Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a potentially life-threatening adverse drug reaction with a mortality rate of 10%. Interstitial nephritis, pneumonitis, myocarditis, meningitis, thyroiditis and pancreatitis are major causes of morbidity and mortality in this syndrome. Cessation of offending medication is paramount. There is paucity in high quality prospective studies guiding the treatment of DRESS, and there are no published therapeutic clinical trials in the treatment of corticosteroid refractory hypersensitivity myocarditis. The authors present a unique case of ciprofloxacin-induced DRESS with concurrent thyroiditis and refractory eosinophilic myocarditis that required mepolizumab and multiple immunosuppressants for successful treatment.
Keywords: unwanted effects / adverse reactions, immunology, skin, dermatology, thyroiditis
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a potentially life-threatening adverse drug reaction with a mortality rate of 10%.1 2 The estimated incidence of DRESS is 2.18 per 100 000 patients.3 Symptoms typically present 2–6 weeks after starting the offending medication, with anticonvulsants and antibiotics identified as common culprits.4–8 The presentation of DRESS is heterogeneous, with patients often presenting with fever, a cutaneous eruption, haematological abnormalities and internal organ involvement.4 Typically, the cutaneous eruption is morbilliform but may feature vesicles, pustules and target lesions.4 9 10 Interstitial nephritis, pneumonitis, myocarditis, meningitis, thyroiditis and pancreatitis are major causes of morbidity and mortality in this syndrome.4
The authors present a unique case of ciprofloxacin-induced DRESS with concurrent thyroiditis and refractory eosinophilic myocarditis that required mepolizumab and multiple immunosuppressants for successful treatment.
Case presentation
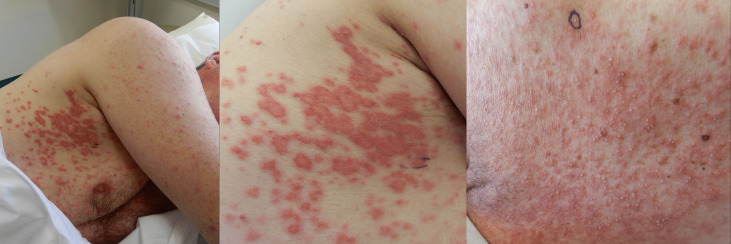
A man in his 50s presented with a 1-week history of progressive monomorphic pustules on an erythematous background over his face, neck and torso (figure 1). There was periorbital swelling but no involvement of conjunctiva or mucosal membranes. He reported diarrhoea and general malaise in the preceding week. On observation, he was febrile to 37.9°C and normotensive. Four weeks prior to presentation, he had been treated with ciprofloxacin for an episode of prostatitis. The patient denied taking any other new medications. His medical history is significant for prostate cancer in 2017 treated with laser ablation and tamsulosin. He denied recent travel abroad. The differential diagnoses for his pustular maculopapular eruption were DRESS, acute generalised exanthematous pustulosis and acute febrile neutrophilic dermatosis.
Figure 1.
Pustular maculopapular eruption over the trunk.
Laboratory investigations revealed raised white cell count of 19.3×109/L (3.7–9.5), with neutrophilia; 14.3×109/L (2.0–8.0) and eosinophilia; 1.5×109/L (0–0.5). Punch biopsies of the skin showed an urticarial reaction pattern with minor interface change, the presence of eosinophils and a pustular reaction compatible with a drug reaction. There were no features of vasculitis. The clinical picture was in keeping with DRESS, and the patient was treated with and responded well to 75 mg (1 mg/kg bodyweight) oral prednisolone with an improvement in his cutaneous eruption. He was discharged at day 5 with a planned reduction of his corticosteroid dose pending review the following week.
Prior to review, the patient presented twice to a peripheral emergency department with tachycardia (110 BPM), shortness of breath and palpitations. He was initially diagnosed with DRESS-induced thyroiditis with laboratory findings of thyroid-stimulating hormone (TSH) <0.01 mIU/L (0.4–4.0), free thyroxine >64 pmol/L (9.0–19.0), free triiodothyronine 22.3 pmol/L (2.6–6.0), negative TSH receptor autoantibodies and an ultrasound demonstrating heterogeneous echotexture of the thyroid gland with normal vascularity and no focal lesion. The patient was initiated on carbimazole and propranolol and discharged for outpatient review. He represented with persisting dyspnoea and palpitations, where it was found that his high-sensitivity troponin-I (hs-TI) rose to 1070 ng/L (<50) despite a normal electrocardiograph and was presumed to have a non-ST-elevation myocardial infarction. He was admitted under cardiology for management, where his eosinophils peaked to 3.8×109/L, ALT 289 U/L (10 – 50) and AST 85 U/L (10-35). A transthoracic echocardiogram (TTE) demonstrated a decreased left ventricular ejection fraction (LVEF) of 33% and a dilated right ventricle with moderately impaired systolic function. A coronary angiogram was subsequently performed which excluded coronary artery disease.
Further laboratory investigations for viral reactivation were unremarkable: parvovirus, CMV, HHV-6 were IgG positive but IgM negative, HSV-1 and HSV-2 IgG were negative, Epstein-Barr virus (EBV) IgM was negative, HHV-7 PCR was not detected and adenovirus, influenza A, influenza B antibodies were negative. An infection screen including quantiFERON, hepatitis B Ag/Ab, hepatitis C PCR, HIV Ag/Ab and strongyloides serology were also negative. The patient’s stool sample tested negative for giardia lamblia, entamoeba histolytica, cryptosporidium, shigella, shiga toxin, campylobacter and salmonella. Serum antineutrophil cytoplasm antibody was negative by both indirect immunofluorescence and by direct measurement of proteinase 3 (PR3) and myeloperoxidase (MPO) antibodies.
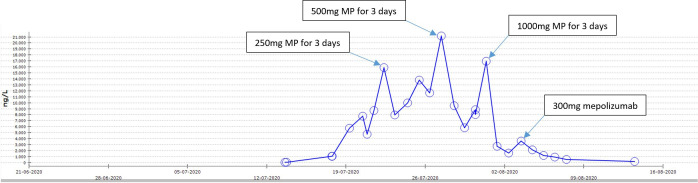
The most likely diagnosis considered in this setting was DRESS-induced eosinophilic myocarditis. While possible, alternate diagnoses such as eosinophilic granulomatosis with polyangiitis (EGPA), hypereosinophilic syndrome (HES), parasitic infection and other non-eosinophilic myocardial process such as lymphocytic myocarditis seemed less likely. The patient’s management at the peripheral hospital was led by immunology as DRESS-induced eosinophilic myocarditis with a 3-day course of intravenous pulsed 250 mg methylprednisolone (MP), with resultant reduction in troponin and resolution of peripheral eosinophilia (figures 2 and 3). With transition to oral prednisolone 1 mg/kg, however, the troponin levels relapsed to 21 172 ng/L necessitating another 3-day course of pulsed intravenous 500 mg MP. The patient’s dyspnoea and fatigue improved and repeat a TTE demonstrated a LVEF of 65% and normal right ventricular cavity size. However, the patient’s troponin relapsed again after completing the second pulse corticosteroid treatment.
Figure 2.
Highly-sensitivity troponin-I rise due to DRESS-induced eosinophilic myocarditis. DRESS, drug reaction with eosinophilia and systemic symptoms; MP, methylprednisolone
Figure 3.
Eosinophil trend in our patient. MP, methylprednisolone.
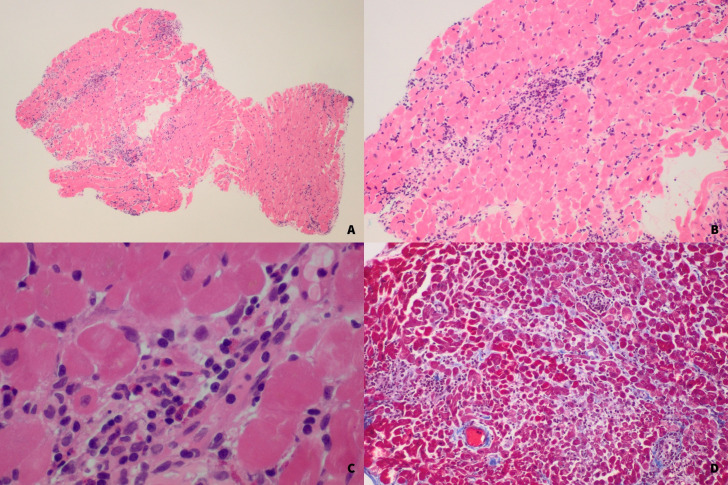
Given the ongoing troponin elevation despite multiple high dose pulses of intravenous corticosteroid, but with an improvement in rash and eosinophil count, the patient underwent a biopsy of the right ventricular endomyocardium for clarification of the underlying diagnosis. The biopsy demonstrated a lymphohistiocytic and eosinophilic infiltrate in the myocardium, but with no myocardial necrosis (figure 4). There was no significant fibrosis, iron overload or amyloidosis on staining. Electron microscopy examination shows no significant abnormalities. The histopathological features were in keeping with DRESS-related myocarditis. Another 3-day course of pulse intravenous MP, this time at a dose of 1 g, was administered after a further increase in troponin 16 972 following biopsy (which was felt not to be solely iatrogenic).
Figure 4.
H&E stain of endomyocardium ventricle biopsy showing mixed inflammatory infiltrate consisting of eosinophils, lymphocytes and histiocytes. There is no significant fibrosis nor necrosis. (A) 4× MAG. (B) 10× MAG. (C) 40× MAG. (D) Gomori trichrome stain showing there is minimal fibrosis.
An intensive regimen of one stat dose of 300 mg mepolizumab plus a 6-month course of monthly intravenous 1500 mg cyclophosphamide was initiated in view of the patient’s aggressive myocarditis that responded only temporarily to multiple courses of high-dose pulse corticosteroids. The addition of mepolizumab to the cyclophosphamide regimen was postulated to lead to greater efficacy and a faster onset of action both to treat the underlying myocarditis and enable potential weaning of corticosteroids. Cyclophosphamide was chosen over ciclosporin (which is often considered the first line steroid sparing agent in DRESS), due to its greater potency and evidence in a potentially life-threatening treatment refractory myocarditis.
The patient’s clinical symptoms resolved, and he was discharged with a hs-TI nadir of 117 ng/L and 75 mg prednisolone. Three weeks later, routine blood monitoring revealed an asymptomatic rise in his troponin to 1760 ng/L. He was readmitted to hospital for further intravenous pulse corticosteroids and a further dose 300 mg dose of mepolizumab, in addition to his next dose of cyclophosphamide as already planned. He was subsequently commenced on ciclosporin as an additional corticosteroid-sparing immunosuppressive agent in the context of a further minor, although asymptomatic troponin rise, both for disease control and to facilitate reduced corticosteroid exposure. His troponin values again improved, and he was discharged.
Outcome and follow-up
The 9 months since discharge were uneventful. The patient completed a 6-month course of cyclophosphamide without complications and his prednisolone and ciclosporin have both been entirely weaned. The patient remains well and asymptomatic with no rash or clinical features of cardiac dysfunction even after finishing all immunosuppressant treatment. He continues to demonstrate normal biomarkers (including troponin, peripheral eosinophil count, thyroid function and liver function testing). He has been counselled on the importance of lifelong strict avoidance of ciprofloxacin and wears a piece of jewellery inscribed with the instruction not to administer ciprofloxacin.
Discussion
The pathophysiology of DRESS is not completely understood, with several underlying mechanisms involving genetic factors, a specific immune response and reactivation of viruses from the herpesviridae family (eg, HH6, HH7, CMV and EBV) thought to play important roles.5 11–13 Eosinophilia and atypical lymphocytes are implicated in visceral organ injury.7 8
DRESS is associated with long-term autoimmune sequalae. Patients may develop autoimmune thyroiditis including Graves’ disease and Hashimoto’s thyroiditis, haemolytic anaemia, alopecia areata and vitiligo.6 14 Dysfunctional regulatory T-cells and HHV-6 reactivation thought to be key players in the pathogenesis of autoimmune sequalae.6 11 14 15 Patients may present with tachycardia and palpitations which can occur from 1 month to several years after the diagnosis of DRESS, therefore, patients should have their thyroid function monitored for at least 2 years.4 Testing for the Herpesviridae family through PCR may be useful in the diagnosis of DRESS as well as a prognostic marker for the development of multiorgan involvement.16–18
Eosinophilic myocarditis associated with a drug reaction, also known as hypersensitivity myocarditis, affects 4%–21% of DRESS patients and has a mortality rate between 31.4% and 55%.19–21 Ampicillin and minocycline are medications commonly implicated.22 Hypersensitivity myocarditis can occur at the onset of, or up to 4 months after, fever and cutaneous eruption.21 22 Interestingly, it may occur in the absence of cutaneous symptoms and resolution of eosinophilia, such as in our case.21 22 Patients presenting with dyspnoea, tachycardia, hypotension and/or chest pain in the context of DRESS should be investigated for myocardial damage, such as a troponin level and ECG, to guide further investigations including imaging and myocardial biopsies.20
There is paucity in high-quality prospective studies guiding the treatment of DRESS, and there are no published therapeutic clinical trials in the management of corticosteroid refractory hypersensitivity myocarditis.19 Cessation of offending medication(s) is paramount.5 Treatment is aimed at arresting the infiltration of eosinophils into tissue, inhibiting eosinophil degranulation and suppressing immune activation.20 Corticosteroids are often employed as first line agents, with a review by Husain et al recommending 0.5–1.0 mg/kg/day of prednisolone with a gradual tapering over several months to reduce the likelihood of relapse and development of autoimmune sequalae.4
Mepolizumab is an anti-interleukin-5 monoclonal antibody that reduces eosinophilic differentiation in the bone marrow and subsequent maturation in the blood.23 It has been used in HES, severe refractory eosinophilic asthma and EGPA.23 In this case, mepolizumab’s use was influenced by its established role in EGPA which can present with eosinophilic myocarditis.24 Mepolizumab has also demonstrated efficacy in the context of DRESS-induced eosinophilia, eosinophilic myocarditis and pneumonitis.25–27
Other novel therapies have also proven efficacious in the context of corticosteroid refractory HES. Janus kinase (JAK) signalling has been demonstrated to activate genes required for Th2 cell differentiation and subsequent development of eosinophils via interleukin-5.28 Case reports have showed efficacy of JAK-inhibitors in corticosteroid refractory HES and eosinophilic fasciitis.29–31
The authors present a unique case of ciprofloxacin-induced DRESS with concurrent thyroiditis and a severe refractory eosinophilic myocarditis, requiring multiple agents, including mepolizumab, to treat successfully. Clinicians need to be aware that DRESS is a potentially life-threatening condition and patients may have concurrent multiorgan involvement, as demonstrated in this case of thyroiditis and myocarditis. It is encumbent on us as clinicians to actively monitor for the short and long-term sequalae of DRESS.
Learning points.
Patients diagnosed with drug reaction with eosinophilia and systemic symptoms (DRESS) are at risk of life-threatening eosinophilic myocarditis, which may present in the absence of cutaneous findings and blood eosinophilia.
Cessation of offending medication is paramount in the management of DRESS. There are limited high-quality studies guiding the treatment of DRESS. Systemic corticosteroids (1 mg/kg/day) are commonly used as first-line agents.
There are no published evidence-based guidelines or therapeutic clinical trials in the treatment of hypersensitivity myocarditis, especially in those cases which are corticosteroid refractory. Interleukin-5 inhibitors may be considered in such circumstances.
Patients may have concurrent multiorgan involvement in DRESS, as demonstrated in this case of thyroiditis and myocarditis. Clinicians need to monitor for the short and long-term sequalae of DRESS.
Footnotes
Contributors: KT, SK, AB and AS made significant contributions to the design, acquisition and write-up of the work. KT, SK, AB and AS contributed to the revision of the work. KT, SK, AB and AS approved the final work. KT, SK, AB and AS agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Chen Y-C, Chiu H-C, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol 2010;146:1373–9. 10.1001/archdermatol.2010.198 [DOI] [PubMed] [Google Scholar]
- 2.Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (dress): a clinical update and review of current thinking. Clin Exp Dermatol 2011;36:6–11. 10.1111/j.1365-2230.2010.03967.x [DOI] [PubMed] [Google Scholar]
- 3.Wolfson AR, Zhou L, Li Y, et al. Drug reaction with eosinophilia and systemic symptoms (dress) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract 2019;7:633–40. 10.1016/j.jaip.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husain Z, Reddy BY, Schwartz RA. Dress syndrome: Part I clinical perspectives. J Am Acad Dermatol 2013;68:706–8. [DOI] [PubMed] [Google Scholar]
- 5.Cho Y-T, Yang C-W, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms (dress): an interplay among drugs, viruses, and immune system. Int J Mol Sci 2017;18. 10.3390/ijms18061243. [Epub ahead of print: 09 Jun 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian research Committee on severe cutaneous adverse reactions (ASCAR). J Dermatol 2015;42:276–82. 10.1111/1346-8138.12770 [DOI] [PubMed] [Google Scholar]
- 7.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (dress): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071–80. 10.1111/bjd.12501 [DOI] [PubMed] [Google Scholar]
- 8.Cacoub P, Musette P, Descamps V, et al. The dress syndrome: a literature review. Am J Med 2011;124:588–97. 10.1016/j.amjmed.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 9.Husain Z, Reddy BY, Schwartz RA. Dress syndrome: Part II. Management and therapeutics. J Am Acad Dermatol 2013;68:18–20. [DOI] [PubMed] [Google Scholar]
- 10.De A, Rajagopalan M, Sarda A, et al. Drug reaction with eosinophilia and systemic symptoms: an update and review of recent literature. Indian J Dermatol 2018;63:30–40. 10.4103/ijd.IJD_582_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Descamps V. Drug reaction with eosinophilia and systemic symptoms and thyroiditis: human herpesvirus-6, the possible common link. Br J Dermatol 2013;169:952. 10.1111/bjd.12456 [DOI] [PubMed] [Google Scholar]
- 12.Hung S-I, Chung W-H, Liou L-B, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;102:4134–9. 10.1073/pnas.0409500102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirmohamed M, Lin K, Chadwick D, et al. Tnfalpha promoter region gene polymorphisms in carbamazepine-hypersensitive patients. Neurology 2001;56:890–6. 10.1212/WNL.56.7.890 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y-C, Chang C-Y, Cho Y-T, et al. Long-Term sequelae of drug reaction with eosinophilia and systemic symptoms: a retrospective cohort study from Taiwan. J Am Acad Dermatol 2013;68:459–65. 10.1016/j.jaad.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Cookson H, Creamer D, Walsh S. Thyroid dysfunction in drug reaction with eosinophilia and systemic symptoms (dress): an unusual manifestation of systemic drug hypersensitivity. Br J Dermatol 2013;168:1130–2. 10.1111/bjd.12169 [DOI] [PubMed] [Google Scholar]
- 16.Kano Y, Hiraharas K, Sakuma K, et al. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol 2006;155:301–6. 10.1111/j.1365-2133.2006.07238.x [DOI] [PubMed] [Google Scholar]
- 17.Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (dress): a multiorgan antiviral T cell response. Sci Transl Med 2010;2:46ra62. 10.1126/scitranslmed.3001116 [DOI] [PubMed] [Google Scholar]
- 18.Tohyama M, Hashimoto K, Yasukawa M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol 2007;157:934–40. 10.1111/j.1365-2133.2007.08167.x [DOI] [PubMed] [Google Scholar]
- 19.Brambatti M, Matassini MV, Adler ED, et al. Treatment, and outcomes. J Am Coll Cardiol 2017;70:2363–75. [DOI] [PubMed] [Google Scholar]
- 20.Thongsri T, Chularojanamontri L, Pichler WJ. Cardiac involvement in dress syndrome. Asian Pac J Allergy Immunol 2017;35:3–10. 10.12932/AP0847 [DOI] [PubMed] [Google Scholar]
- 21.Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol 2012;66:e229–36. 10.1016/j.jaad.2010.11.057 [DOI] [PubMed] [Google Scholar]
- 22.Bourgeois GP, Cafardi JA, Groysman V, et al. Fulminant myocarditis as a late sequela of dress: two cases. J Am Acad Dermatol 2011;65:889–90. 10.1016/j.jaad.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faverio P, Bonaiti G, Bini F, et al. Mepolizumab as the first targeted treatment for eosinophilic granulomatosis with polyangiitis: a review of current evidence and potential place in therapy. Ther Clin Risk Manag 2018;14:2385–96. 10.2147/TCRM.S159949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. New England Journal of Medicine 2017;376:1921–32. 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ange N, Alley S, Fernando SL, et al. Drug reaction with eosinophilia and systemic symptoms (dress) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract 2018;6:1059–60. 10.1016/j.jaip.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 26.Kowtoniuk R, Pinninti M, Tyler W, et al. Dress syndrome-associated acute necrotizing eosinophilic myocarditis with giant cells. BMJ Case Rep 2018;66. 10.1136/bcr-2018-226461. [Epub ahead of print: 08 Oct 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thein OS, Sutton B, Thickett DR, et al. Mepolizumab rescue therapy for acute pneumonitis secondary to dress. BMJ Case Rep 2019;12:e231355. 10.1136/bcr-2019-231355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker S, Wang C, Walradt T, et al. Identification of a gain-of-function STAT3 mutation (p.Y640F) in lymphocytic variant hypereosinophilic syndrome. Blood 2016;127:948–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King B, Lee AI, Choi J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the JAK inhibitors tofacitinib and ruxolitinib. J Invest Dermatol 2017;137:951–4. 10.1016/j.jid.2016.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X-Y, Zhao J-L, Hou Y, et al. Janus kinase inhibitor tofacitinib is a potential therapeutic option for refractory eosinophilic fasciitis. Clin Exp Rheumatol 2020;38:567–8. [PubMed] [Google Scholar]
- 31.Kim SR, Charos A, Damsky W, et al. Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep 2018;4:443–5. 10.1016/j.jdcr.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]