Abstract
Introduction
New targeted therapies have changed cancer treatment in the past decades. However, high prices of targeted anticancer medications have increased economic burden for both patients and health insurance systems. In July 2017, China implemented combined medication price negotiation and mandatory reimbursement policies for 15 targeted anticancer medications. This study assesses effects of the policy on hospital procurement prices, volumes and spending.
Methods
Using a quasi-experimental interrupted time series design, we analysed procurement data from the Chinese Medical Economic Information of 789 public hospitals in 30 provinces between January 2016 and September 2018. The intervention group consisted of 15 targeted anticancer medications with negotiated prices in 2017. The comparison group consisted of six targeted anticancer medications without negotiated prices by 2018. The effective date of the policy was September 2017.
Results
After the implementation of the 2017 medication price negotiation and reimbursement policy, cost per defined daily dose (DDD) of the 15 targeted anticancer medications dropped US$71.21 on average from an average US$169.24/DDD before (p=0.000). Compared with what would have happened without the intervention, cost/DDD of price-negotiated medications decreased by 48.9% (p=0.000), procurement volumes increased by 143.0% (p=0.000) and hospital medication spending decreased by 6.9% (p=0.146).
Conclusions
The 2017 medication price negotiation and reimbursement policy decreased targeted medication procurement costs per DDD, increased volumes procured and at least temporarily contained spending. These changes should result in better access to and affordability of targeted anticancer medications in China.
Keywords: cancer, health policy, intervention study
Key questions.
What is already known?
As cancer burden is increasing across the world, high prices of new targeted anticancer medications have raised major concerns regarding access and affordability, especially in emerging and expanding universal coverage systems.
It has been widely recommended that governments should use their bargaining power to reduce procurement prices of anticancer medicines.
China has implemented in 2017 medication price negotiation as a criterion for mandatory insurance reimbursement. Impacts of the negotiation policy on targeted anticancer medication prices, volumes and spending are unknown.
What are the new findings?
Using a quasi-experimental interrupted time series design, this study demonstrates that in China national medication price negotiation as a condition for mandatory insurance reimbursement decreased medication prices, increased volumes procured and controlled hospital spending on anticancer medications.
What do the new findings imply?
China’s approach to promoting affordability of new anticancer medications provides valuable experience for health policy decision-makers.
Introduction
With increasing incidence, cancers are a major public health problem worldwide.1 Since 2010, cancers are the leading cause of death in China.2 New targeted therapies have significantly changed the treatment of some cancers in the past decades.3 However, high prices of targeted anticancer medications have increased economic burden for both patients and health insurance systems.4
To promote access to healthcare, including access to cancer care and anticancer medications, China has since 2009 implemented a series of linked policies (see box 1). As medications listed in the national reimbursement list must be paid at least in part by China’s health insurance system,5 including new medications in the National Reimbursement Drug List (NRDL) is the main approach for improving access and patient affordability. Before 2017, medications were listed in the NRDL based on expert review and some would not be recommended due to high prices. Aiming to increase coverage by the Basic Medical Insurance (BMI) while containing BMI spending, China has since 2017 used medication price negotiation as one of the conditions for medication inclusion in the NRDL (box 1). Following national price negotiations and inclusion of medicines in the NRDL, provinces are required to update their Provincial Reimbursement Drug Lists (PRDLs).6 All price-negotiated medications are mandated to be listed in the PRDLs.7 Public sector hospitals must purchase these medications via the provincial procurement websites and negotiated prices are maximum prices. City or county-level insurance funding is then required to reimburse medication costs based on the negotiated prices (minus beneficiaries’ copayments).
Box 1. Main policies and actions of the Chinese government to promote medication access and affordability.
March 2009: Beginning of comprehensive health system reforms
Aiming to establish an equitable and effective health system for all people (universal health coverage) by 2020,44 the government established Basic Medical Insurance (BMI) coverage with a target of enrolling 90% of the population by 2010.45
November 2009: 2009 Edition of the National Reimbursement Drug List (NRDL) for the BMI
The NRDL is the guiding standard for BMI to pay for medications. Prior to 2017, experts created the NRDL based on safety, efficacy and need, without price negotiation of included products.46
February 2017: 2017 Edition NRDL6
Medications included in the February 2017 Edition NRDL were based on expert review.
July 2017: First-round of drug price negotiations for the 2017 Edition NRDL17
The Chinese government conducted price negotiations with manufacturers whose medications had been suggested for inclusion in the NRDL based on expert review. Thirty-six medications were listed in the NRDL after successful price negotiations in July 2017 with negotiated medication maximum procurement prices reduced by 44% on average.47
October 2018: Second round of drug price negotiations for the 2017 Edition NRDL48
The Chinese government conducted the second round of price negotiations in October 2018. Seventeen medications were added to the NRDL in October 2018 with negotiated medication maximum procurement prices reduced by 57%.49
December 2018: BMI enrolment
The BMI enrolled more than 95% of Chinese citizens by the end of 2018.33
Effects of centralised negotiation or other regulation approaches on curbing medication prices have been described in China8 and other countries.8–11 Previous studies have also shown that insurance coverage of medications without price negotiation led to a rise in medication use volumes as well as expenditures.12 13 Expectations are that China’s combined price negotiation and mandatory reimbursement policies decrease procurement cost per medication unit and increase utilisation and thereby improve access to and affordability of expensive targeted anticancer medications. In this study, we used an interrupted time series (ITS) design and segmented regression analyses to assess the effects of the 2017 national price negotiations on targeted anticancer medication cost per defined daily dose (DDD), hospital procurement volumes and spending in China.
Methods
Study design
We used an ITS design covering the period from January 2016 to September 2018 to analyse changes in daily costs and hospital purchasing volumes of and spending on 15 targeted anticancer medications for which procurement prices were negotiated in July 2017 and which were subsequently included in the NRDL and PRDLs.14–16 The sample included all targeted anticancer medications identified from the total national price-negotiated medications in 2017.17 To further strengthen the ITS design, we selected as comparison group all targeted anticancer medications approved by the National Medical Products Administration (NMPA) before 2016 and which were not price negotiated by the end of the study period. Provinces in mainland China were mandated to list the 2017 price-negotiated medications in their PRDLs before 31 July 2017. Considering that policy effects may lag, we selected September 2017 as the time point when price negotiation and mandatory reimbursement would have taken effect.
Data and outcome measures
We used hospital procurement data captured in the Chinese Medicine Economic Information (CMEI) database. The CMEI captures monthly medicines purchases reported by 594 tertiary and 195 secondary public sector hospitals,18 which respectively accounted for 28.1% and 3.2% of tertiary and secondary public hospitals in China in 2017.19
In this study, we assessed three main outcome measures: cost per DDD, procurement volume and spending. We calculated cost per DDD of each product, for example, the ratio of procurement spending and volume procured (in DDDs), as a surrogate measure of actual medication price paid. DDDs, recommended by WHO for drug utilisation monitoring and research,20 constituted the measure of purchased volumes. DDDs were the number of daily doses of each medication based on dosage regimens recommended in the manufacturers’ product labels and as approved by the NMPA.21 We prepared data for ITS analysis by summing up monthly hospital spending and DDDs of medications procured between January 2016 and September 2018 and calculated average cost per DDD for each medication and across the intervention and comparison groups.22 Hospital average procurement volumes and spending were calculated to reflect drug consumption. All expenditures were converted to 2016 US dollars using the Consumer Price Index and average annual exchange rates.23 24
Statistical analysis
Statistical model
We used segmented regression models to assess whether the 2017 national medication price negotiation policy affected hospital procurement costs and consumption of intervention and comparison group medications across 30 of 31 provinces (data of Qinghai province was not available).14 25 The regression model assumes the following form.
Yt is the dependent variable measured at each monthly time point t, Tt is the time since the start of the study, Xt is a dummy variable representing the intervention (preintervention periods 0, otherwise 1), and XtTt is an interaction term of the time and intervention.26 Z is a dummy variable to denote the cohort assignment (treatment or comparison group). When a comparison group is available, Z for the intervention group is set as 1.27
We performed the Durbin-Watson test to estimate residual autocorrelations28 and used the Cochrane-Orcutt autoregression procedure to correct for first order serially correlated errors when needed.29 The results of the segmented regression models are presented as changes in the levels and slopes of average daily cost, DDDs and spending after the implementation of 2017 medication price negotiation policy. In addition, based on level and trend change parameter estimates, we calculated absolute and relative differences in outcomes at 6 months after the intervention as well as at the end of the observation period compared with what would have happened without the policy (the counterfactual).14 26
To analyse policy impacts on use and costs of and spending on different medications, we conducted the ITS regression analyses separately for individual medications. Pearson correlation coefficient was also estimated to examine the correlation between relative price change at the intervention time and medication launch time in China.
Statistical analysis software
All models were run using the statistical software Stata/MP V.14.0 (Revision 2 April 2015), StataCorp.
Patient and public involvement
Patients were not involved in this study.
Results
Fifteen targeted anticancer medications had price negotiations in 2017 and constituted the intervention group. The comparison group consisted of all six targeted anticancer medications included in the CMEI database for which prices were not negotiated and which were not listed in the NRDL by the end of the observation period (table 1, for more detailed information, please see online supplemental appendix 1). Launch years of the intervention and comparison group medications ranged from 2000 to 2015. The majority of intervention group medications were indicated for the most common solid tumours in China, such as lung cancer (N=3), breast cancer (N=2), kidney cancer (N=2). Prices of four medications for haematological malignancies including lymphoma and multiple myeloma treatment were also negotiated in 2017. As for the comparison group, 4 medications were indicated for solid tumours including lung cancer, colorectal cancer and kidney cancer, and two medications were leukaemia therapies.
Table 1.
Characteristics of targeted anticancer medication in the intervention group and comparison group
| No | Generic name | Launch time in China | Indication |
| Intervention group | |||
| 1 | Rituximab | April 2000 | Lymphoma |
| 2 | Trastuzumab | September 2002 | Breast cancer; stomach cancer |
| 3 | Bortezomib | February 2005 | Multiple myeloma; lymphoma |
| 4 | Recombinant Human Endostatin | September 2005 | Lung cancer |
| 5 | Erlotinib | April 2006 | Lung cancer |
| 6 | Sorafenib | September 2006 | Liver cancer; kidney cancer; thyroid cancer |
| 7 | Nimotuzumab | April 2008 | Nasopharynx cancer |
| 8 | Bevacizumab | February 2010 | Colorectal cancer; lung cancer |
| 9 | Fulvestrant | June 2010 | Breast cancer |
| 10 | Lapatinib | January 2013 | Breast cancer |
| 11 | Everolimus | January 2013 | Brain cancer; kidney cancer; pancreatic neuroendocrine tumor |
| 12 | Lenalidomide | January 2013 | Multiple myeloma |
| 13 | Apatinib | October 2014 | Stomach cancer |
| 14 | Chidamide | December 2014 | Lymphoma |
| 15 | Abiraterone | May 2015 | Prostate cancer |
| Comparison group | |||
| 1 | Cetuximab | July 2006 | Colorectal cancer |
| 2 | Sunitinib | October 2007 | Kidney cancer; stomach cancer; pancreatic neuroendocrine tumor |
| 3 | Pegaspargase | January 2009 | Leukaemia |
| 4 | Nilotinib | July 2009 | Leukaemia |
| 5 | Crizotinib | January 2013 | Lung cancer |
| 6 | Axitinib | April 2015 | Kidney cancer |
bmjgh-2021-006196supp001.pdf (630KB, pdf)
Impacts on cost per DDD, volume and spending
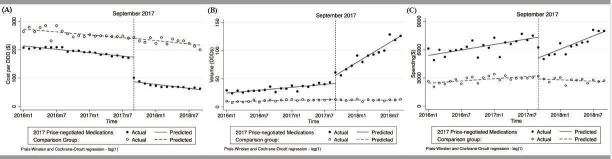
The aggregated controlled ITS analysis showed that the cost per DDD of medications in the intervention group decreased abruptly and significantly in September 2017 while the cost of comparison group medications did not. (figure 1A). With the implementation of the negotiation policy, cost per DDD of the fifteen 2017 price-negotiated medications had dropped by US$71.21(p<0.01) on average from an average pre-policy cost of US$169.24/DDD just before the policy change (table 2). At 6 months after the price-negotiation policy, cost per DDD of negotiated medications had dropped by US$76.91 (p<0.01) and the relative change compared with expected cost without the policy was −48.9%. When the comparison group was included in the model, at the end of the observation period, average cost per DDD of the intervention group medications had declined by US$88.36 (p<0.01) compared with the estimated cost without price negotiation. Table 2 lists coefficients for level and trend changes for all outcome measures.
Figure 1.
(A) Observed and predicted cost per DDD of 2017 price-negotiated and comparison group medications. (B) Observed and predicted hospital average volume (in DDDs) of 2017 price-negotiated and comparison group medications. (C) Observed and predicted hospital average spending ($) on 2017 price-negotiated and comparison group medications. DDDs, defined daily doses.
Table 2.
Changes in levels and trends of and absolute and relative changes in medication cost per DDD, procurement volume and spending, for 2017 price-negotiated and comparison group medications
| 2017 negotiation medications | 2017 price-negotiated medications | Comparison group medications | Change relative to comparison group | ||||||
| Coefficient | P value | 95% CI | Coefficient | P value | 95% CI | Coefficient | P value | 95% CI | |
| Cost per DDD (US$/DDD)* | |||||||||
| Baseline level | 215.58 | 0.000 | 204.37 to 226.80 | 274.83 | 0.000 | 267.18 to 282.48 | −59.16 | 0.000 | −68.55 to −49.77 |
| Baseline trend | −2.24 | 0.000 | −3.11 to −1.37 | −1.71 | 0.000 | −2.35 to −1.07 | −0.29 | 0.463 | −1.08 to 0.50 |
| Level change after intervention | −71.21 | 0.000 | −81.56 to −60.85 | 1.54 | 0.801 | −10.81 to 13.89 | −87.69 | 0.000 | −102.76 to −72.61 |
| Trend change after intervention | −0.95 | 0.316 | −2.85 to 0.95 | −0.24 | 0.723 | −1.63 to 1.14 | −0.05 | 0.951 | −1.75 to 1.64 |
| Absolute change at 6 months after intervention | −76.91 | 0.000 | −90.25 to −63.57 | 0.08 | 0.989 | −11.60 to 11.76 | −88.00 | 0.000 | −102.32 to −73.68 |
| Relative change at 6 months after intervention | −48.88 | 0.000 | 53.71 to 44.04 | 0.04 | 0.989 | 4.82 to 4.89 | −32.94 | 0.000 | −36.24 to −29.64 |
| Absolute change by end of observation period | −83.56 | 0.000 | −107.86 to −59.27 | −1.62 | 0.848 | −18.70 to 15.47 | −88.36 | 0.000 | −109.37 to −67.36 |
| Relative change by end of observation period | −58.99 | 0.000 | 69.05 to 48.92 | −0.74 | 0.846 | 8.19 to 6.71 | −33.33 | 0.000 | −38.55 to −28.11 |
| Volume (no of DDDs)* | |||||||||
| Baseline level | 23.69 | 0.000 | 20.01 to 27.37 | 8.76 | 0.000 | 7.83 to 9.70 | 14.92 | 0.000 | 11.21 to 18.64 |
| Baseline trend | 0.86 | 0.000 | 0.55 to 1.17 | 0.22 | 0.000 | 0.14 to 0.30 | 0.64 | 0.000 | 0.33 to 0.95 |
| Level change after intervention | 9.38 | 0.003 | 3.45 to 15.31 | −1.11 | 0.143 | −2.62 to 0.40 | 10.49 | 0.001 | 4.51 to 16.48 |
| Trend change after intervention | 4.99 | 0.000 | 4.33 to 5.66 | −0.21 | 0.017 | −0.38 to −0.04 | 5.20 | 0.000 | 4.53 to 5.88 |
| Absolute change at 6 months after intervention | 39.35 | 0.000 | 33.73 to 44.97 | −2.36 | 0.002 | −3.79 to −0.94 | 41.72 | 0.000 | 36.04 to 47.39 |
| Relative change at 6 months after intervention | 85.59 | 0.000 | 65.26 to 105.93 | −16.41 | 0.000 | −24.65 to −8.18 | 164.19 | 0.000 | 82.47 to 245.92 |
| Absolute change by end of observation period | 74.31 | 0.000 | 66.09 to 82.54 | −3.83 | 0.001 | −5.92 to −1.74 | 78.14 | 0.000 | 69.84 to 86.45 |
| Relative change by end of observation period | 142.98 | 0.000 | 110.18 to 175.78 | −24.04 | 0.000 | −34.47 to −13.62 | 261.47 | 0.000 | 134.94 to 387.99 |
| Spending (US$)* | |||||||||
| Baseline level | 5279.02 | 0.000 | 4892.18 to 5665.86 | 2408.59 | 0.000 | 2199.23 to 2617.96 | 2879.15 | 0.000 | 2449.58 to 3308.72 |
| Baseline trend | 97.36 | 0.000 | 64.96 to 129.76 | 38.78 | 0.000 | 21.25 to 56.31 | 57.75 | 0.002 | 21.77 to 93.73 |
| Level change after intervention | −2347.96 | 0.000 | −2972.24 to −1723.67 | −270.61 | 0.110 | −606.29 to 65.07 | −2061.56 | 0.000 | −2754.26 to −1368.86 |
| Trend change after intervention | 135.79 | 0.000 | 65.81 to 205.77 | −62.48 | 0.002 | −100.30 to −24.65 | 198.35 | 0.000 | 120.66 to 276.05 |
| Absolute change at 6 months after intervention | −1533.22 | 0.000 | −2123.59 to −942.85 | −645.46 | 0.000 | −964.59 to −326.33 | −871.43 | 0.010 | −1526.98 to −215.87 |
| Relative change at 6 months after intervention | −19.63 | 0.000 | −25.72 to −13.54 | −18.89 | 0.000 | −26.46 to −11.32 | −22.32 | 0.000 | −34.13 to −10.51 |
| Absolute change by end of observation period | −582.68 | 0.178 | −1446.50 to 281.13 | −1082.79 | 0.000 | −1551.16 to −614.41 | 517.06 | 0.285 | −442.46 to 1476.58 |
| Relative change by end of observation period | −6.86 | 0.146 | −16.12 to 2.39 | −29.36 | 0.000 | −38.99 to −19.72 | 12.00 | 0.346 | −12.98 to 36.98 |
US$ in 2016
*Except for relative changes which are expressed in percent.
CI, confidence interval; DDD, defined daily doses.
After the negotiation policy, the procured volume of price-negotiated medications increased significantly in terms of level and trend (figure 1B)(). Meanwhile, the volume of comparison group medication was slightly decreasing (p<0.05). (table 2) At the intervention point, the consumption volume of the price-negotiated medications showed a significant increase (p<0.01). (figure 1B)Compared with what would have happened without the intervention, the volume of use (in DDDs) had increased by 85.6% at 6 months after the negotiation policy took effect and by 143.0% at the end of the observation period (table 2).
Figure 1C illustrates that, with the price decrease after the negotiation policy, medication spending dropped significantly at the time of the policy implementation. Given increasing consumption volumes, medication spending over time increased and the change in spending by the end of observation period was slightly less than predicted spending without the negotiation (p=0.146). Compared with what would have been expected without the negotiation, spending on intervention group medications had decreased by 19.6% at 6 months after the intervention and 6.9% by the end of the observation period. Spending on comparison group medications slightly increased before September 2017 and decreased after the intervention (table 2).
Impacts on individual medication costs per DDD, volumes, spending
For individual medications, our results show that all price-negotiated medications had significant decreases in cost per DDD, ranging from 23.4% to 69.9% by the end of the observation period. After implementation of the negotiation policy, consumption volumes of 14 of the 15 medications (except bortezomib), trended significantly upward. (table 3 and online supplemental appendix 2) By the end of the observation period, the Pearson correlation coefficient between the relative price change and medication launch date was 0.309 (p=0.262, online supplemental appendix 2.1.2).
Table 3.
Changes in levels and trends of, and absolute and relative changes in individual price-negotiated medication cost per DDD, procurement volume and spending
| Price-negotiated medications | Baseline | Change after intervention | Change 6 months after intervention | Change at the end of observation | ||||
| Level | Trend | Level | Trend | Absolute | Relative (%) | Absolute | Relative (%) | |
| Cost per DDD (US$/DDD) | ||||||||
| Trastuzumab | 155.57*** | −1.65*** | −55.75*** | −0.26*** | −57.29*** | −50.83 | −59.08*** | −58.40 |
| Bortezomib | 460.65*** | −5.55*** | −108.07*** | −0.23*** | −109.47*** | −34.59 | −111.11*** | −40.02 |
| Recombinant human endostatin | 228.69*** | −2.02*** | −35.62*** | 0.58*** | −32.12*** | −18.24 | −28.04*** | −17.31 |
| Erlotinib | 94.15*** | −1.87*** | −22.69*** | 1.02*** | −16.58*** | −36.48 | −9.46 | −29.25 |
| Sorafenib | 246.21*** | −1.23* | −111.12*** | −2.51*** | −126.16*** | −58.87 | −143.7*** | −69.85 |
| Nimotuzumab | 146.75*** | −1.98*** | −26.46*** | 0.57*** | −23.02*** | −24.20 | −19.01 | −23.40 |
| Rituximab | 951.44*** | −7.49*** | −215.69*** | 3.79*** | −192.94*** | −25.50 | −166.4*** | −23.62 |
| Bevacizumab | 198.88*** | −1.94*** | −64.31*** | −0.58*** | −67.80*** | −45.68 | −71.88*** | −53.30 |
| Fulvestrant | 26.83*** | −0.08*** | −13.30*** | −0.05*** | −13.63*** | −55.05 | −14.01*** | −57.89 |
| Lapatinib | 86.40*** | −0.10 | −24.98*** | −3.09*** | −43.52*** | −52.00 | −65.15*** | −78.52 |
| Everolimus | 75.30*** | −0.33*** | −27.16*** | 0.12*** | −26.46*** | −39.63 | −25.63*** | −39.76 |
| Lenalidomide | 185.76*** | −0.02 | −114.32*** | −0.69*** | −118.43*** | −63.94 | −123.23*** | −66.59 |
| Apatinib | 105.38*** | −0.24** | −35.50*** | −0.15*** | −36.43*** | −36.74 | −37.52*** | −38.49 |
| Chidamide | 144.03*** | −0.64*** | −38.90*** | −0.14*** | −39.74*** | −31.19 | −40.73*** | −33.13 |
| Abiraterone | 187.17*** | −0.79*** | −93.69*** | 0.72*** | −89.38*** | −53.67 | −84.35*** | −52.39 |
| Volume (no of DDDs) | ||||||||
| Trastuzumab | 93.35*** | 3.75 | −23.27 | 33.63*** | 178.52*** | 93.59 | 413.94*** | 190.79 |
| Bortezomib | 7.89*** | 0.51*** | 17.98*** | −1.34*** | 9.96*** | 47.00 | 0.60 | 2.41 |
| Recombinant human endostatin | 21.89*** | 0.31** | 6.76** | 0.4*** | 9.17*** | 30.70 | 11.99*** | 37.44 |
| Erlotinib | 30.23*** | 0.53*** | 2.35 | 1.37*** | 10.57*** | 24.09 | 20.17*** | 42.39 |
| Sorafenib | 21.67*** | 0.29 | 12.96 | 5.78*** | 47.64*** | 163.19 | 88.10*** | 282.22 |
| Nimotuzumab | 26.14*** | 0.34 | 17.74*** | 2.34*** | 31.77*** | 90.68 | 48.13*** | 128.60 |
| Rituximab | 8.41*** | 0.29*** | 2.34 | 1.53*** | 11.53*** | 72.11 | 22.26*** | 123.44 |
| Bevacizumab | 32.77*** | 1.97*** | 19.62*** | 15.05*** | 109.93*** | 130.83 | 215.3*** | 220.08 |
| Fulvestrant | 41.79*** | 0.38 | 34.09*** | 16.7*** | 134.26*** | 260.44 | 251.14*** | 463.54 |
| Lapatinib | 10.25* | 0.75 | −7.02 | 4.46*** | 19.77** | 66.53 | 51.02*** | 145.97 |
| Everolimus | 13.27*** | −0.10 | −0.01 | 0.41*** | 2.44 | 22.74 | 5.30** | 52.70 |
| Lenalidomide | 5.84*** | 0.21 | −5.54* | 2.3*** | 8.29*** | 72.69 | 24.42*** | 189.26 |
| Apatinib | 5.34*** | 1.47*** | 0.48 | 1.37*** | 8.69*** | 19.96 | 18.27*** | 33.94 |
| Chidamide | −0.30 | 0.17** | 4.29*** | 0.63*** | 8.07*** | 198.04 | 12.49*** | 237.57 |
| Abiraterone | 3.92* | 0.19 | 8.79** | 6.56*** | 48.17*** | 549.62 | 94.12*** | 934.69 |
| Spending (US$) | ||||||||
| Trastuzumab | 15377.32*** | 310.38** | −12360.62*** | 1108.81*** | −5707.79** | −24.34 | 2053.84 | 8.02 |
| Bortezomib | 3911.54*** | 128.15*** | 1507.93*** | −423.41*** | −1032.53** | −14.25 | −3996.39*** | −49.09 |
| Recombinant human endostatin | 4983.48*** | 21.99 | −276.99 | 37.53*** | −51.83 | −0.93 | 210.86 | 3.69 |
| Erlotinib | 2970.76*** | −32.48*** | −833.64*** | 40.52*** | −590.52** | −27.77 | −306.87 | −16.16 |
| Sorafenib | 5306.88*** | 37.32* | −1882.34*** | 281.62*** | −192.60 | −3.07 | 1778.75*** | 27.20 |
| Nimotuzumab | 3974.38*** | −16.77 | −0.72 | 157.25*** | 942.80 | 26.65 | 2043.57** | 59.74 |
| Rituximab | 8350.76*** | 171.62*** | −2785.27*** | 804.79*** | 2043.47*** | 15.95 | 7676.99*** | 54.78 |
| Bevacizumab | 6962.73*** | 258.54*** | −4440.06*** | 719.03*** | −125.89 | −0.92 | 4907.31*** | 31.67 |
| Fulvestrant | 1126.59*** | 5.97 | −247.60 | 164.26*** | 737.93*** | 57.57 | 1887.71*** | 142.63 |
| Lapatinib | 877.3*** | 64.37** | −682.04 | −31.6*** | −871.64*** | −34.17 | −1092.84 | −36.41 |
| Everolimus | 997.63*** | −11.09* | −296.67** | 20.01*** | −176.58 | −24.89 | −36.48 | −5.77 |
| Lenalidomide | 1201.93*** | 29.48 | −1353.46*** | 114.9*** | −664.09 | −33.74 | 140.19 | 6.45 |
| Apatinib | 596.74*** | 145.21*** | −1171.08*** | 9.64*** | −1113.27*** | −25.46 | −1045.82*** | −19.41 |
| Chidamide | −44.18 | 15.7** | 443.86*** | 42.48*** | 698.76*** | 192.00 | 996.14*** | 210.24 |
| Abiraterone | 735.93*** | 30.16* | −54.39 | 483.01*** | 2843.68*** | 187.08 | 6224.77*** | 359.58 |
US$ in 2016.
*P<0.05, **p<0.01, ***p<0.001.
CI, confidence interval; DDD, defined daily doses.
Before September 2017, 12 of 15 medications in the intervention group showed an increasing trend in procurement spending. After implementation of the negotiation policy, trends in spending on erlotinib, nimotuzumab and everolimus increased while spending on bortezomib decreased (online supplemental appendix 2.3). At 6 months after the intervention, spending on five price-negotiated medications was significantly lower than spending estimated without the policy (table 3).
Discussion
Principal findings
Our results indicate that the introduction of the Chinese medication price negotiation and reimbursement policies led to significant decreases in cost per DDD and sharp increases in procured volumes of targeted anticancer medications, while at least temporarily containing overall spending on these medications.
The increasing economic burden of cancer treatments has become a source of worldwide concern for patients, prescribers, payers and policy-makers.30 Various approaches have been proposed to mitigate effects of increasing drug prices.31 32 Aiming to improve access to healthcare and medications, the Chinese government published the Opinions on Deepening Health System Reform in 2009, a political commitment to establishing an accessible, equitable, affordable, and efficient health system to cover all people by 2020.33 Insurance coverage expansion and better medication access were two foci of the 2009 reforms.34 The 2017 medication price negotiation and mandatory reimbursement policies jointly targeted both policy goals. Prior to 2017, most insured patients needed to pay for expensive targeted anticancer medications entirely out-of-pocket (OOP) except in few provinces or cities which had included the medications in their reimbursement lists.5 To improve patient and system affordability and decrease health inequity, the Chinese government implemented a series of policies, including reimbursement-linked central medication price negotiations.7
We found that after national medication price negotiations in 2017, procurement volumes of all price-negotiated anticancer medications increased abruptly and significantly, except for bortezomib for which use increased initially and declined in 2018. Nonetheless, cost per DDD of all price-negotiated medication decreased between 17.3% and 78.5% by the end of the study period regardless of duration of the drugs on the Chinese market. Increased volumes of use suggest that patients’ access to these cancer medications may have improved.35 In addition to price reduction, insurance coverage should have further improved affordability of expensive medications, as demonstrated before.13 36 A previous study showed that estimated patient OOP spending decreased after a patient assistance programme in Zhejiang Province, China.37 However, challenges to equitable access likely remain in China since insurance schemes differ in the amounts of patient copayments.38 39 Further study, based on individual-level data, including claims data, is needed to evaluate equity in access to and quality of use of new anticancer medications following price and reimbursement negotiations.
With price reductions and volume increases, our study showed that spending on targeted anticancer medications in the intervention group did not increase 1 year after the policy took effect. Considering the significant increase in use of most of the sample medications, further follow-up is needed to estimate the long-term policy effects, including the impact on insurance budgets.
New anticancer medications come to markets at high and increasing prices and many with limited evidence of benefit at time of approval.40 41 It is therefore imperative for payers to negotiate prices, to assess whether new cancer therapies result in the expected clinical benefits, and to estimate the opportunity costs of cancer therapy spending in health and social systems. Our results are consistent with previous studies that have demonstrated price negotiation as a strategy to improve medication affordability in both developed and low/middle-income countries.42 43 With more bargaining power, centralised national price negotiation seems more effective in constraining medication prices.9 While impacts of the policy changes on access to, quality, and outcomes of cancer care require further study, China’s reimbursement-linked cancer medication price negotiation approach may constitute a valuable experience for healthcare decision-makers elsewhere.
Strengths and limitations
To our knowledge, this is the first analysis to evaluate impacts of national reimbursement-linked price negotiations on targeted cancer medication costs, volumes and spending in China. We used an ITS design, a quasi-experimental approach for evaluating the effects of interventions, increasing internal validity. In addition, we strengthened the ITS design by adding a comparison group to separate intervention effects from other potential influences on the outcomes that may have occurred at the same time as the price negotiations.
This study has several limitations related to its data source. First, our study is based on aggregated medication purchase data of hospitals. We, therefore, cannot assess access in terms of numbers of patients treated or affordability in terms of OOP spending. In addition, we cannot assess whether treatment for patients with indications for bortezomib changed to explain the volume decline of that medication in 2018. Third, few targeted anticancer medications were available in China before 2016 and the comparison group is imperfect in that intervention and comparison group targeted anticancer medications have different indications. Different incidences of the diseases for which the medications are indicated may influence changes in use and spending over time. However, the quasi-experimental design we used controls for preintervention levels and trends of medication costs, use and spending.
Further research is needed to evaluate the actual financial burden of new anticancer medications on households and the health system and clinical outcomes among patients after the implementation of the policy.
Conclusion
Our results suggest that the 2017 medication price negotiation policy, linked to mandatory reimbursement, significantly changed costs and use of and spending on selected anticancer medications. The decline in per-unit medication procurement costs combined with at least partial coverage by the BMI should improve patients’ access to these anticancer medications, although this remains to be demonstrated.
Acknowledgments
We thank CMEI for providing the raw data and related information on the hospital purchase data.
Footnotes
Handling editor: Lei Si
Contributors: YZ and XG conceptualised and undertook the analyses, and wrote the first draft of the manuscript. AKW provided input into the analyses and critically reviewed and modified the initial and subsequent drafts. All authors refined versions of and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by National Natural Science Foundation of China (Grant No.71774005). AKW received partial support from the Department of Population Medicine Ebert Award.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data was obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was considered not human subjects research by the Harvard Pilgrim Health Care Institutional Review Board and Peking University Health Science Review Board.
References
- 1.GBD 2016 Causes of Death Collaborators . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1151–210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Sledge GW. What is targeted therapy? J Clin Oncol 2005;23:1614–5. 10.1200/JCO.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 4.Shih Y-CT, Xu Y, Liu L, et al. Rising prices of targeted oral anticancer medications and associated financial burden on Medicare beneficiaries. J Clin Oncol 2017;35:2482–9. 10.1200/JCO.2017.72.3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan X, Zhang Y, Wushouer H, et al. Differences in reimbursement listing of anticancer therapies in China: an observational study. BMJ Open 2020;10:e031203–e03. 10.1136/bmjopen-2019-031203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notice of the Ministry of Human Resources and Social Security on Issuing the National Drug Catalog for Basic Medical Insurance, Work-Related Injury Insurance, and Maternity Insurance [updated 21 Feb 2017]. Available: http://www.mohrss.gov.cn/gkml/zlbmxgwj/ylbx_3063/201702/t20170223_266775.html [Accessed Jun 2021].
- 7.Tang M, Song P, He J. Progress on drug pricing negotiations in China. Biosci Trends 2020;13:464–8. 10.5582/bst.2019.01339 [DOI] [PubMed] [Google Scholar]
- 8.Guan X, Wushouer H, Yang M, et al. Influence of government price regulation and deregulation on the price of antineoplastic medications in China: a controlled interrupted time series study. BMJ Open 2019;9:e031658. 10.1136/bmjopen-2019-031658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkemeier F, Whaley C, Robinson JC. Increasing divergence in drug prices between the United States and Germany after implementation of comparative effectiveness analysis and collective price negotiations. J Manag Care Spec Pharm 2019;25:1310–7. 10.18553/jmcp.2019.25.12.1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moye-Holz D, van Dijk JP, Reijneveld SA, et al. Policy approaches to improve availability and affordability of medicines in Mexico - an example of a middle income country. Global Health 2017;13:53. 10.1186/s12992-017-0281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limwattananon C, Waleekhachonloet O. Access to and price trends of antidiabetic, antihypertensive, and antilipidemic drugs in outpatient settings of the universal coverage scheme in Thailand. PLoS One 2019;14:e0211759. 10.1371/journal.pone.0211759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Y-L, Lee C-T, Tain Y-L, et al. The impact of adoption of a new urate-lowering agent on trends in utilization and cost in practice. PLoS One 2019;14:e0221504. 10.1371/journal.pone.0221504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garabedian LF, Ross-Degnan D, Ratanawijitrasin S, et al. Impact of universal health insurance coverage in Thailand on sales and market share of medicines for non-communicable diseases: an interrupted time series study. BMJ Open 2012;2. 10.1136/bmjopen-2012-001686. [Epub ahead of print: 28 11 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Wei Q, Zhou Y, et al. A systematic analysis of FDA-approved anticancer drugs. BMC Syst Biol 2017;11:87. 10.1186/s12918-017-0464-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limaverde-Sousa G, Sternberg C, Ferreira CG. Antiangiogenesis beyond VEGF inhibition: a journey from antiangiogenic single-target to broad-spectrum agents. Cancer Treat Rev 2014;40:548–57. 10.1016/j.ctrv.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Human Resources and Social Security . Notice of the Ministry of Human Resources and Social Security on Including 36 Drugs in the National Reimbursement Drug List for Basic Medical Insurance, Work-Related Injury Insurance, and Maternity Insurance 2017 [updated July 19, 2017]. Available: http://www.mohrss.gov.cn/SYrlzyhshbzb/shehuibaozhang/zcwj/yiliao/201707/t20170718_274153.html [Accessed June 2021].
- 18.Guan X, Tian Y, Ross-Degnan D, et al. Interrupted time-series analysis of the impact of generic market entry of antineoplastic products in China. BMJ Open 2018;8:e022328–e28. 10.1136/bmjopen-2018-022328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.China National health accounts report: China National health development research center 2018.
- 20.WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2019. 2019.
- 21.National Medical Products Administration . Available: http://app1.sfda.gov.cn/datasearchcnda/face3/base.jsp?tableId=25&tableName=TABLE25&title=%B9%FA%B2%FA%D2%A9%C6%B7&bcId=152904713761213296322795806604 [Accessed Jan 2020].
- 22.O'Donnell A, Anderson P, Jané-Llopis E, et al. Immediate impact of minimum unit pricing on alcohol purchases in Scotland: controlled interrupted time series analysis for 2015-18. BMJ 2019;366:l5274. 10.1136/bmj.l5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Bureau of Statistics of China . Available: http://data.stats.gov.cn/easyquery.htm?cn=A01 [Accessed Jun 2021].
- 24.Fu H, Li L, Yip W. Intended and unintended impacts of price changes for drugs and medical services: evidence from China. Soc Sci Med 2018;211:114–22. 10.1016/j.socscimed.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 25.Jandoc R, Burden AM, Mamdani M, et al. Interrupted time series analysis in drug utilization research is increasing: systematic review and recommendations. J Clin Epidemiol 2015;68:950–6. 10.1016/j.jclinepi.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Wagner AK, Soumerai SB, et al. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol 2009;62:143–8. 10.1016/j.jclinepi.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J 2015;15:480–500. 10.1177/1536867X1501500208 [DOI] [Google Scholar]
- 28.Durbin J, Watson GS. Testing for serial correlation in least squares regression. I. Biometrika 1950;37:409–28. [PubMed] [Google Scholar]
- 29.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. 4th ed. New York; Boston: McGraw-Hill/Irwin, 2004. [Google Scholar]
- 30.World Health Organization . Technical report: pricing of cancer medicines and its impacts: a comprehensive technical report for the world health assembly resolution 70.12: operative paragraph 2.9 on pricing approaches and their impacts on availability and affordability of medicines for the prevention and treatment of cancer. Geneva: World Health Organization, 2018: 112. [Google Scholar]
- 31.Kantarjian H, Rajkumar SV. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin Proc 2015;90:500–4. 10.1016/j.mayocp.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Gómez-Dantés O, Wirtz VJ, Reich MR, et al. A new entity for the negotiation of public procurement prices for patented medicines in Mexico. Bull World Health Organ 2012;90:788–92. 10.2471/BLT.12.106633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng Q, Yin D, Mills A, et al. China's encouraging commitment to health. BMJ 2019;365:l4178. 10.1136/bmj.l4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang H, Eggleston K, Hanson K. Enhancing financial protection under China's social health insurance to achieve universal health coverage. BMJ 2019;365:l2378–l78. 10.1136/bmj.l2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moye-Holz D, van Dijk JP, Reijneveld SA, et al. The impact of price negotiations on public procurement prices and access to 8 innovative cancer medicines in a middle-income country: the case of Mexico. Value Health Reg Issues 2019;20:129–35. 10.1016/j.vhri.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 36.Emmerick ICM, Campos MR, Luiza VL, et al. Retrospective interrupted time series examining hypertension and diabetes medicines usage following changes in patient cost sharing in the 'Farmácia Popular' programme in Brazil. BMJ Open 2017;7:e017308. 10.1136/bmjopen-2017-017308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diao Y, Qian J, Liu Y, et al. How government insurance coverage changed the utilization and affordability of expensive targeted anti-cancer medicines in China: an interrupted time-series study. J Glob Health 2019;9:020702. 10.7189/jogh.09.020702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng Q, Fang H, Liu X, et al. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet 2015;386:1484–92. 10.1016/S0140-6736(15)00342-6 [DOI] [PubMed] [Google Scholar]
- 39.Zhang A, Nikoloski Z, Mossialos E. Does health insurance reduce out-of-pocket expenditure? heterogeneity among China's middle-aged and elderly. Soc Sci Med 2017;190:11–19. 10.1016/j.socscimed.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 40.Davis C, Naci H, Gurpinar E, et al. Availability of evidence of benefits on overall survival and quality of life of cancer drugs Approved by European medicines agency: retrospective cohort study of drug approvals 2009-13. BMJ 2017;359:j4530–j30. 10.1136/bmj.j4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tibau A, Molto C, Ocana A, et al. Magnitude of clinical benefit of cancer drugs Approved by the US food and drug administration. J Natl Cancer Inst 2018;110:486–92. 10.1093/jnci/djx232 [DOI] [PubMed] [Google Scholar]
- 42.Kwon H-Y, Kim J. Consistency of new drug pricing in Korea: bridging variations among personnel in price negotiations. Health Policy 2020;124:965–70. 10.1016/j.healthpol.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Kim J, Cho H, et al. Trends in the pricing and reimbursement of new anticancer drugs in South Korea: an analysis of listed anticancer drugs during the past three years. Expert Rev Pharmacoecon Outcomes Res 2021;21:479–88. 10.1080/14737167.2021.1860023 [DOI] [PubMed] [Google Scholar]
- 44.Meng Q, Mills A, Wang L, et al. What can we learn from China's health system reform? BMJ 2019;365:l2349. 10.1136/bmj.l2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y, Shibuya K, Cheng G, et al. Tracking China's health reform. Lancet 2010;375:1056–8. 10.1016/S0140-6736(10)60397-2 [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Shi L, Sawhney M, et al. Evidence for the effectiveness of anti-hypertensive medicines included on the Chinese national reimbursement drug list. BMC Health Serv Res 2019;19:112. 10.1186/s12913-019-3937-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ministry of Human Resources and Social Security Releases Results of Negotiations on National Reimbursement Drug List Entry [updated July 19, 2017]. Available: http://www.mohrss.gov.cn/SYrlzyhshbzb/dongtaixinwen/buneiyaowen/201707/t20170719_274189.html [Accessed Jun 2021].
- 48.Notice of the National Healthcare Security Administration on the Inclusion of 17 Anticancer Medications in the Category B of National Reimbursement Drug List for Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance [updated October 10, 2018]. Available: http://www.nhsa.gov.cn/art/2018/10/10/art_37_1057.html [Accessed Jun 2021].
- 49.Statistical Communiqué on the Development of National Basic Medical Insurance in 2018 [updated June 30, 2019]. Available: http://www.nhsa.gov.cn/art/2019/6/30/art_47_1476.html [Accessed Jun 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-006196supp001.pdf (630KB, pdf)
Data Availability Statement
Data was obtained from a third party and are not publicly available.