measurements.
Assessments for cough and other conditions predisposing to cough
Patients completed the Leicester Cough Questionnaire (LCQ) – a validated cough questionnaire assessing cough-related quality of life scores in three domains – physical, psychological and social (12). The LCQ was administered at enrolment into the study, after 6 weeks of sham CPAP or regular CPAP therapy and at 12 weeks (after 6 weeks of regular CPAP using insurance-approved CPAP) (Fig. 1). In addition to the LCQ, patients were administered questionnaires to assess for sinonasal disease – sinonasal test 20 (SNOT-20) (13), gastroesophageal reflux disease (GERD-QoL) (14), and asthma (Asthma Life Questionnaire - ALQ) (15). These questionnaires were completed by the patients at baseline, after 6 weeks of either CPAP or sham CPAP therapy and following 6 weeks of regular CPAP therapy (Figure 1).
In addition to above questionnaires, patients also underwent cough monitoring for 24 hours at baseline, 6 weeks and 12 weeks. Due to inadequate duration of cough monitoring data in study patients (only 6/9 of sham CPAP and 2/9 of regular CPAP patients had cough recordings of 4 or more hours), comparisons of cough frequency at 6 weeks between these two groups could not be done in this study. Also given that there is no validation of objective cough monitors in patients on CPAP and the possibility of that CPAP noise interfering with cough monitor assessments, LCQ change was chosen as the primary end-point.
Sham CPAP was implemented in specially designed Phillips-Respironics CPAP machines in which a modified mask elbow between the mask and the tubing provided a larger than usual air leak. When assembled, this modification was not noticeable and was compatible with a limited selection of nasal masks. Based on manufacturers testing, this kind of setup reached static pressures of 0.75cm H2O between 7 and 30 breaths per minute and peak pressures of 2.5cm at volumes of 750ml and 30 breaths/minute. Pressure in these devices were locked at 10cm and the operating system was not accessible to the patient.
CPAP users were placed on 10cm of CPAP (5/9) or autotitrating CPAP (2/9) based on whether there was significant positional variations in OSA (these patients received autoCPAP). 2 patients that underwent split-night PSGs and were titrated received 15cm of CPAP pressure.
Exhaled breath condensate measurement
Exhaled breath condensate (EBC) was obtained using the RTube™ breath condensate collection device (Respiratory Research, Inc. Austin, TX, USA) and assessed for different makers of airway inflammation (16, 17). Following collection of up to 100 −1000ul of EBC through RTube system (https://respiratoryresearch.com/rtube), the sample was aliquoted into 100μl vials at −70C and later used for measurement of 8-isoprostanes (8isopg) and (Cayman Chemical, 8-isoprostane ELISA Kit, assay range - 0.8–500 pg/mL), Interleukin-8 (IL-8) (Invitrogen IL-8 Human ELISA Kit, Analytical Sensitivity < 5pg/mL), by enzyme-linked immunoassay (ELISA), nitrates/nitrates (NOX) (Cayman Chemical Nitrate/Nitrite Colorimetric Assay Kit, detection limit for total nitrate/nitrite = 2.5 μM) and hydrogen peroxide (H2O2) (Pierce™ Quantitative Peroxide Assay Kit, assay range - 1 μM – 1 mM) were measured by reactive colorimetric assay (17). The 96-well plate assays were read on a Synergy HTX multi-mode 96-well plate reader (BioTek Instruments Inc., Vermont, USA).
Statistical methods
Subjects that reported CPAP or sham CPAP usage in the first few weeks were only included in this study. None of the devices used in first 6 weeks of the study had any remote tracking facility for monitoring CPAP usage. Therefore patient reported usage as assessed by the study coordinator through phone calls and/or manual downloads from their CPAP devices in the first 3 weeks was used to gauge patient adherence to sham CPAP or regular CPAP. Given that CPAP non-adherence is not unusual during OSA therapy (18), an intent-to-treat analysis design was used to compare the primary end-point of LCQ score change from baseline to 6 weeks between subjects randomized to the CPAP and sham CPAP arms. Based on the results of a longitudinal study of CPAP effect on total LCQ score, the mean LCQ change from CPAP intervention was +4.1 (±SD 4.4) (8). A mean change in total LCQ from placebo therapy has been 1.1 in previously randomized trials of chronic cough (19). Based on this, an expected difference between mean LCQs of two groups was projected to be 3.0. To achieve 80% difference to detect a significant difference of 0.05 level for a difference means of 2.58, 19 subjects per group were needed assuming no attrition using a mixed models analysis of repeated measures data.
Comparisons of demographic data, LCQ and other questionnaires were done using the Wilcoxon test to ensure that subjects enrolled were matched in both groups. The primary end-point of LCQ change between the CPAP and sham CPAP group was compared adjusting for a number of parameters including the baseline LCQ scores. Secondary analyses included comparisons of EBC markers between the CPAP and sham CPAP groups.
Results
Participant characteristics
A total of 78 patients were screened for this study out of which 22 were randomized into the CPAP vs. sham CPAP arms (Consort diagram – Figure 2). Due to non-usage with CPAP (3/12) and sham CPAP (1/10), 9 patients underwent six weeks of sham CPAP and 9 patients six weeks of regular CPAP therapy. Demographics of patients is outlined in Table 1. Patients enrolled to the sham CPAP group were significantly older as compared to the regular CPAP group. There were no other significant demographic differences noted between two groups– groups were matched in terms of their sex ratio and the body mass index (BMI) (Table 1). There were also no significant differences in OSA severity between groups. Based on validated scores for GERD, sinonasal disease and asthma, both groups did not show any differences (Table 1).
Figure 2.
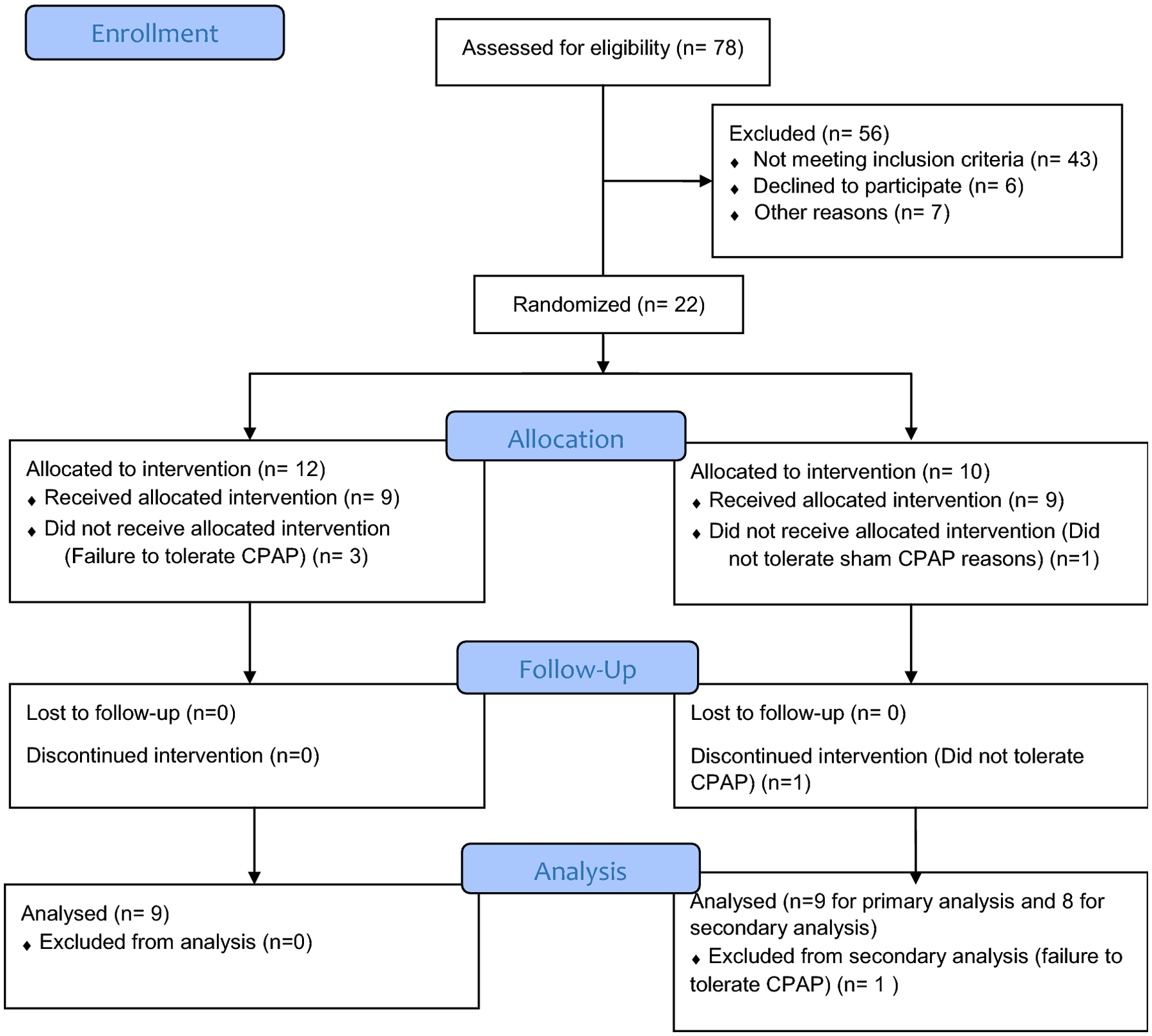
CONSORT Flow Diagram for study enrolment, participation and analysis.
Table 1.
Demographic data of patients randomized to continuous positive airway pressure (CPAP) vs. sham continuous positive airway pressure (sham CPAP). Groups were compared using Wilcoxon t test.
| CPAP (n=9) | Sham CPAP (n=9) | p value | |
|---|---|---|---|
| Age (range) | 52.4 ± 10.9 (33–67) | 62.7 ± 6.3 (54–75) | 0.007 |
| Sex | 7F; 2M | 4F; 5M | 0.147 |
| BMI (range) | 38.3±9.1 (23–52) | 33.2 ± 6 (25–40) | 0.311 |
| OSA diagnosis | 5PSGs and 4HSTs | 4PSGs and 5HSTs | 0.637 |
| AHI (range) | 35.4 ± 37.4 (6.5–107) | 30.3 ± 36.7 (7–124) | 0.783 |
| GERD QoL score (0–50) | 9.4 ± 22.25 | 6.3 ± 6.7 | 0.474 |
| SNOT-20 score (0–120) | 46 ± 14.8 | 34.9 ± 14.6 | 0.202 |
| ALQ score (0–20) | 8.9 ± 2.5 | 7.4 ± 3.5 | 0.292 |
In terms of cough severity, patients in the sham CPAP group had significantly longer mean cough durations and this difference occurred due to a single cough patient with duration of cough of 35 years (Table 2). There were however no significant differences in terms of cough quality of life questionnaire scores based on the total LCQ score or the individual LCQ domain scores between the two groups (Table 2). While the compliance to sham CPAP was lower, there was no significant difference between the two groups in terms of average duration of CPAP vs. sham CPAP usage or the percentage of nights used for more than 4 hours (Table 2)
Table 2.
Comparison of cough characteristics including duration, Leicester Cough Questionnaire (LCQ) scores and CPAP compliance rates between CPAP and sham CPAP treated groups.
| CPAP | Sham CPAP | p value | |
|---|---|---|---|
| Duration of cough (months) | 35.1±57.1 (3–180) | 162.7±153.8 (24–420) | 0.023 |
| Total LCQ | 10.63±3.94 | 12.62±4.13 | 0.185 |
| - Physical | 3.54±0.96 | 4.43±1.07 | 0.053 |
| - Psychological | 3.65±1.61 | 4.09±1.51 | 0.439 |
| - Social | 3.44±1.6 | 4.09±1.51 | 0.359 |
| CPAP compliance | |||
| - % days used | 47% (±37%) | 40% (±28%) | 0.302 |
| - Avg. minutes per night | 246.9 ± 143.6 (>6hrs) | 192.6± 114.7 (>3hrs) | 0.272 |
Relationship between CPAP use and cough
In terms of the primary end-point, there was significant difference in LCQ change between CPAP and sham CPAP groups using both the two-sample t test (with the Kolmogorov-Smirnov test) and ANCOVA that adjusted for baseline LCQ scores – p values of 0.017 and 0.016 respectively. The p value remained significant using the ANCOVA even when adjusted for BMI and gender but not when adjusted for age. A significant LCQ change that met the minimal clinically important difference of 1.3 (19) was seen in all patients treated with CPAP and 4/9 patients treated with sham CPAP (Table 3). 8 patients in sham CPAP group went on to complete 6 weeks of subsequent CPAP therapy but there was no significant difference in the LCQ change after CPAP therapy as compared to the change in LCQ after sham CPAP (p=0.11), due to significant change in 4/9 patients’ LCQ score in the sham CPAP group. Similarly when comparisons of those that were treated with CPAP (n=17; 9 in the first 6 weeks and 8 following transition from sham CPAP to regular CPAP) were done to patients treated with sham CPAP (n=9 in the first 6 weeks), no significant difference in the total LCQ change was noted (p=0.11).
Table 3.
Comparisons of mean changes in LCQ scores between CPAP treated and sham CPAP treated groups.
| CPAP | Sham CPAP | |
|---|---|---|
| Baseline LCQ | 10.63±3.94 | 12.62±4.13 |
| 6 week LCQ | 17.24±3.97 | 14.69±3.94 |
| Mean LCQ change | 6.61±1.36 (SE) | 2.07±0.98 (SE) |
| No. with LCQ Δ of 1.3 | 9/9 | 4/9 |
There were no correlations between baseline AHI values of the 18 patients that completed the study and total LCQ scores (p=0.68) or scores in individual LCQ domains. Neither was there a significant association between severity of OSA (based on AHI) and the degree of LCQ change after 6 weeks of therapy with CPAP (r=0.69 and p=0.07). While there was a correlation between LCQ change and CPAP compliance based on average number of CPAP usage in minutes (r=0.37), this was not significant (p=0.3). There was also no correlation between CPAP usage in terms of percentage used more than 4 hours and LCQ change in the CPAP treated group (p=0.27).
CPAP and effects on exhaled breath condensate
Results of exhaled breath condensate analysis showed no differences in baseline values of IL-8, 8-isoprostanes, NOX or H2O2 values between the CPAP vs. the sham treated groups (Table 4). Following CPAP treatment, there were decreases in mean values of IL-8, NOX and H2O2 whereas there was increases in mean values of 8-isoprostanes, even though these changes were not significant (Table 4). There were no significant difference between CPAP-treated vs. sham-CPAP treated subjects after 6 weeks of intervention when adjusted for baseline values (ANCOVA p values >0.05). Additionally, no significant differences were noted in the sham CPAP group in mean change of airway inflammatory marker values after 6 weeks of sham CPAP therapy (p values > 0.05).
Table 4.
Measurements of airway inflammatory markers, nitrite/nitrate (NOX), interleukin-8 (IL-8), 8-isoprostanes (8iso), hydrogen peroxide (H2O2) in patients treated with CPAP and sham CPAP.
| NOX (μmol/l) | IL- 8 (pg/ml) | 8iso (pg/ml) | H2O2 nmol/L | |
|---|---|---|---|---|
| CPAP – treated (n=9) | ||||
| Baseline | 3.34 ± 2.07 | 1.52 ± 1.41 | 4.92 ± 2.23 | 2458.02 ± 324.88 |
| Post-6 weeks | 2.91 ± 2.32 | 1.00 ± 0.21 | 7.35 ± 3.47 | 1654.07 ± 239.71 |
| p value* | 0.685 | 0.299 | 0.212 | 0.097 |
| Sham CPAP – treated (n=8) | ||||
| Baseline | 3.35 ± 2.81 | 1.02 ± 0.24 | 3.99 ± 1.89 | 1714.42 ± 337.1 |
| Post-6 weeks | 5.26 ± 0.18 | 1.04 ± 0.18 | 5.04 ± 2.13 | 1468.04 ± 143.58 |
| p value* | 0.334 | 0.771 | 0.199 | 0.061 |
| Difference between CPAP – treated and sham CPAP – treated groups (p value#) | 0.258 | 0.594 | 0.156 | 0.643 |
For comparing intragroup change with either CPAP or sham-CPAP therapy, p value* was calculated using paired-sample t test for measurement of significant differences of means. For differences of change after 6 weeks of either CPAP or sham-CPAP therapy, p value# was calculated using ANCOVA that adjusted for baseline values of each marker.
CPAP and effects on coexisting disease scores
Changes in GERD, UACS and asthma-related symptoms/quality of life scores were compared between the CPAP and sham-CPAP treated groups (n=9 in each group). There were no significant differences in mean change in GERD-Qol scores (p=0.27), SNOT-20 scores (p=0.27) and the ALQ scores (p=0.090) using the ANCOVA even though bigger improvements in all three categories were noted in CPAP group as compared to sham-CPAP treated group.
Discussion
This is the first study to compare effects of CPAP and sham CPAP therapy in patients with chronic cough. While both CPAP and sham CPAP use resulted in improvement in cough based on validated cough quality of life scores using the LCQ, the improvement with CPAP was significantly better. Patients randomized to CPAP vs. sham CPAP were comparable in most aspects especially in terms of LCQ scores. Differences in age and duration in cough between two groups was attributed to effects from outliers in the sham CPAP group and the small size of study populations. The improvement in LCQ with CPAP was noted over a 6-week duration and there was poor correlation between duration of CPAP usage and cough improvement.
Prior studies studying the relationship between OSA and cough have undertaken therapy for OSA while patients were undergoing a number of other therapies for chronic cough due to which it was difficult to separate out the contribution of CPAP therapy to resolution of chronic cough (4, 5, 7, 9). Despite this, a substantial effect of CPAP treatment has been purported based upon the subjective improvement in cough in studies that have evaluated CPAP effects in a retrospective manner (5, 7, 9). Despite a higher male preponderance seen in patients with OSA and a higher female preponderance seen in chronic cough patients, the prevalence of OSA in chronic cough patients is substantial (2, 3). Studies have reported OSA prevalence ranging from 39% to 68% that is substantially higher than seen in the general population (5, 8). Except for one study from Thailand that showed a higher male preponderance (7), most studies of chronic cough and OSA have included populations that have a higher female to male ratio as seen in chronic cough (5, 8, 9, 21).
The role of CPAP was assessed as a sole additional intervention in chronic cough patients in one study that followed LCQ as a primary end point, however this study was not randomized and did not have a placebo arm (8). In this study, patients with chronic unexplained cough were randomized to either CPAP or sham CPAP with both the investigator and patient blinded to the nature of CPAP therapy. Only the study coordinator, a sleep technologist with experience to address mask and machine issues was unblinded given that CPAP therapy requires education and support which is impossible without the awareness of the nature of CPAP therapy being rendered. The use of sham CPAP has been advocated as an acceptable placebo for CPAP intervention studies examining the contribution of OSA to disease or outcome (22). Studies examining the usage of sham CPAP technology have shown that sham CPAP application does not result in significant effects on sleep quality that can result in independent effects on the disease or outcomes under study (23). Our study also showed an improvement in cough scores in patients receiving sham CPAP and while this was unexpected, randomized studies on chronic cough have demonstrated a significant placebo effect on chronic cough measures (24). Due to the improvement in the LCQ values in sham CPAP patients, further analyses looking at LCQ change in the sham CPAP group after CPAP therapy did not show any significant differences. A lack of association between OSA severity and cough severity as assessed LCQ have been reported in other studies (8, 9) and this may be due to AHI being not reflective of how OSA contributes to ongoing cough.
The CPAP and sham CPAP groups were matched in almost all aspects especially LCQ scores, validated cough scales for GERD, UACS and asthma, and OSA severity although the sham CPAP group was slightly older and with a longer duration of cough. This study was also notable for higher BMI values in enrolled study participants due to the need for enrolling patients with both chronic cough and OSA. While the relationship between obesity and OSA is well-established, recent reports suggest there is a significant contribution from obesity to the occurrence of chronic cough (25, 26).
OSA can lead to number of local and systemic effects that can contribute to perpetuation of cough. These have includes effects on conditions predisposing to chronic cough such as UACS, GERD and cough-variant asthma as well as novel pathways by which OSA can lead to cough (21). Amongst the etiologies closely associated with chronic cough, GERD is the one that has been commonly reported with OSA. Even though relationship between OSA and GERD is not causal (27), CPAP therapy appears to improve GERD in a number of studies (28). One study that examined CPAP effect in a patient with chronic cough showed that CPAP improved cough sensitivity and cough measures although the change in cough sensitivity occurred almost a year after placement on CPAP therapy (29). Unless 24-hour esophageal pH monitoring is performed in patients with chronic cough, ongoing acid reflux contributing the cough cannot be excluded. This mechanistic pathway will need to be explored in greater detail to understand how CPAP therapy influences the course of cough.
OSA also leads to inflammation in the airways and while this inflammation has been demonstrable in both upper and lower airways, upper airway inflammation may be particularly relevant to the occurrence of chronic unexplained cough (30). OSA is characterized by recurrent upper airway collapse during sleep that leads to upper airway trauma and inflammation which can potentially contribute to persistence of chronic cough (21). Upper airway dysfunction particularly laryngeal dysfunction is being increasingly being reported in patients with OSA (31, 32). One study that systematically assessed laryngeal sensory abnormalities in OSA patients showed a sensory impairment in 61% of OSA patients with significant correlation between AHI and laryngeal sensory values (33). OSA is also characterized by rapid changes in oxygenation due to recurrent upper airway closure, the effects of which may be most pronounced in the lower airway epithelial cells. Airway inflammation has been demonstrated in OSA patients exhaled breath condensates (17) and in our analyses, there was a change in airway inflammatory markers after CPAP therapy (Table 4) that has been demonstrated in prior studies (17). However unlike prior studies, there was no significant change with CPAP therapy as this study was not powered to assess changes for inflammatory markers with CPAP therapy. Finally, CPAP usage leads to lung inflation which may have direct effects on the cough reflex. Studies on expiration and cough reflexes in anesthetized animals have shown effects on coughing in response to punctate mechanical stimulation following CPAP (34) and a role for lung volumes in the modulation of cough motor pattern in the presence inspiratory resistance or expiratory occlusion (35).
Limitations
This study has a number of limitations that include small numbers, single-center enrolment, inability to get adequate objective cough monitoring data and limited CPAP compliance in the sham CPAP arm. Despite these limitations, the study did have similar numbers of patients as enrolled in other pilot studies establishing roles for novel therapies in chronic cough (24). In comparing to other studies where randomization to CPAP or sham CPAP has been done, average CPAP compliance in our study was significantly higher in those randomized to CPAP (>6hrs) as compared to the CPAP compliance of 4–4.2 hours to in other studies (36, 37). The sham CPAP compliance in our study was comparable to that seen in other sham CPAP studies (36, 37). Although sham CPAP has been advocated as a suitable placebo, the problem of lower adherence to sham CPAP has been notable with sham CPAP usage in multiple studies (36–38).
In terms of the study population, while a higher proportion of males and higher body mass indices were noted due to the need for these subjects to have OSA in addition to underlying chronic cough, recent prevalence studies of OSA have shown significant occurrence of moderate to severe OSA in the general population with lower BMIs (39).
Conclusions
This study establishes a role for treatment of comorbid OSA in the management of chronic cough patients. OSA may lead to cough by a number of above outlined pathways although much work remains to be done to understand how OSA treatment leads to improvement or resolution in cough. Larger studies looking at this association need to be done to understand the role of OSA therapy in chronic unexplained cough. Future research should emphasize upon the effects of OSA treatment on cough hypersensitivity given that this is a key mechanism for cough perpetuation in the majority of patients with UCC (40). Given the high prevalence of OSA in chronic cough population that typically affects middle-aged adults (39), screening and therapy for OSA should occur in conjunction with other therapies directed at cough.
Acknowledgments
KM Sundar takes responsibility for the content of the manuscript including data and analysis. KMS and SSB contributed to study design and write-up. AMW tabulated all study participant data, kept track of enrolment and was involved in the analysis. SS was the study coordinator who administered all study procedures including collection of exhaled breath condensates. SS was the unblended sleep tech who managed patients after they were randomized to CPAP or sham CPAP. JPK performed all the exhaled breath condensate analyses. NH performed all statistical analyses.
The authors wish to thank the physicians and advanced care practitioners in the Pulmonary Division at the University of Utah for referring patients with chronic cough for this study. We appreciate the help by Dr. Cheryl Pirozzi and University of Utah Lung research with the exhaled breath condensate collection and storage.
Funding
Phillips-Respironics Inc. (only equipment support)
Summary of Conflicts of Interest
KM Sundar - Site PI for study of iVAPS EPAP algorithm funded by Resmed Inc.; Co-founder, Hypnoscure LLC (no conflicts of interest with this study)
SS Birring - Received personal fees from Merck, Bayer, Shionogi, Nerre, Boehringer Ingelheim, GSK and Respivant, unrelated to this study and an investigator initiated research grant from Merck.
Rest of the authors do not have any conflicts of interest to report.
Abbreviations list
- UCC
Unexplained chronic cough
- OSA
Obstructive sleep apnea
- CPAP
continuous positive airway pressure
- AHI
Apnea-hypopnea index
- BMI
Body mass index
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Pratter MR. Unexplained (idiopathic) cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129 (1 suppl):220S–221S. [DOI] [PubMed] [Google Scholar]
- 2.Pratter MR, Brightling CE, Boulet LP, Irwin RS. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129 (1 suppl):222S–231S. [DOI] [PubMed] [Google Scholar]
- 3.Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ 2015;351:h5590. [DOI] [PubMed] [Google Scholar]
- 4.Birring SS, Ing AJ, Chan K, et al. Obstructive sleep apnoea: a cause of chronic cough. Cough 2007;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar KM, Daly SE, Pearce MJ, Alward WT. Chronic cough and obstructive sleep apnea in a community-based pulmonary practice. Cough 2010;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan KK, Ing AJ, Laks L, Cossa G, Rogers P, Birring SS. Chronic cough in patients with sleep-disordered breathing. Eur Respir J 2010;35:368–372. [DOI] [PubMed] [Google Scholar]
- 7.Wang T-Y, Lo Y-L, Liu W-T, et al. Chronic cough and obstructive sleep apnoea in a sleep laboratory-based pulmonary practice. Cough 2013;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundar KM, Daly SE, Willis AM. A longitudinal study of CPAP therapy for patients with chronic cough and obstructive sleep apnoea. Cough 2013;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Good JT Jr, Rollins DR, Kolakowski CA, Stevens AD, Denson JL, Martin RJ. New insights in the diagnosis of chronic refractory cough. Respir Med 2018;141:103–110. [DOI] [PubMed] [Google Scholar]
- 10.Obstructive sleep apnea disorders. In: International Classification of Sleep Disorders. 3rd edition. Darien IL: American Academy of Sleep Medicine, 2014. Sateia M. ed. Pp 53–62. [Google Scholar]
- 11.Won CHJ, Qin L, Selim B, Yaggi HK. Varying hypopnea definitions affect obstructive sleep apnea severity classification and association with cardiovascular disease. J Clin Sleep Med 2018;14:1987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picirillo JF, Merritt MG, Richards ML. Psychometric and clinimetric validity of the 20-item sinonasal outcome test (SNOT-20). Otolaryngol Head Neck Surg 2002;126:41–7. [DOI] [PubMed] [Google Scholar]
- 14.Chan Y, Ching JYL, Cheung CMY, et al. Development and validation of a disease specific quality of life questionnaire for gastro-oesophageal reflux disease: The GERD-QoL questionnaire. Aliment Pharmcol Ther 2009;31:452–60. [DOI] [PubMed] [Google Scholar]
- 15.Winder JA, Nash K, Winder Brunn J. Validation of a life quality (LQ) test for asthma. Ann Allergy Asthma Immunol 2000;85:467–472. [DOI] [PubMed] [Google Scholar]
- 16.Finamore P. Scarlata S, Cardaci V, Incalzi RA. Exhaled breath analysis in obstructive sleep apnea syndrome: A review of the literature. Medicina (Kaunas) 2019;55 (9).pii:E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpagnano G. Exhaled Breath Analysis and Sleep J Clin Sleep Med. 2011. October 15; 7(5 suppl): S34–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver T, Grunstein R. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc 2008;5:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet 2012;380:1583–9. [DOI] [PubMed] [Google Scholar]
- 20.Raj AA, Pavord DI, Birring SS. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol 2009;187:311–20. [DOI] [PubMed] [Google Scholar]
- 21.Sundar KM, Daly SE. Chronic cough and OSA: a new association? J Clin Sleep Med 2011;7:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodway GM, Weaver TE, Mancini C, et al. Effect of sham-CPAP as a placebo in CPAP intervention studies. Sleep 2010;33:260–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid ML, Gleason KJ, Bakker JP, Wang R, Mittleman MA, Redline S. The role of sham continuous positive airway pressure as a placebo in controlled trials: Best Apnea Interventions for Research Trial. Sleep 2019;42(8) pii: zsz099. doi: 10.1093/sleep/zsz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomized, double-blind, placebo-controlled phase 2 study. Lancet 2015;385:1198–205. [DOI] [PubMed] [Google Scholar]
- 25.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross-sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006;61:975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colak Y, Nordestgaard BG, Laursen LC, Afzal S, Lange P, Dahl M. Risk factors for chronic cough among 14,669 individuals from the general population. Chest 2017;152:563–573. [DOI] [PubMed] [Google Scholar]
- 27.Fujiara Y, Arakawa T, Fass R. Gastroesophageal reflux and sleep. Gastroenterol Clin N Am 2013;42:57–70. [DOI] [PubMed] [Google Scholar]
- 28.Tamanna S, Campbell D, Warren R, Ullah MI. Effect of CPAP therapy on symptoms of nocturnal gastroesophageal reflux among patients with obstructive sleep apnea. J Clin Sleep Med 2016;12:1257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faruqi S, Fahim A, Morice AH. Chronic cough and obstructive sleep apnoea: reflux-associated cough hypersensitivity? Eur Respir J 2012;40:1049–50. [DOI] [PubMed] [Google Scholar]
- 30.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Care Med 2004;170:541–546 [DOI] [PubMed] [Google Scholar]
- 31.Roy N, Merrill RM, Pierce J, Sundar KM. Voice disorders in obstructive sleep apnea: Prevalence, risk factors, and role of CPAP. Ann Otol Rhinol Laryngol 2018. December 21:3489418819541. doi: 10.1177/0003489418819541 (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 32.Gouveia CJ, Yalamanchili A, Ghadersohi S, Price CPE, Bove M, Attarian HP, Tan BK. Are chronic cough and laryngopharyngeal reflux more common in obstructive sleep apnea patients? Laryngoscope 2019;2019:1244–1249. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. SLEEP 2005:28:585–593. [DOI] [PubMed] [Google Scholar]
- 34.Ioan I, Demoulin B, Leblanc AL, et al. Modulation of defensive airway reflexes during CPAP in the rabbit. Respir Physiol Neurobiol 2018;257:87. [DOI] [PubMed] [Google Scholar]
- 35.Poliacek I, Kotmanova Z, Veternik M, et al. The motor pattern of tracheobronchial cough is affected by inspiratory resistance and expiratory occlusion – The evidence for volume feedback during cough expiration. Respir Physiol Neurobiol 2019;261:9–14. [DOI] [PubMed] [Google Scholar]
- 36.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea Result of the CPAP apnea trial North American Program (CATNAP) Randomized clinica trial. Am J Respir Crit Care Med 2012;186;677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The apnea positive pressure long-term efficacy study (APPLES). Sleep 2012;35:1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chasens ER, Drumheller OJ, Strollo PJ. Success in blinding to group assignment with sham-CPAP. Biol Res Nurs 2013;15:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung K, McGarvey L, Mazzone S. Chronic cough as a neuropathic disorder. Lancet Respir Med 2013;1:412–422. [DOI] [PubMed] [Google Scholar]


