Abstract
Elevated CO2 (eCO2) and high temperatures are known to affect plant nitrogen (N) metabolism. Though the combined effects of eCO2 and chronic warming on plant N relations have been studied in some detail, a comprehensive statistical review on this topic is lacking. This meta-analysis examined the effects of eCO2 plus warming on shoot and root %N, tissue protein concentration (root, shoot and grain) and N-uptake rate. In the analyses, the eCO2 treatment was categorized into two classes (<300 or ≥300 ppm above ambient or control), the temperature treatment was categorized into three classes (<1.5, 1.5–5 and >5 °C above ambient or control), plant species were categorized based on growth form and functional group and CO2 treatment technique was also investigated. Elevated CO2 alone or in combination with warming reduced shoot %N (more so at ≥300 vs. <300 ppm above ambient CO2), while root %N was significantly reduced only by eCO2; warming alone often increased shoot %N, but mostly did not affect root %N. Decreased shoot %N with eCO2 alone or eCO2 plus warming was greater for woody and non-woody dicots than for grasses, and for legumes than non-legumes. Though root N-uptake rate was unaffected by eCO2, eCO2 plus warming decreased N-uptake rate, while warming alone increased it. Similar to %N, protein concentration decreased with eCO2 in shoots and grain (but not roots), increased with warming in grain and decreased with eCO2 and warming in grain. In summary, any benefits of warming to plant N status and root N-uptake rate will generally be offset by negative effects of eCO2. Hence, concomitant increases in CO2 and temperature are likely to negate or decrease the nutritional quality of plant tissue consumed as food by decreasing shoot %N and shoot and/or grain protein concentration, caused, at least in part, by decreased root N-uptake rate.
Keywords: Climate change, elevated CO2, heat stress, meta-analysis, nitrogen metabolism, nitrogen translocation, nitrogen-uptake rate, protein, warming
In the future, concomitant increases in CO2 and temperature are likely to affect plant N metabolism by lowering plant %N, root N-uptake rate and tissue protein concentration. In addition, different growth forms will respond differently to concomitant increases in CO2 and temperature. Elevated CO2 plus warming is likely to enhance root-to-shoot N translocation in grasses but decrease it in non-woody dicots and woody species. Further, in addition to the FACE technique, other enclosed techniques such as GC, TGT and CTC can produce reliable results for studies examining the effects of eCO2 plus warming.
Introduction
Present-day atmospheric carbon dioxide (CO2) levels (ca. 400 ppm) are unprecedented over the past 420 000 years (Petit et al. 1999). With industrialization, and its expansion due to economic and population growth, atmospheric CO2 levels have increased up to 46 % in the last 170 years. According to low and intermediate CO2-emission scenarios, atmospheric CO2 is likely to be in the range of 450–1000 ppm by the end of this century (IPCC 2014). Carbon dioxide is a greenhouse gas and its emissions account for ca. two-thirds of current global warming. Due to global warming, the increase in Earth’s mean surface temperature is likely to be in the range of 1.5–6 °C by 2100 (IPCC 2014). The concomitant increases in CO2 and temperature are expected to have various impacts on plant species, including those used in agriculture and forestry. Though the interactive effects of CO2 enrichment and warming on plant growth and function have been studied in some detail (Morison and Lawlor 1999; Wang et al. 2012), nitrogen (N) metabolism in response to concomitant increases in CO2 and temperature is still poorly understood.
Plant N relations in response to eCO2 have been extensively studied. Due to natural variation among species and differences among experimental protocols, most plant responses to eCO2 are highly variable; one exception to this pattern is tissue N concentration (Bassirirad 2000; Ainsworth and Long 2005; Taub and Wang 2008; Ainsworth and Long 2021). Elevated CO2 stimulates photosynthesis which then stimulates production of non-structural carbohydrates. When the sugar production exceeds the plant sink capacity, it induces a negative feedback on the transcription of Ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco), resulting in reduced Rubisco concentrations (and thus leaf N; see also Moore et al. 1999), and thus net CO2 assimilation (which may still be higher compared to plants grown under ambient CO2) (Ainsworth and Long 2005; Taub and Wang 2008). According to the progressive N limitation (PNL) hypothesis, eCO2 could enhance the sequestration of N into long-lived plant biomass and soil organic matter which in turn reduce the available soil N for plant growth resulting lower tissue N (Luo et al. 2004). In addition, several other mechanisms, such as decreased root N uptake, less efficient root architecture and increased N loss, have been proposed to contribute to lower tissue N concentrations at eCO2 (Taub and Wang 2008). As with tissue %N, eCO2 is also likely to reduce tissue protein concentration (Taub et al. 2008; Feng et al. 2015). However, legumes are hypothesized to have an advantage over other C3 species when grown at eCO2 due to their ability to exchange carbon for N with their N-fixing symbionts (Rogers et al. 2009). Though eCO2 tends to decrease root N-uptake rate, especially in plants rooted in solid media, past studies collectively have shown that both N-uptake kinetics and root N-uptake rate in response to eCO2 can be highly variable (Bassirirad 2000; Taub and Wang 2008).
Plant N relations in response to chronic warming have been studied in some detail. A meta-analysis conducted by Zvereva and Kozlov (2006) found a non-significant negative effect of warming (≥4 or <4 °C above control) on above-ground N concentration. Heat stress is known to cause rates of protein degradation to exceed rates of new protein synthesis (Huang et al. 2012). Huang et al. (2012) further showed that some cultivars were capable of producing more thermostable proteins and maintaining low levels of proteolytic enzyme activities to protect against warming. Therefore, though warming is likely to reduce protein concentration, variation in plant protein levels in response to warming could be either interspecies- or intraspecies-specific. Optimal growth temperature is species-specific, and hence, warming from suboptimal to optimal temperatures is likely to increase root N-uptake rate (Clarkson and Warner 1979; Tindall et al. 1990; Cruz et al. 1993; Atkin and Cummins 1994), while warming (acute or chronic) from optimal to supra-optimal temperatures is likely to decrease this rate (Tindall et al. 1990; Delucia et al. 1992; Bassirirad et al. 1993; Mainali et al. 2014; Giri et al. 2017).
Combined eCO2 plus warming is likely to decrease above-ground N concentration in plant tissue, and the magnitude of this decrease has been greater for C3 and woody species than C4 and herbaceous species (Zvereva and Kozlov 2006; Wang et al. 2012); however, root %N is likely to be unaffected by eCO2 plus warming (Wang et al. 2012). A limited number of studies suggests that the effects of eCO2 plus warming on N-uptake rate can be variable, within and among species (Coleman and Bazzaz 1992; Dijkstra et al. 2010; Arndal et al. 2014; Jayawardena et al. 2017). Few studies have looked at the effects of eCO2 plus warming on tissue protein concentration (total protein per g dry mass). They suggest that grain protein concentration can vary in response to eCO2 plus warming (Abebe et al. 2016; Jing et al. 2016; Palacios et al. 2019; Qiao et al. 2019). Previously, we examined the effects of eCO2 plus warming on total root protein concentration of tomato (Solanum lycopersicum) provided either nitrate (NO3−) or ammonium (NH4+) as the sole N source and noted a significant decrease in root protein concentration in both sets of plants (Jayawardena et al. 2017). To the best of our knowledge, total shoot protein concentration in response to eCO2 plus warming has not been studied before. Collectively, these studies indicate that the effects of eCO2 plus warming on root N uptake and assimilation are not fully understood.
Free-air CO2 enrichment (FACE) experiments are thought to provide the most realistic measures of the effects of eCO2 on crop yields (Ainsworth et al. 2008; Ainsworth and Long 2021). However, the high cost associated with FACE experiments (ca. US$1 million in maintenance per year plus investigation costs) (DaMatta et al. 2010) makes it inaccessible to many researchers who are interested in investigating environmental variables such as CO2 and temperature on plant responses. Though many available enclosure techniques produce a ‘chamber effect’ (Ainsworth et al. 2008), they have often produced results similar to those of FACE experiments (e.g., see Taub et al. 2008). This comparison has mostly been assessed only for eCO2 and not eCO2 plus other factors, so it would be useful to compare plant responses (e.g. plant %N) to eCO2 plus warming using different eCO2 treatment delivery techniques.
Our current understanding of the effects of eCO2 plus warming on plant N metabolism has important knowledge gaps. Therefore, the main objective of this study was to narrow these knowledge gaps using a comprehensive meta-analysis of the effects of eCO2 plus warming on variables related to plant N metabolism, such as shoot and root %N, tissue protein concentration and root N-uptake rate. This includes a subgroup analysis of the effects of eCO2 plus warming on different growth forms, functional groups and eCO2 treatment techniques. Results of this study will help crop scientists, plant breeders and molecular biologists better understand how plant N metabolism will likely respond to future predicted climate conditions and provide targets for developing new genotypes with improved N-relation traits suited for future climate conditions.
Methods
Data collection
This meta-analysis mostly followed methods described by Wang et al. (2012). A literature search was conducted between November 2019 and March 2020 using the search engines PubMed and Google Scholar to construct the database for this meta-analysis (Table 1). The obtained peer-reviewed research or review papers were cross-referenced to help ensure the inclusion of all relevant articles. Research papers published in English which met the following criteria were included in the meta-analysis: (i) CO2 × temperature treatment interaction (full factorial or at least the interaction and the control treatment), (ii) warming treatment that was chronic (i.e. growing plants for a longer period of time ranging from weeks to years at higher than ambient/near-optimal temperatures; abrupt or short-term heating was not considered here, i.e. heat-shocking plants with supra-optimal temperatures for hours to a few days), (iii) whole-plant warming throughout the day (soil or night-time-only warming was excluded) and (iv) reporting of standard error or standard deviation and number of replicates. For all studies considered, ambient or control CO2 concentration (aCO2) ranged between 350 and 400 ppm, while eCO2 ranged between 490 and 800 ppm. If aCO2 was not reported for a study, mean annual CO2 concentration for the year in which the study was conducted was estimated using CO2.earth (https://www.co2.earth/monthly-co2). The lowest elevated temperature was ambient temperature (Tamb) + 0.6 °C, while the highest elevated temperature was Tamb + 19 °C. However, there were only four experimental observations with elevated temperatures >10 °C above ambient. Response variables extracted for analysis included shoot and root %N (per unit dry mass), root N-uptake rate (rate per unit dry mass per unit time) and tissue protein concentration (total protein per unit dry mass). Shoot %N data had four experimental observations with partial irrigation, eight experimental observations with insect feeding and one experimental observation with elevated Ultraviolet-B light treatment. Root %N data had one experimental observation with partial irrigation. When taking these observations into account, the non-CO2 or non-temperature-stress treatment alone was used as the control. These observations were not excluded from the database in order to increase sample size (and response patterns did not change with the exclusion of these observations). Root N-uptake rate included total N, NO3− or NH4+ uptake rates of intact or excised roots. Graphically presented data were extracted using the data extraction software WebPlotDigitizer version 4.2 (Rohatgi 2017). Standard errors of the mean (SEM) were converted to standard deviations (SDs) using the equation; , where n is the number of replicates [seeSupporting Information—Appendix S1].
Table 1.
Plant species, response variables extracted, subgroup of the response variable extracted and references used in the meta-analysis.
| Species | Response variable extracted | Subgroup of the response variable extracted | References | |||||
|---|---|---|---|---|---|---|---|---|
| Shoot %N | Root %N | Root N-uptake rate | Protein concentration | Growth form | Functional group | Treatment technique | ||
| Abutilon theophrasti | * | * | NWD | NL | GC | Coleman and Bazzaz (1992) | ||
| Acer rubrum | * | W | NL | OTC | Norby et al. (2000) | |||
| Acer rubrum | * | W | NL | OTC | Wan et al. (2004) | |||
| Acer rubrum | * | W | NL | OTC | Williams et al. (2003) | |||
| Acer rubrum | * | W | NL | OTC | Williams et al. (2000) | |||
| Acer saccharum | * | W | NL | OTC | Norby et al. (2000) | |||
| Acer saccharum | * | W | NL | OTC | Wan et al. (2004) | |||
| Acer saccharum | * | W | NL | OTC | Williams et al. (2000) | |||
| Alliaria petiolata | * | NWD | NL | GC | Anderson and Cipollini (2013) | |||
| Amaranthus retroflexus | * | * | NWD | NL | GC | Coleman and Bazzaz (1992) | ||
| Betula pendula | * | W | NL | GC | Kellomaki and Wang (2001) | |||
| Betula pendula | * | W | NL | CTC | Kuokanen et al. (2001) | |||
| Betula pendula | * | W | NL | CTC | Kuokanen et al. (2003) | |||
| Betula pendula | * | W | NL | CTC | Lavola et al. (2013) | |||
| Brassica juncea | * | NWD | NL | GC | Seth and Misra (2014) | |||
| Calluna vulgaris | * | * | W | NL | FACE | Andresen et al. (2009) | ||
| Calluna vulgaris | * | * | W | NL | FACE | Andresen et al. (2010) | ||
| Calluna vulgaris | * | W | NL | FACE | Arndal et al. (2014) | |||
| Coffea arabica | * | G | NL | GC | Ramalho et al. (2018) | |||
| Deschampsia flexuosa | * | * | W | NL | FACE | Andresen et al. (2009) | ||
| Deschampsia flexuosa | * | * | G | NL | FACE | Andresen et al. (2010) | ||
| Deschampsia flexuosa | * | G | NL | FACE | Arndal et al. (2014) | |||
| Echinium plantagineum | * | NWD | NL | GC | Johns and Hughes (2002) | |||
| Eucalyptus globulus | * | W | NL | CTC | Crous et al. (2013) | |||
| Eucalyptus globulus | * | W | NL | OTC | Sharwood et al. (2017) | |||
| Eucalyptus robusta | * | W | NL | GH | Gherlenda et al. (2015) | |||
| Eucalyptus saligna | * | W | NL | GH | Ayub et al. (2011) | |||
| Eucalyptus saligna | * | W | NL | GH | Ghannoum et al. (2010a) | |||
| Eucalyptus saligna | * | * | W | NL | GH | Ghannoum et al. (2010b) | ||
| Eucalyptus sideroxylon | * | W | NL | GH | Ghannoum et al. (2010a) | |||
| Eucalyptus sideroxylon | * | * | W | NL | GH | Ghannoum et al. (2010b) | ||
| Eucalyptus tereticornis | * | W | NL | GH | Gherlenda et al. (2015) | |||
| Eucalyptus tereticornis | * | W | NL | GH | Gherlenda et al. (2016) | |||
| Eucalyptus tereticornis | * | W | NL | GH | Murray et al. (2013) | |||
| Geum vernum | * | NWD | NL | GC | Anderson and Cipollini (2013) | |||
| Glycine max | * | NWD | L | OTC | Palacios et al. (2019) | |||
| Glycine max | * | NWD | L | OTC | Qiao et al. (2019) | |||
| Glycine max | * | NWD | L | FACE | Rosenthal et al. (2014) | |||
| Gossypium hirsutum | * | W | NL | GC | Zhang et al. (2017) | |||
| Grasses | * | G | NL | GC | Johnson and Hartley (2018) | |||
| Lantana camara | * | W | NL | GC | Johns et al. (2003) | |||
| Lolium perenne | * | * | G | NL | CTC | Soussana et al. (1996) | ||
| Lolium perenne | * | G | NL | CTC | Zavalloni et al. (2012) | |||
| Lotus corniculatus | * | NWD | L | CTC | Zavalloni et al. (2012) | |||
| Medicago lupulina | * | NWD | L | CTC | Zavalloni et al. (2012) | |||
| Medicago sativa | * | NWD | L | TGT | Aranjuelo et al. (2005) | |||
| Medicago sativa | * | NWD | L | TGT | Aranjuelo et al. (2008) | |||
| Medicago sativa | * | NWD | L | GH | Ariz et al. (2015) | |||
| Oryza sativa | * | G | NL | FACE | Jing et al. (2016) | |||
| Oryza sativa | * | G | NL | TGT | Kim et al. (2011) | |||
| Oryza sativa | * | * | G | NL | FACE | Li et al. (2017) | ||
| Oryza sativa | * | G | NL | OTC | Liu et al. (2019) | |||
| Panicum maximum | * | G | NL | FACE | de Assis Prado et al. (2016) | |||
| Phalaris aquatica | * | G | NL | TGT | Lilley et al. (2001) | |||
| Phalaris aquatica | * | G | NL | TGT | Volder et al. (2015) | |||
| Phaseolus vulgaris | * | NWD | L | CTC | Prasad et al. (2004) | |||
| Pinus ponderosa | * | W | NL | GH | King et al. (1997) | |||
| Pinus sylvestris | * | W | NL | CTC | Luomala et al. (2003) | |||
| Pinus taeda | * | W | NL | GH | King et al. (1997) | |||
| Plantago lanceolata | * | G | NL | CTC | Zavalloni et al. (2012) | |||
| Poa pratensis | * | G | NL | CTC | Zavalloni et al. (2012) | |||
| Pseudotsuga menziesii | * | W | NL | CTC | Chen et al. (2008) | |||
| Pseudotsuga menziesii | * | W | NL | CTC | Hobbie et al. (2001) | |||
| Quercus robur | * | W | NL | GH | Dury et al. (1998) | |||
| Rumex acetosa | * | NWD | NL | CTC | Zavalloni et al. (2012) | |||
| Salix myrsinifolia | * | W | NL | CTC | Veteli et al. (2002) | |||
| Semiarid grasses | * | * | G | NL | FACE | Dijkstra et al. (2010) | ||
| Solanum lycopersicum | * | * | * | * | NWD | NL | GC | Jayawardena et al. (2017) |
| Solanum lycopersicum | * | * | * | * | NWD | NL | GC | Jayawardena et al. (2021) |
| Species mix | * | N/A | NL | CTC | Kandeler et al. (1998) | |||
| Species mix | * | N/A | NL | FACE | Mueller et al. (2016) | |||
| Species mix | * | G | NL | CTC | Zavalloni et al. (2012) | |||
| Trifolium subterraneum | * | NWD | L | TGT | Lilley et al. (2001) | |||
| Triticum aestivum | * | * | * | * | G | NL | GC | Jayawardena et al. (2020) |
| Triticum durum | * | NWD | NL | TGT | Jauregui et al. (2015) | |||
| Vitis vinifera | * | * | W | NL | GH | Salazar-Parra et al. (2015) | ||
| Zea mays | * | G | NL | OTC | Abebe et al. (2016) | |||
| Zea mays | * | G | NL | CTC | Kim et al. (2007) | |||
| Zea mays | * | G | NL | OTC | Qiao et al. (2019) |
References included in the meta-analysis are available in Supporting Information—Appendix S2.
* denotes the response variable extracted from each reference. Different growth forms are denoted as woody, W; grassy, G; and non-woody dicot, NWD. Different functional groups are denoted as legume, L; and non-legume, NL. Different treatment techniques are denoted as open-top chambers, OTC; closed-top chambers, CTC; greenhouses, GH; free-air CO2 enrichment, FACE; temperature-gradient tunnels, TGT; and growth chambers, GC.
Categorization of data
The increase in global mean surface temperature is likely to exceed 1.5 °C by 2100 under all emission scenarios. It is also likely to be in the range of 1.5–4.5 °C, and very unlikely to be greater than 6 °C, by 2100 (IPCC 2014). Based on these predictions, the warming treatments were categorized into three temperature classes as ambient or control plus: <1.5 °C (TL), 1.5–5 °C (TM) and >5 °C (TH). According to the low and intermediate CO2 emission scenarios, atmospheric CO2 is likely to increase between 450 and 1000 ppm by 2100 (IPCC 2014). Therefore, a breakpoint for categorization of eCO2 treatments into two levels was arbitrarily selected as <300 ppm or ≥300 ppm above ambient or control (350–400 ppm CO2). Plant species were categorized based on growth form (woody, grassy or non-woody dicots), functional group (legumes or non-legumes) and treatment technique (open-top chambers, OTC; closed-top chambers, CTC; greenhouses, GH; FACE; temperature-gradient tunnels, TGT; or growth chambers, GC). Only shoot and root %N data were categorized into temperature or CO2 subclasses (growth form, functional group and treatment technique) due to the high availability of experimental observations for these two response variables. Protein concentration was categorized based on tissue type (grain, shoot or root).
Meta-analytic method
In this meta-analysis, the natural-log response ratio between the means of experimental and control groups was used as the metric of the effect size (Hedges et al. 1999). The effect size was graphically presented as the mean % change (Ainsworth et al. 2002) with its 95 % confidence interval (CI). The meta-analysis was performed using OpenMEE an open-source software for meta-analysis in ecology and evolutionary biology (Wallace et al. 2017). A continuous random-effects model with Hedges–Olkin method that relies on inverse-variance weighting to account for variation in precision (sampling error) within and between studies was used (Hedges and Olkin 1985; Wallace et al. 2017). The independent variables (eCO2, warming and eCO2 plus warming) were considered to have a significant effect on the dependent variables if CIs did not overlap the reference line at 0 % change. The outcome was considered significant if P < 0.05. Normality assumption was checked using normal quantile–quantile plots. Publication bias was checked using Rosenthal’s fail-safe number and funnel plots. Rosenthal’s fail-safe number was calculated using OpenMEE software. This number indicates the number of non-significant and unpublished studies required for the meta-analysis to change the statistical significance of the meta-analytic result to a non-significant result (Rosenthal 1979). If this number was greater than , where n is the number of experimental observations, publication bias could be safely ignored (Rosenberg 2005). In addition, if data were symmetrically distributed in the funnel plot, publication bias was safely ignored.
Results
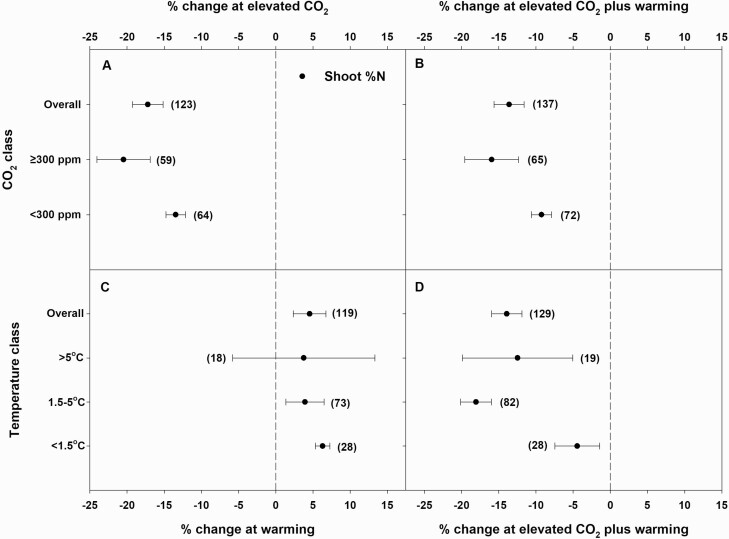
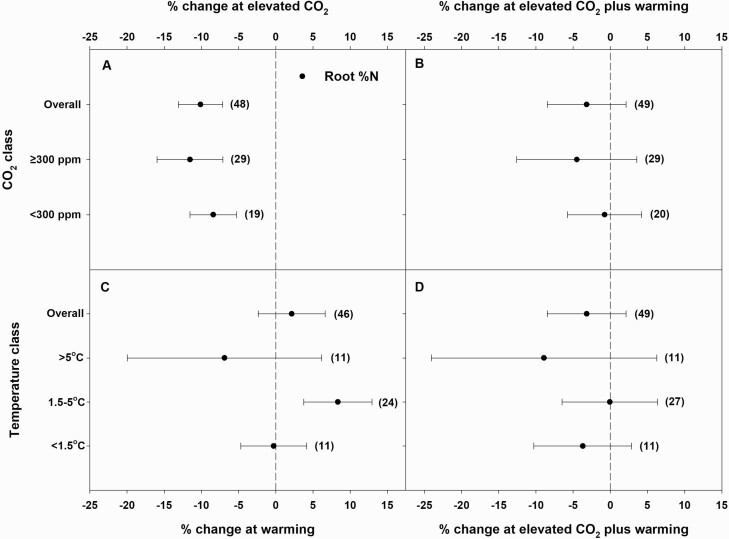
Elevated CO2 alone significantly reduced both shoot and root %N, by 17 and 10 % overall, respectively. The magnitude of the decrease was greater at high eCO2 (≥300 ppm above control) than at low eCO2 (<300 ppm above control), although it was significant only for shoots (Figs 1A and 2A). Warming alone significantly increased shoot %N by 5 % and non-significantly increased root %N by 2 %. The magnitude of a warming-driven increase in shoot %N decreased as temperature increases from TL to TH, although CIs overlapped for all three temperature classes. Notably, the increase in shoot %N at TH was non-significant (Fig. 1C). Meanwhile, root %N neither increased nor decreased at TL, but significantly increased at TM and non-significantly decreased at TH (Fig. 2C). There was a publication bias for the effect of temperature on root %N. Irrespective of the CO2 or temperature classes, eCO2 plus warming significantly reduced shoot %N by 14 %. The magnitude of decrease was greater at high eCO2 or TM (1.5–5 °C above control) than at low eCO2 or TL (<1.5 °C above control) (Fig. 1B and D). Elevated CO2 plus warming non-significantly decreased root %N by 3 % and trended towards a greater decrease at high eCO2 or TH (>5 °C above control) than at low eCO2 or TM (Fig. 2B and D). There was a publication bias for the effects eCO2 plus warming on root %N.
Figure 1.
Percent change (compared to ambient or controls) in shoot %N in response to elevated CO2 (eCO2) (A) or eCO2 plus warming (B) at different eCO2 classes (ambient + <300 or ≥300 ppm) and warming (C) or eCO2 plus warming (D) at different temperature classes (ambient + <1.5, 1.5–5 or >5 °C). Each data point represents the mean ± 95 % CI. Numbers within parentheses represent the number of experimental observations. The dashed vertical line is the reference line at 0 % change. Treatment effects are non-significant at P < 0.05 if CIs overlap the zero line, and differences among treatments are non-significant if CIs overlap.
Figure 2.
Percent change (compared to ambient or controls) in root %N in response to elevated CO2 (eCO2) (A) or eCO2 plus warming (B) at different eCO2 classes (ambient + <300 or ≥300 ppm) and warming (C) or eCO2 plus warming (D) at different temperature classes (ambient + <1.5, 1.5–5 or >5 °C). Each data point represents the mean ± 95 % CI. Numbers within parentheses represent the number of experimental observations. The dashed vertical line is the reference line at 0 % change. Treatment effects are non-significant at P < 0.05 if CIs overlap the zero line, and differences among treatments are non-significant if CIs overlap.
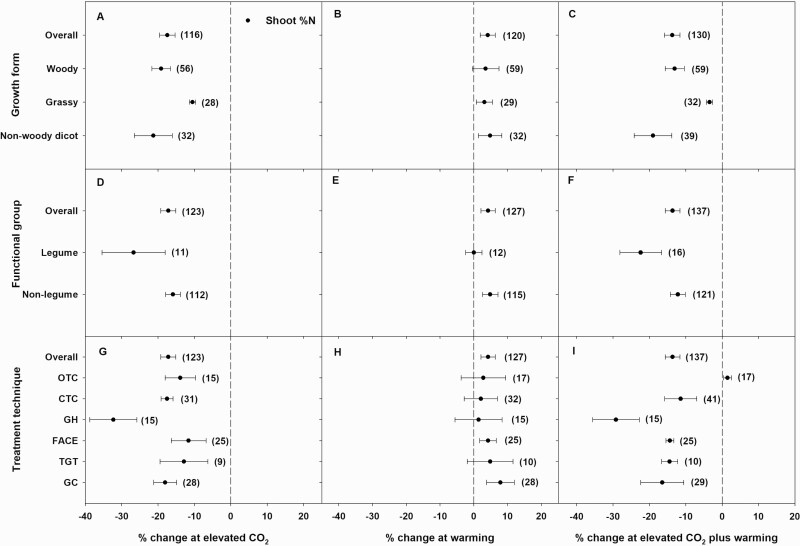
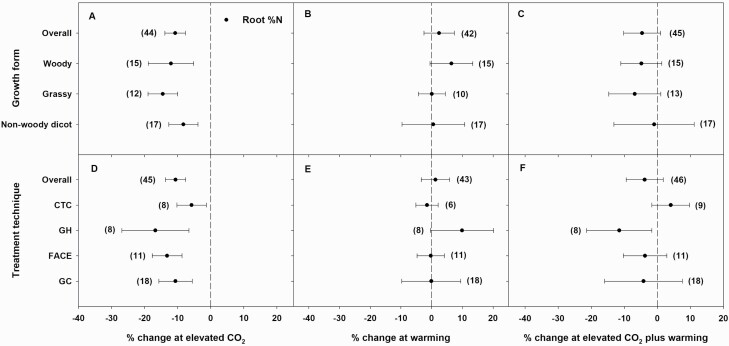
Elevated CO2 alone or in combination with warming significantly reduced shoot %N in all woody, grassy and non-woody dicot growth forms (Fig. 3A and C). Grasses had the smallest decrease in shoot %N in response to eCO2 (11 %) and to eCO2 plus warming (3 %), while non-woody dicots had the largest decrease in shoot %N in response to eCO2 (21 %) and eCO2 plus warming (19 %). Woody species had an intermediate decrease in shoot %N in response to eCO2 (19 %) and eCO2 plus warming (13 %) (Fig. 3A and C). Warming significantly increased shoot %N in both grasses (3 %) and non-woody dicots (5 %), while it non-significantly increased shoot %N in woody species (3 %) (Fig. 3B). Elevated CO2 (significantly, 12 % in woody; 15 % in grassy; and 8 % in non-woody dicot) and eCO2 plus warming (non-significantly, 5 % in woody; 7 % in grassy; and 1 % in non-woody dicot) reduced root %N in all three growth forms (Fig. 4A and C). Warming non-significantly increased root %N in woody species by 6 %, but it did not influence root %N in grasses or non-woody dicots (Fig. 4B).
Figure 3.
Percent change (compared to ambient or controls) in shoot %N in different growth forms (A–C), functional groups (D–F) and elevated CO2 (eCO2) treatment techniques (G–I, OTC = open-top chambers; CTC = closed-top chambers; GH = greenhouses; FACE = free-air CO2 enrichment; TGT = temperature-gradient tunnels; GC = growth chambers) in response to eCO2 (A, D, G), warming (B, E, H) and eCO2 plus warming (C, F, I). Each data point represents the mean ± 95 % CI. Numbers within parentheses represent the number of experimental observations. The dashed vertical line is the reference line at 0 % change. Treatment effects are non-significant at P < 0.05 if CIs overlap the zero line, and differences among treatments are non-significant if CIs overlap.
Figure 4.
Percent change (compared to ambient or controls) in root %N in different growth forms (A–C) and elevated CO2 (eCO2) treatment techniques (D–F, CTC = closed-top chambers; GH = greenhouses; FACE = free-air CO2 enrichment; GC = growth chambers) in response to eCO2 (A, D), warming (B, E) and eCO2 plus warming (C, F). Each data point represents the mean ± 95 % CI. Numbers within parentheses represent the number of experimental observations. The dashed vertical line is the reference line at 0 % change. Treatment effects are non-significant at P < 0.05 if CIs overlap the zero line, and differences among treatments are non-significant if CIs overlap.
Elevated CO2 alone or in combination with warming significantly decreased shoot %N in legumes (by 27 and 22 %, respectively) and non-legumes (16 and 12 %, respectively). However, warming by itself significantly increased shoot %N in non-legumes by 5 % but did not influence shoot %N of legumes (Fig. 3D–F).
In each eCO2 treatment technique, eCO2 significantly reduced both shoot and root %N, and this decrease was greatest for plants grown in GH (Figs 3G and 4D). Excluding GH, the magnitude of eCO2-driven decreases in shoot %N was similar across the different eCO2 techniques. Meanwhile, eCO2-driven decreases in root %N were similar for FACE (13 %) and GC (11%), both of which were similar to the overall decrease in root %N (11%). Except for plants grown in OTC, eCO2 plus warming significantly reduced shoot %N in plants grown using all eCO2 treatment techniques (Fig. 3I). Similar to eCO2 alone, this decrease in shoot %N was also greatest when plants were grown in GH. Likewise, similar decreases in shoot %N in response eCO2 plus warming were observed for plants grown using FACE (14 %), TGT (14 %) and GC (16 %), which were also similar to the overall decrease in shoot %N (14 %). A significant decrease in root %N in response to eCO2 plus warming was found only in plants grown in GH; all other treatment techniques were not significantly affected by eCO2 plus warming (Fig. 4F). Plants grown using FACE and growth-chamber techniques had similar decreases in root %N (4 %) in response to eCO2 plus warming which were similar to the overall decrease in root %N (3 %). Warming alone significantly (FACE and GC) or non-significantly (all other eCO2 treatment techniques) increased shoot %N, while, except for GH, warming did not influence root %N in plants grown using any other technique (Fig. 4E).
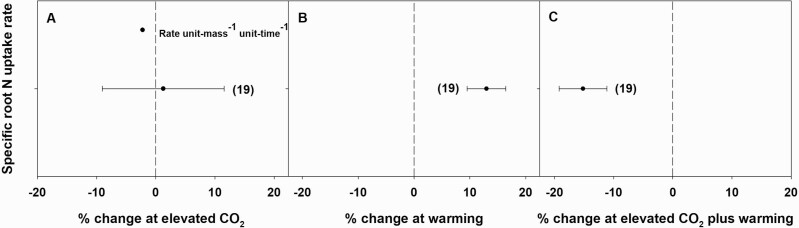
Specific root N-uptake rate was not influenced by eCO2, but it did significantly increase by 13 % in response to warming and significantly decrease by 15 % in response to eCO2 plus warming (Fig. 5). The publication bias could not be ignored for the individual effect of eCO2 on root N-uptake rate.
Figure 5.
Percent change (compared to ambient or controls) in root N-uptake rate in response to elevated CO2 (A), warming (B) and elevated CO2 plus warming (C). Each data point represents the mean ± 95 % CI. Numbers within parentheses represent the number of experimental observations. The dashed vertical line is the reference line at 0 % change. Treatment effects are non-significant at P < 0.05 if CIs overlap the zero line.
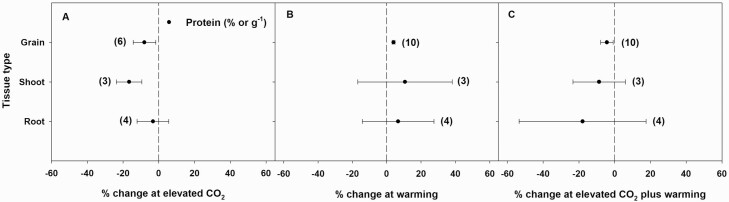
Elevated CO2 alone or in combination with warming significantly or non-significantly decreased protein concentration in shoots, roots and grains, while warming alone significantly or non-significantly increased protein concentrations in all three tissue types (Fig. 6).
Figure 6.
Percent change (compared to ambient or controls) in the concentration of total protein in different tissue types in response to elevated CO2 (A), warming (B) and elevated CO2 plus warming (C). Each data point represents the mean ± 95 % CI. Numbers within parentheses represent the number of experimental observations. The dashed vertical line is the reference line at 0 % change. Treatment effects are non-significant at P < 0.05 if CIs overlap the zero line, and differences among treatments are non-significant if CIs overlap.
Discussion
The effects of eCO2 on N concentration of plant tissues have been extensively studied and the findings often show a negative effect (Cotrufo et al. 1998; Curtis and Wang 1998; Ainsworth and Long 2005; Taub and Wang 2008). In agreement with previous reports, in this meta-analysis, eCO2 significantly reduced both shoot and root %N and the decrease was greater for shoots (17 %) than roots (10 %). These results are consistent with Cotrufo et al. (1998), who also found a 14 and 9 % decrease in above- and below-ground tissue N concentrations, respectively, in response to eCO2. As with the effects of eCO2 on %N, eCO2 plus warming also decreased %N in shoots (significantly) and roots (non-significantly). However, the magnitude of the negative effect of eCO2 plus warming on tissue %N was smaller than that of eCO2 alone. Previously, with smaller sample sizes, Zvereva and Kozlov (2006) and Wang et al. (2012) also reported negative effects of eCO2 plus warming on above-ground %N. The current meta-analysis further revealed that tissue quality (i.e. %N) can be persistently decreased with continuous exposure to eCO2, regardless of the temperature. Therefore, in the future, eCO2 is likely to reduce shoot %N regardless of the temperature, and, as a result, herbivores are likely to be N-limited and so would be required to consume more leaf tissues in order to meet their N requirement. This would eventually reduce photosynthetic rate followed by plant growth. One of the widely accepted hypotheses for low tissue %N at eCO2 is the dilution of N by increased photosynthetic assimilation of carbon (Ainsworth and Long 2005; Taub and Wang 2008). In addition, decreased root N-uptake rate has also been hypothesized as a potential cause of low tissue %N in plants grown at eCO2 (Taub and Wang 2008; Feng et al. 2015). Though the results of this meta-analysis do not support this hypothesis (Fig. 5A), it cannot be completely ruled out due to the large variation observed for N-uptake rate in response to eCO2 alone. However, in this meta-analysis, root N-uptake rates showed a positive relationship with shoot or root %N with warming or eCO2 plus warming, suggesting a greater dependence of N-uptake rate on temperature than CO2. Previously, Zvereva and Kozlov (2006), using 42 experimental observations, showed a negative but non-significant effect of warming on above-ground %N. In contrast, based on 119 experimental observations, the current meta-analysis reports a significant increase in above-ground %N in response to warming. Warming with <1.5 °C (TL) significantly increased shoot %N but this increase was neutralized as the magnitude of temperature elevation increased from TL to TM to TH (<1.5, 1.5–5 or >5 °C above control), suggesting the inability of plants to maintain tissue quality (i.e. %N) at higher than optimal temperatures. Likewise, the magnitude of the negative effect of eCO2 plus warming on shoot %N further increased as temperature increased from TL to TM, suggesting an inability of plants to maintain tissue quality at higher temperatures, even when combined with eCO2. TM alone or in combination with eCO2 showed a tendency to increase root %N relative to the effect of TL or its combination with eCO2 on root %N. However, TH alone or in combination with eCO2 showed a tendency to decrease root %N relative to the effect of TL or its combination with eCO2 on root %N. These results suggest that although shoot or root %N respond to different levels of CO2 similarly, irrespective of the temperature, they may not respond to different temperatures in a similar way, irrespective of the CO2 level.
Elevated CO2 is likely to reduce the protein concentration of many plant species, including those grown for human consumption. The C3 grasses such as wheat and rice are more likely to be negatively affected by eCO2 than legumes (Myers et al. 2014). Though the mechanism by which eCO2 decreases tissue protein concentration is not well understood, one possible explanation could be the increased concentration of non-structural carbohydrates relative to protein when plants are grown under eCO2 (Taub et al. 2008). In this meta-analysis, eCO2 alone or in combination with warming significantly or non-significantly reduced total protein concentrations in all shoots, roots and grains. These results are in conformity with the results observed for C3 grasses by Myers et al. (2014). Decreased protein concentration in edible portions of the crops can cause malnutrition among humans (Myers et al. 2014). In contrast, warming alone significantly or non-significantly increased the total protein concentration in all three tissue types. Interestingly, the variation in shoot or root protein concentration in response to eCO2 and/or warming scaled with the variation in shoot or root %N in response to these independent variables, suggesting a dependence of tissue protein concentration and, hence the nutritional quality, on tissue %N. At warming alone or eCO2 plus warming, tissue protein concentration also showed a positive relationship with root N-uptake rate, suggesting the dependence of nutritional quality on N-uptake rate when temperature is involved.
Grasses had the smallest decrease in shoot %N, but the largest decrease in root %N, in response to both eCO2 and eCO2 plus warming. In contrast, non-woody dicots had the largest decrease in shoot %N, but the smallest decrease in root %N, in response to both eCO2 and eCO2 plus warming. These results suggest a potential enhancement of net N translocation from roots-to-shoots in grasses, while a potential inhibition of net N translocation in non-woody dicots, in response to both eCO2 and eCO2 plus warming. Based on shoot and root %N data of woody species, both eCO2 and eCO2 plus warming are also likely to inhibit net N translocation in woody species. In addition, based on shoot and root %N data of grasses and non-woody dicots, warming is likely to enhance net N translocation in both growth forms. Collectively, these results suggest that the net translocation of N from roots-to-shoots will respond differently among plants of different growth forms to future climate conditions. Root-to-shoot translocation in woody species involves long-distance transportation compared to grasses or non-woody dicots. As eCO2 is likely to reduce xylem volume (Cohen et al. 2019), the observed decrease in net N translocation in woody species could be in part due to decreased xylem volume when plants grown at eCO2.
Legumes are known to have the ability to withstand the eCO2-driven leaf N dilution which is typically observed in C3 plants grown at eCO2 (Rogers et al. 2009). However, results of this meta-analysis oppose this view as eCO2 caused a greater decrease in legume shoot %N than non-legume shoot %N, regardless of the temperature. Notably, in this meta-analysis, a greater proportion of experimental observations of legume shoot %N were taken from studies which were conducted under natural soil nutrient conditions (FACE, OTC or TGT techniques with no additional nutrients supplied). As van Groenigen et al. (2006) explained, eCO2 will not have an effect on N2 fixation when legumes are grown under natural conditions with no fertilizer additions, and this could be one of the potential reasons for the observed result in this study. Meanwhile, though warming significantly increased non-legume shoot %N, it did not have an effect on legume shoot %N. As Hungria and Kaschuk (2014) explained, warming can limit N2 fixation by inhibiting NH4+ assimilation and nitrogenase activity, which could be one of the potential reasons for the observed neutral effect of warming on shoot %N.
In this meta-analysis, a subgroup analysis of different treatment techniques was conducted to find those more suitable for climate studies involving the interaction of CO2 and temperature. All of these techniques have their own advantages and disadvantages. Both shoot and root %N were investigated here because, apart from biomass measures, these have been widely measured in plants grown with these treatment techniques. Since this meta-analysis focused primarily on the effects of eCO2 plus warming on plant N relations, the suitability of these techniques is mainly discussed in response to eCO2 plus warming. The FACE technique is thought to provide the most realistic measure of the effects of eCO2 on crop yields because enclosure techniques can produce a ‘chamber effect’ that can exceed the effects of eCO2 (Ainsworth et al. 2005, 2008; Ainsworth and Long 2021). However, in this meta-analysis, some enclosure studies produced results similar to those of FACE studies (e.g. shoot %N in TGT and root %N in GC). Previously, a meta-analysis conducted by Taub et al. (2008) also reported similar protein concentrations in response to eCO2 in crops grown using either FACE or other techniques (OTC, CTC, GH, GC). In the current meta-analysis, the overall decreases in shoot and root %N in response eCO2 plus warming were 14 and 3 %, respectively. Interestingly, FACE and TGT studies also showed 14 % decreases in shoot %N, and GC and CTC studies showed 16 and 11 % decreases in shoot %N, respectively, in response to eCO2 plus warming. Meanwhile, the 4 % decrease in root %N observed for FACE and GC was similar to the overall decrease in root %N in response to eCO2 plus warming. These results suggest that in addition to FACE technique, other enclosed techniques such as GC, TGT and CTC can produce reliable results when studying the effects of eCO2 plus warming. Additionally, these three enclosed techniques produced similar results to those of the FACE technique in response to warming alone. This meta-analysis further suggests that the GH technique is likely unsuitable for studies involved with eCO2 plus warming, due to its overestimation of the negative impacts of eCO2 plus warming on plant %N.
In the future, global environmental changes such as CO2 enrichment, warming, drought, N deposition, etc. will occur concomitantly. Therefore, multi-factor manipulation approaches will be necessary to understand the combined effects of these various factors on plant growth, metabolism and production. This meta-analytic review was designed to improve understanding of the effects of eCO2 plus warming on plant N metabolism as this area of research has important knowledge gaps. However, one of the limitations of this study was the analysis of the effects of only two predictor variables on plant N metabolism-related response variables. Therefore, future research should focus on incorporating more predictor variables, such as drought, when investigating the impacts of environmental change on plant N metabolism. In addition, it will be interesting to see how experimental duration and the level and form of N affect plant N metabolism under these conditions, and how plant N metabolism-related variables respond to different levels of CO2 and temperature.
Conclusions
In the future, concomitant increases in CO2 and temperature are likely to affect plant N metabolism by lowering plant %N, root N-uptake rate and tissue protein concentration. Therefore, when developing plants for future climates, plant-improvement efforts should focus on generating new genotypes with more-resilient N metabolism.
Supporting Information
The following additional information is available in the online version of this article—
Appendix S1. Database.
Appendix S2. References included in the meta-analysis.
Acknowledgement
Authors would like to thank the anonymous reviewers for their constructive feedback.
Plants, Ecosystems & Climate. Chief Editor: Mary Heskel
Sources of Funding
None declared.
Conflict of Interest
None declared.
Contributions by the Authors
D.M.J. and S.A.H. were involved in the conceptual design of the study. D.M.J. constructed the database, conducted the meta-analysis, generated graphs and wrote the draft manuscript. S.A.H. is the faculty advisor of D.M.J., and was involved in data interpretation and manuscript revision. J.K.B. was involved in manuscript revision and provided valuable feedback.
Data Availability
The data are available as Supporting Information.
Literature Cited
- Abebe A, Pathak H, Singh SD, Bhatia A, Harit RC, Kumar V. 2016. Growth, yield and quality of maize with elevated atmospheric carbon dioxide and temperature in north-west India. Agriculture, Ecosystems & Environment 218:66–72. [Google Scholar]
- Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Yoora HS, Zhu XG, Curtis PS, Long SP. 2002. A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Global Change Biology 8:695–709. [Google Scholar]
- Ainsworth EA, Leakey ADB, Ort DR, Long SP. 2008. FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. The New Phytologist 179:5–9. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. The New Phytologist 165:351–371. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. 2021. 30 years of free-air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Global Change Biology 27:27–49. [DOI] [PubMed] [Google Scholar]
- Arndal MF, Schmidt IK, Kongstad J, Beier C, Michelsen A. 2014. Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland-grass ecosystem. Functional Plant Biology 41:1–10. [DOI] [PubMed] [Google Scholar]
- Atkin O, Cummins WR. 1994. The effect of root temperature on the induction of nitrate reductase activities and nitrogen uptake rates in arctic plant species. Plant and Soil 159:187–197. [Google Scholar]
- Bassirirad H. 2000. Kinetics of nutrient uptake by roots: responses to global change. The New Phytologist 147:155–169. [Google Scholar]
- Bassirirad H, Caldwell MM, Bilbrough C. 1993. Effects of soil temperature and nitrogen status on kinetics of 15NO3- uptake by roots of field-grown Agropyron desertorum (Fisch. ex Link) Schult. The New Phytologist 123:485–489. [DOI] [PubMed] [Google Scholar]
- Clarkson DT, Warner AJ. 1979. Relationships between root temperature and the transport of ammonium and nitrate ions by Italian and perennial ryegrass (Lolium multiflorum and Lolium perenne). Plant Physiology 64:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Halpern M, Yermiyahu U, Bar-Tal A, Gendler T, Rachmilevitch S. 2019. CO2 and nitrogen interaction alters root anatomy, morphology, nitrogen partitioning and photosynthetic acclimation of tomato plants. Planta 250:1423–1432. [DOI] [PubMed] [Google Scholar]
- Coleman JS, Bazzaz FA. 1992. Effects of CO2 and temperature on growth and resource use of co-occurring C3 and C4 annuals. Ecology 73:1244–1259. [Google Scholar]
- Cotrufo MF, Ineson P, Scott A. 1998. Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biology 4:43–54. [Google Scholar]
- Cruz C, Lips SH, Martins-Loução MA. 1993. Uptake of ammonium and nitrate by carob (Ceratonia siliqua) as affected by root temperature and inhibitors. Physiologia Plantarum 89:532–543. [Google Scholar]
- Curtis PS, Wang X. 1998. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313. [DOI] [PubMed] [Google Scholar]
- DaMatta FM, Grandis A, Arenque BC, Buckeridge MS. 2010. Impacts of climate changes on crop physiology and food quality. Food Research International 43:1814–1823. [Google Scholar]
- DeLucia EH, Heckathorn SA, Day TA. 1992. Effects of soil temperature on growth, biomass allocation and resource acquisition of Andropogon gerardii Vitman. The New Phytologist 120:543–549. [Google Scholar]
- Dijkstra FA, Blumenthal D, Morgan JA, Pendall E, Carrillo Y, Follett RF. 2010. Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. The New Phytologist 187:426–437. [DOI] [PubMed] [Google Scholar]
- Feng Z, Rütting T, Pleijel H, Wallin G, Reich PB, Kammann CI, Newton PC, Kobayashi K, Luo Y, Uddling J. 2015. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Global Change Biology 21:3152–3168. [DOI] [PubMed] [Google Scholar]
- Giri A, Heckathorn S, Mishra S, Krause C. 2017. Heat stress decreases levels of nutrient-uptake and-assimilation proteins in tomato roots. Plants 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156. [Google Scholar]
- Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis, 1st edn. Orlando, FL: Academic Press. [Google Scholar]
- Huang B, Rachmilevitch S, Xu J. 2012. Root carbon and protein metabolism associated with heat tolerance. Journal of Experimental Botany 63:3455–3465. [DOI] [PubMed] [Google Scholar]
- Hungria M, Kaschuk G. 2014. Regulation of N2 fixation and NO3-/NH4+ assimilation in nodulated and N-fertilized Phaseolus vulgaris L. exposed to high temperature stress. Environmental and Experimental Botany 98:32–39. [Google Scholar]
- IPCC . 2014. Climate change 2014: impacts, adaptation and vulnerability, contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press. [Google Scholar]
- Jayawardena DM, Heckathorn SA, Bista DR, Mishra S, Boldt JK, Krause CR. 2017. Elevated CO2 plus chronic warming reduce nitrogen uptake and levels or activities of nitrogen-uptake and -assimilatory proteins in tomato roots. Physiologia Plantarum 159:354–365. [DOI] [PubMed] [Google Scholar]
- Jing L, Wang J, Shen S, Wang Y, Zhu J, Wang Y, Yang L. 2016. The impact of elevated CO2 and temperature on grain quality of rice grown under open-air field conditions. Journal of the Science of Food and Agriculture 96:3658–3667. [DOI] [PubMed] [Google Scholar]
- Luo Y, Su BO, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren Ram, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB. 2004. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739. [Google Scholar]
- Mainali KP, Heckathorn SA, Wang D, Weintraub MN, Frantz JM, Hamilton EW 3rd. 2014. Impact of a short-term heat event on C and N relations in shoots vs. roots of the stress-tolerant C4 grass, Andropogon gerardii. Journal of Plant Physiology 171:977–985. [DOI] [PubMed] [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR. 1999. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant, Cell & Environment 22:567–582. [Google Scholar]
- Morison JIL, Lawlor DW. 1999. Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell & Environment 22:659–682. [Google Scholar]
- Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey AD, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T, Holbrook NM, Nelson RL, Ottman MJ, Raboy V, Sakai H, Sartor KA, Schwartz J, Seneweera S, Tausz M, Usui Y. 2014. Increasing CO2 threatens human nutrition. Nature 510:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios CJ, Grandis A, Carvalho VJ, Salatino A, Buckeridge MS. 2019. Isolated and combined effects of elevated CO2 and high temperature on the whole-plant biomass and the chemical composition of soybean seeds. Food Chemistry 275:610–617. [DOI] [PubMed] [Google Scholar]
- Petit J-R, Jouzel J, Raynaud D, Barkov NI, Barnola JM, Basile I, Bender M, Chappellaz J, Davis M, Delaygue G, Delmotte M, Kotlyakov VM, Legrand M, Lipenkov VY, Lorius C, Pepin L, Ritz C, Saltzman E, Stievenard M. 1999. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399:429–436. [Google Scholar]
- Qiao Y, Miao S, Li Q, Jin J, Luo X, Tang C. 2019. Elevated CO2 and temperature increase grain oil concentration but their impacts on grain yield differ between soybean and maize grown in a temperate region. The Science of the Total Environment 666:405–413. [DOI] [PubMed] [Google Scholar]
- Rogers A, Ainsworth EA, Leakey AD. 2009. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiology 151:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi A. 2017. WebPlotDigitizer.https://automeris.io/WebPlotDigitizer/ (28 October 2019).
- Rosenberg MS. 2005. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59:464–468. [PubMed] [Google Scholar]
- Rosenthal R. 1979. The file drawer problem and tolerance for null results. Psychological Bulletin 86:638. [Google Scholar]
- Taub DR, Miller B, Allen H. 2008. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biology 14:565–575. [Google Scholar]
- Taub DR, Wang X. 2008. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology 50:1365–1374. [DOI] [PubMed] [Google Scholar]
- Tindall JA, Mills HA, Radcliffe DE. 1990. The effect of root zone temperature on nutrient uptake of tomato. Journal of Plant Nutrition 13:939–956. [Google Scholar]
- van Groenigen KJ, Six J, Hungate BA, de Graaff MA, van Breemen N, van Kessel C. 2006. Element interactions limit soil carbon storage. Proceedings of the National Academy of Sciences of the United States of America 103:6571–6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC, Lajeunesse MJ, Dietz G, Dahabreh IJ, Trikalinos TA, Schmid CH, Gurevitch J. 2017. OpenMEE: intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods in Ecology and Evolution 8:941–947. [Google Scholar]
- Wang D, Heckathorn SA, Wang X, Philpott SM. 2012. A meta-analysis of plant physiological and growth responses to temperature and elevated CO(2). Oecologia 169:1–13. [DOI] [PubMed] [Google Scholar]
- Zvereva EL, Kozlov MV. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Global Change Biology 12:27–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available as Supporting Information.