Abstract
Introduction
Optimal treatment for ‘potentially resectable’ stage III-N2 non-small cell lung cancer (NSCLC) requires multimodality treatment: local treatment (surgery or radiotherapy) and systemic anticancer therapy. There is no clear evidence of superiority for survival between the two approaches and little research has explored quality of life (QOL). This study will inform the design of a phase III randomised trial of surgery versus no surgery as part of multimodality treatment for stage III-N2 NSCLC with QOL as a primary outcome.
Methods and analysis
Patient participants will be randomised to receive multimodality treatment (1) with surgery OR (2) without surgery. The Quintet Recruitment Intervention will be used to maximise recruitment. Eligible patients will have ‘potentially resectable’ N2 NSCLC and have received a multidisciplinary team recommendation for multimodality treatment. Sixty-six patients and their carers will be recruited from 8 UK centres. Patient/carer QOL questionnaires will be administered at baseline, weeks 6, 9, 12 and month 6. Semistructured interviews will be conducted. Quantitative data will be analysed descriptively and qualitative data will be analysed using framework analysis.
Ethics and dissemination
Ethical approval has been obtained. Results will be disseminated via publications, national bodies and networks, and patient and public involvement groups.
Trial registration
Keywords: lung cancer chemotherapy, non-small cell lung cancer, thoracic surgery
Introduction
There are over 46 000 new cases of lung cancer every year in the UK and almost 36 000 deaths.1 The most common histological subtype is non-small cell lung cancer (NSCLC) representing approximately 80% of all lung cancers. Stage III-N2 NSCLC describes lung cancer with nodal metastases to the mediastinum on the ipsilateral side to the primary tumour. This patient group represents approximately 6% of all lung cancer cases.2 Curative-intent treatment is recommended for stage III-N2 NSCLC but outcomes remain poor with only 15%–20% surviving more than 5 years following treatment.2 3 The optimal curative-intent treatment for stage III-N2 NSCLC requires a combination of (1) local treatment (surgery or radiotherapy) for local control of the primary tumour and nodal disease and (2) systemic anticancer therapy with the intention of preventing distant metastatic disease. Previous randomised controlled trials (RCTs) investigating which treatment regimen (surgical vs non-surgical) provides the best overall survival have failed to show that one treatment regimen is markedly superior to another.4–7 Furthermore, stage III NSCLC is a rapidly changing paradigm. Recent guidelines from the National Institute for Health and Care Excellence (NICE) have recommended patients with Stage III-N2 NSCLC planned for surgical management should be considered for chemoradiotherapy, followed by surgery (trimodality treatment) on the basis of the cost-effectiveness of this approach due to an improved progression free survival of 4.5 months in a network meta-analysis.8 Importantly, trimodality treatment did not show a statistically significant improvement in overall survival. NICE has also recently approved maintenance Durvalumab (anti programmed death ligand 1 (PD-L1)immunotherapy) in patients with stage III NSCLC treated with concurrent chemoradiotherapy (non-surgical treatment) and a PD-L1 expression >1%, based on improved overall survival versus placebo in a global study assessing the effects ofMEDI4736 following concurrent chemoradiation (PACIFIC study).9 In summary, although it is clear that the optimal treatment for stage III-N2 NSCLC is multimodality, including both local and systemic treatment, patients and clinicians are faced with uncertainty as to the optimal strategy between a surgical versus non-surgical approach.
Health-related quality of life in stage III-N2 NSCLC treatment
Multimodality treatment in stage III-N2 NSCLC is intensive and may have a considerable impact on patients’ health-related quality of life (HRQOL). Given the markedly different treatment pathways between surgery and no surgery (radiotherapy) there are likely to be differences in HRQOL of patients undergoing such treatments. However, such metrics are severely lacking from the stage III-N2 NSCLC related literature that has largely focused on survival outcomes.10 Caregivers play an important role in supporting patients’ through treatment decisions and pathways.11 12 The small number of studies that have explored caregiver HRQOL during lung cancer treatment have found evidence of high caregiving burden and reduced HRQOL.13–16 However, no research has been conducted to explore whether caregiver experience varies depending on type of treatment. Having comparative HRQOL and treatment burden information from both patients and caregivers undergoing surgical and non-surgical treatment regimens for stage III-N2 NSCLC will help future patients, their carers and clinicians to make informed decisions about their cancer treatment. This is particularly relevant when survival outcomes have been shown to be similar and a choice of treatment pathway exists. There is, therefore, a clear need to study HRQOL in patients and caregivers undergoing surgical and non-surgical multimodality treatment for stage III-N2 NSCLC.
Methods and analysis
Aim
The PIONEER trial aims to determine the feasibility and acceptability of conducting a phase III RCT comparing the impact on HRQOL of surgery versus no-surgery as part of multimodality treatment, in patients with stage III-N2 NSCLC and their carers. Two treatment groups will be compared: (1) multimodality treatment including surgery or (2) multimodality treatment without surgery. This trial, therefore, is focused on those patients with stage III-N2 in whom surgery is technically feasible (termed ‘potentially resectable’). Patients with stage III-N2 in whom the cancer is technically ‘unresectable’ are not eligible for the trial and are not the patient cohort for whom the decision between surgical and non-surgical treatments exist. The following research questions will be addressed: (A) what is the total number and proportion of patients with ‘potentially resectable’ N2-NSCLC, and fit for multimodal therapy, in an unselected population of lung cancer patients? (B) Are patients (and carers) willing to consent to the study and once randomised do they undergo allocated treatment, and if not, why not? (C) Are clinicians willing to recruit eligible patients to the trial, what are clinician-related barriers/facilitators to recruitment? (D) What is the optimum strategy to facilitate trial recruitment? (E) What is the optimal primary outcome assessment and design for a full phase III trial?
Inclusion and exclusion criteria
Participants are eligible to be included in the study only if all of the following criteria apply: Patients with potentially resectable T1-4 N2 M0 NSCLC who have received a multidisciplinary team (MDT) recommendation for multimodality treatment; MDT consensus that the patient has adequate physiological reserve for multimodality treatment and either treatment arm is both technically and clinically appropriate; patient over the age of 18 years. Participants are excluded from the study if any of the following criteria apply: Patients unable to provide informed consent; Patients where the recommendation is for treatment other than those multimodality treatments listed; patients in whom a specific multimodality treatment regimen is recommended over another by the MDT for individualised reasons.
Definition of ‘potentially resectable stage III-N2 NSCLC’
A standardised definition of potentially resectable is provided to all sites (box 1). This definition has been proposed and validated with a high level of agreement in a National survey of practice in stage III lung cancer across the UK.17 18 Patients with stage III-N2 NSCLC will be deemed ‘potentially resectable’ and eligible for the trial if they fulfil all of the criteria set out in the standardised definition.
Box 1. Standardised definition of potentially resectable stage III-N2 non-small cell lung cancer.
✓Pathologically confirmed non-small cell lung cancer.
✓Systematic invasive nodal staging completed (surgical or endoscopic).
✓Thorough radiological staging completed including at least PET-CT and contrast enhanced brain imaging.
✓Primary tumour resectable with high probability of clear pathological margins and complete resection.
✓N2 nodal disease is discrete, easily measurable and defined, free from major mediastinal structures including the great vessels and trachea with no individual lymph node measuring >3 cm.
Treatment arms
Both treatment arms are offered as standard practice. There is no experimental and control arm in this study as no one treatment regimen has been found to be clearly superior to another and therefore multiple treatment options exist as standard of care. This is reflected by international lung cancer guidelines which confirm both surgical and non-surgical multimodality treatment regimens as appropriate strategies for stage III-N2 NSCLC.19 20
Surgical multimodality treatment arm
The surgical arm can include any form of multimodality treatment that includes surgical resection. This includes induction chemotherapy followed by surgery, induction chemoradiotherapy followed by surgery and surgery followed by adjuvant chemotherapy. The choice of surgical multimodality regimen is left to the discretion of the local treating MDT. In the UK, surgery followed by adjuvant chemotherapy is the most common form of multimodality surgical treatment performed and is likely to form a significant component of the surgical arm in this trial.2 However, the recent NICE guideline recommendation for induction chemoradiotherapy followed by surgery may lead to a higher contribution of trimodality treatment to the surgical arm.
Non-surgical multimodality treatment arm
The non-surgical arm can include any form of multimodality treatment that does not involve surgery. This includes sequential chemoradiotherapy and concurrent chemoradiotherapy. Within this treatment arm, there is an important discussion point in regards to the use of durvalumab following concurrent chemoradiotherapy. There is considerable debate among the lung cancer community about how the PACIFIC trial results impact treatment decisions for patients with stage III NSCLC considered suitable for surgery. The PACIFIC trial recruited patients with ‘unresectable’ stage III NSCLC but the protocol provided no standardised definition of ‘unresectable’ and this was left at the discretion of the local MDT. Some MDTs consider any N2/mediastinal nodal metastases as unresectable whereas other MDTs will recommend surgery in N2 NSCLC. In the PACIFIC trial, no data were provided to help clinicians understand the study population in terms of the extent of nodal disease and tumour burden. Everyday practice in the UK has reflected this uncertainty with oncologists considering the use of durvalumab in patients with stage III NSCLC that have completed concurrent chemoradiotherapy regardless of whether the disease could have been considered technically suitable for surgery at the outset, that is, the ‘unresected’ rather than the ‘unresectable’. The PIONEER trial provides a standardised definition of ‘potentially resectable’ N2 NSCLC that is broad and will likely include some patients that are similar to those treated in the PACIFIC trial. Therefore, if a patient within the PIONEER trial is randomised to the non-surgical arm and they complete concurrent chemoradiotherapy, the local oncologist will consider their eligibility for durvalumab. The local oncologist may wish to rediscuss that patient within their local MDT (perhaps with the PD-L1 status) to clarify eligibility for durvalumab towards the end of the chemoradiotherapy treatment.
Recruitment
The eight recruiting sites will be in Manchester (Wythenshawe and Salford), Leeds, Sheffield, University College London Hospitals (UCLH), Birmingham, Glasgow and Nottingham. There are approximately 2500 new cases of N2 NSCLC every year in the UK. Our conservative estimate for recruitment is one patient every second month from each site. Participants will be eligible for the study if they have ‘potentially resectable’ Stage III-N2 lung cancer and have received an MDT recommendation for multimodality treatment. We aim to recruit 66 patients over a 20-month staggered recruitment period. This sample size was chosen to ensure that we can estimate key parameters with sufficient precision based on recommendations by Lancaster et al.21 Recruitment will be led by the respiratory physicians at eight recruiting centres (all high volume lung cancer MDTs). The consenting process will be conducted by respiratory physicians and supported by lung cancer nurse specialists. Our ambition is that the majority of recruitment is undertaken by respiratory physicians as impartial advocates for the patient and to minimise treatment related bias. However, recruitment within surgical and oncology clinics will be allowed to provide flexibility and maximise recruitment opportunities. All potential study participants will be provided with a participant information sheet (PIS) and invited to voluntarily consent to participate in the study. They will also be asked if they have a caregiver who may also want to take part in the study. Due to the anticipated challenges with recruitment we will proactively monitor, evaluate and react to quickly rectify emerging issues. We will apply the principles of the Quintet Recruitment Intervention (QRI) to maximise recruitment as much as possible and inform the recruitment strategy for the follow-on trial.22 Key steps within the QRI process include: (1) understanding the recruitment process; (2) documenting barriers to recruitment and trying to understand them; (3) assessing how the RCT is being delivered by recruiters; (4) presenting evidence of recruitment difficulties to the trial management group and developing strategies to overcome these problems and (5) implementing the plan of improvement. This will include audio recording of recruitment appointments, with real-time data analysis and feedback to the clinical teams to proactively inform recruitment practices including ongoing training. We will conduct trial recruitment workshop sessions with recruiting clinicians across all sites and provide refresher sessions as required based on the results of the QRI process.22 Figure 1 presents the flow of patients through the study.
Figure 1.
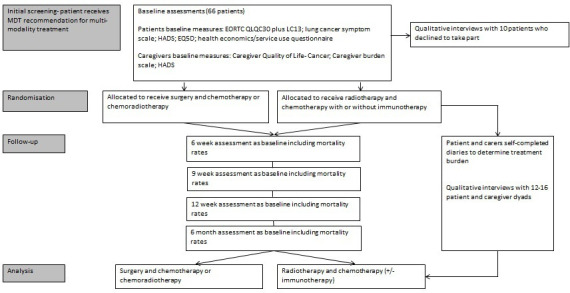
CONSORT flow diagram. CONSORT, Consolidated Standards of Reporting Trials; MDT, multidisciplinary team. Hospital Anxiety and Depression Scale (HADS). EuroQOL 5 Dimensions (EQ5D)
Randomisation
Eligible patients and caregivers will provide written informed consent and complete baseline assessments. The recruiting clinician will contact the research team at The Christie National Health Service Foundation Trust who will manage the randomisation process using an online randomisation system. The person responsible for randomisation will have no involvement in the recruiting process. Participants will be randomised on a 1:1 basis to receive either surgery or no surgery as part of multimodality treatment. Once a patient has been randomised, both the clinical team and the patient will be made aware of the treatment allocation; given the nature of the intervention there will be no masking of patients, carers or clinical staff
Assessments and data collection
Various outcomes and success metrics (table 1) will be collected via questionnaires returned by post or completed over the telephone by a member of the clinical team. The outcomes and success metrics collected will determine if it is feasible to do a follow-on randomised controlled trial in the future. A summary of required data, assessment tools and collection time points is provided in table 2.
Table 1.
Feasibility success metrics providing support for a future trial
| Green light (proceed with minor changes) | Amber light (proceed with significant modifications) | Red light (do not proceed) | |
| Recruitment | 50 or more patients recruited | 25–49 patients recruited | 24 or fewer patients recruited |
| Treatment fidelity | At least 60% of randomised participants receive allocated treatments | At least 40% of randomised participants receive allocated treatments | Less than 40% of randomised participants receive allocated treatments |
| Rate of assessment attrition at 3 and 6 months | Less than 30% at 3 and 6 months | Less than 60% at 3 and 6 months | 61% or more at 3 and 6 months |
| Qualitative data | Positive opinions and/or feasibility recommendations to improve the follow-on trial. We will hold an end of study workshop with all coinvestigators, site PIs, patient/carer representatives and other stakeholders to present the outcomes and discuss approaches to address issues noted as green or amber and reasons for red lights. | ||
Table 2.
Summary of required data, assessment tools, collection time points and processes
| Baseline | Week 6 | Week 9 | 3 months | 6 months | |
| EORTC QLQC30 (plus LC13) | X | X | X | X | X |
| SF-36 | X | X | X | X | X |
| HADS | X | X | X | X | X |
| EQ-5D | X | X | X | ||
| Service use/health economics questionnaire | X | X | X | ||
| Demographics | X | ||||
| Clinical data | X | ||||
| Mortality rates | X | X | X | X | |
| Caregiver outcomes | |||||
| Carer Quality of Life-Cancer | X | X | X | X | X |
| Zarit Caregiver-Burden | X | X | X | X | X |
| HADS | X | X | X | X | X |
*European Organisation for Research and Treatment of Cancer (EORTC)
EQ5D, EuroQOL 5 dimensions; HADS, Hospital Anxiety and Depression Scale; SF-36, Short Form Health Survey.
Feasibility outcomes
Proportion of patients with N2 disease that are potentially resectable as defined by the lung cancer MDT.
Proportion of patients with potentially resectable N2 NSCLC that have adequate physiological reserve for multimodality treatment.
Number and proportion of eligible patients recruited—assessment of screening logs to determine numbers eligible versus not randomised and reasons (such as clinician reluctance, patient/family decline, other).
Proportion of people lost to follow-up due to withdrawal, death or failure to return questionnaires (ie, assessment attrition) by 3 and 6 months by arm.
Percentage of participants with complete data at end of trial (6 months), by arm.
Proportion of randomised patients who receive allocated treatment, by arm.
Adherence to the allocated treatment arm will be monitored closely (any patients that crossover to receive treatment in the other arm will remain in the trial for data collection but will be recorded as failing to complete the allocated treatment).
Qualitative data recording recruitment consultations and interviews with participants and non-participants and their carers.
Clinical data
Clinical data will be collected directly from the patients’ hospital records. Data collected will include: age; gender; performance status at recruitment; clinical frailty score; co-morbidities (aligned to the Charlson Comorbidity Index); diagnosis of interstitial lung disease; body mass index (BMI), smoking status; forced expiratory volume (% predicted); diffusing capacity of the lungs for carbon monoxide % predicted; blood results at recruitment; NSCLC histology; clinical (and pathological where available) TNM8 staging; location, size and standard uptake value (SUV) of primary tumour; N2 nodes involved; N2 sub classification (single station vs multistation); Highest stage N2 nodal size (short axis); Highest stage N2 nodal SUVmax and mediastinal blood pool SUVmax. When performed the following additional data will be collected, accepting this is not mandated: 6 min walk test (metres) or incremental shuttle walk (metres); Vo2max (mls/kg/min) V02max%predicted; left and right ventricular function on transthoracic echocardiogram (% ejection fraction and/or categorised as normal, mild impairment, moderate impairment and severe impairment).
Participant completed data
Patient and caregiver reported outcome measures are collected at baseline 6, 9 and 12 weeks and 6 months. Unless the participant has a coinciding clinic appointment, questionnaires will be sent by post. Reminder phone calls will be made if questionnaires are not returned. Real-time completion of questionnaires via a telephone call with research staff may also be considered if this is the preference of the patient. Prior to contacting the participant by telephone or post, the participants’ survival status will be checked with the clinical team. Patients and caregivers will also complete diaries describing their experiences in more detail.
Qualitative interviews
Qualitative interviews will be conducted at various stages of the follow-up process with 12–16 patient and caregiver dyads. Participants will be asked at the time of consenting to the study if they are happy to be approached to participate in an interview. The interview will explore how the treatment has impacted on the daily lives of patient and caregivers from a practical and psychological perspective, their thoughts on the randomisation process and the treatment options will also be explored. Interviews will last approximately 60 min; they will be audiorecorded and transcribed verbatim. Interviews will be conducted by a member of The Christie Research team. We will also interview 8–10 patients who declined to take part in the study or opted not to receive their allocated treatment and explore their decision-making processes. Interviews will be conducted face to face or over the telephone. A topic guide will be developed and interviews audiorecorded with consent and transcribed verbatim.
Data analysis
Given that this is a feasibility study, we will not conduct hypothesis testing to determine if either treatment option has a greater impact on HRQOL. Instead, analyses will be descriptive, focusing on calculating confidence intervals for key trial parameters.23 For example: (1) estimating eligible participant numbers, recruitment, fidelity and attrition rates, rate of missing data; (2) estimating the SD for HRQOL and likely trajectory over time, in order to inform/refine a sample size calculation for a future phase III trial and (3) determining whether the study design was acceptable to participants and their caregivers. A feature of the study data will be that some participants will have their HRQOL outcomes censored due to death. The study will allow us to estimate the frequency of this occurring, and to develop an analytical strategy for a phase III trial. We plan to use joint modelling techniques (joineR package in R24), which allows for the joint analysis of survival data and endogenous repeated measures. Our analysis will focus on estimating variability, correlation between repeated measurements from the same individual and modelling the trajectory of HRQOL over time. We will adjust for baseline functional status. We will use the data from this study to design a detailed analysis plan for a confirmatory study. Qualitative data generated from the audiorecorded interviews with patient–carer dyads and clinicians will be analysed using framework analysis.25 Audiorecordings of recruitment consultations will be analysed using content analysis26 to highlight specific issues and enable immediate feedback to the trial management group.
Patient and public involvement
Patients have played a central role in the design and development of this study. Our early work with the Wythenshawe/Christie lung cancer Patient and Public Invovlement and Engagement (PPIE) group and North Trent Consumer Research Panel clearly supported the need for quality of life and burden to be included in treatment decision making. The issue of recruitment with previous trials comparing surgery with radiotherapy has been fully explored with our patient groups. The randomisation process was a concern for some patients so it will be clearly specified in the patient information sheet and at the time of consent that the trial has been suggested to them because there was no clear indication which treatment would have the best outcome for them. Given the patients concern, it was also decided that a clinician would lead the recruitment consultation.
Anticipated risks and mitigation
As described earlier, optimising recruitment will be addressed using the QRI methodology. Other risks include that the treatment paradigm in stage III-N2 NSCLC may change during the course of this feasibility trial (eg, immunotherapy in surgical treatment pathways). However, the research question will remain relevant regarding the impact on HRQOL from surgical versus non-surgical based multimodality treatment regimens. We have designed this feasibility study in a pragmatic way which allows different types of regimens within each arm that reflect changing standards of care. If we can prove this trial is feasible to recruit to then for a phase III trial we will need to clearly define the standard of care in both the surgical and non-surgical arms that reflects the evidence base and guidelines at that moment. Finally, data completeness will be important particularly in the first 9 weeks where multiple different HRQOL questionnaires are needed. The ability to complete this information via telephone and the use of reminder telephone calls should help to mitigate this risk.
Ethics and dissemination
The trial has been approved by the West of Scotland Research Ethics Committee and Health Research Authority. The sponsor and the REC have approved the trial protocol, PIS, consent form and submitted supporting documents. Any agreed substantial amendments will be submitted for ethical approval prior to implementation. The trial will be conducted in accordance with the principles of Good Clinical Practice. Data will be collected and processed in accordance with the Data Protection Act 2018. Trial documents will be retained for 5 years after the end of the study then they will be destroyed. All personal identifiable information will be kept in secure password protected electronic files or locked storage cabinets. We intend to submit the summary results of the feasibility to a peer-reviewed, open-access journal. After publication, the deidentified individual patient data will be made available on request to researchers who provide a methodologically sound proposal.
The current study represents the first randomised trial in the N2 lung cancer population to assess quality of life and treatment burden as an outcome for both patients and family carers. We will disseminate the result of this study widely at national and international clinical meetings and academic conferences. We have coapplicants that are members of the organising committee for various national and international organisations and will ensure full dissemination. The trial has already been discussed at the EORTC trials meeting with positive feedback and a potential to collaborate on a phase III European wide trial. We will also engage with patient and public involvement groups, including those who helped to develop the application and disseminate results.
Conclusion
This feasibility trial aims to provide the pivotal information that will inform the design and scale of a phase III randomised trial of surgery versus no surgery as part of multimodality treatment in stage III-N2 with HRQOL as a primary outcome, as well as to determine whether it is feasible to recruit patients at the required level to deliver such a trial.
Footnotes
Contributors: ST, JY and ME drafted the initial manuscript. All authors reviewed the manuscript prior to submission. All authors were involved in study conception and the development of the protocol.
Funding: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG- 1217-20039).
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Cancer Research UK . Lung cancer statistics. Available: http://www.cancerresearchuk.org/healthprofessional/%0Acancer-statistics/statistics-by-cancer-type/lung-cancer
- 2.Adizie JB, Khakwani A, Beckett P, et al. Stage III non-small cell lung cancer management in England. Clin Oncol 2019;31:688–96. 10.1016/j.clon.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 4.Pless M, Stupp R, Ris H-B, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049–56. 10.1016/S0140-6736(15)60294-X [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379–86. 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Meerbeeck JP, Kramer GWPM, Van Schil PEY, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442–50. 10.1093/jnci/djk093 [DOI] [PubMed] [Google Scholar]
- 7.Eberhardt WEE, Pöttgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194–201. 10.1200/JCO.2015.62.6812 [DOI] [PubMed] [Google Scholar]
- 8.NICE . NICE: National Institute for health and care excellence. Lung Cancer: Diagnosis and Management, 2019. [PubMed] [Google Scholar]
- 9.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 10.Evison M, Clive A, Castle L, et al. Resectable clinical N2 non-small cell lung cancer; what is the optimal treatment strategy? an update by the British thoracic Society lung cancer specialist Advisory group. J Thorac Oncol 2017;12:1434–41. 10.1016/j.jtho.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 11.Shin DW, Cho J, Roter DL, et al. Preferences for and experiences of family involvement in cancer treatment decision-making: patient-caregiver dyads study. Psychooncology 2013;22:2624–31. 10.1002/pon.3339 [DOI] [PubMed] [Google Scholar]
- 12.Hobbs GS, Landrum MB, Arora NK, et al. The role of families in decisions regarding cancer treatments. Cancer 2015;121:1079–87. 10.1002/cncr.29064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant M, Sun V, Fujinami R, et al. Family caregiver burden, skills preparedness, and quality of life in non-small cell lung cancer. Oncol Nurs Forum 2013;40:337–46. 10.1188/13.ONF.337-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray SA, Kendall M, Boyd K, et al. Archetypal trajectories of social, psychological, and spiritual wellbeing and distress in family care givers of patients with lung cancer: secondary analysis of serial qualitative interviews. BMJ 2010;340:c2581. 10.1136/bmj.c2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Houtven CH, Ramsey SD, Hornbrook MC, et al. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist 2010;15:883–93. 10.1634/theoncologist.2010-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancini J, Baumstarck-Barrau K, Simeoni M-C, et al. Quality of life in a heterogeneous sample of caregivers of cancer patients: an in-depth interview study. Eur J Cancer Care 2011;20:483–92. 10.1111/j.1365-2354.2010.01227.x [DOI] [PubMed] [Google Scholar]
- 17.Evison M, McDonald F, Batchelor T. What is the role of surgery in potentially resectable N2 non-small cell lung cancer? Thorax 2018;73:1105–9. 10.1136/thoraxjnl-2018-212287 [DOI] [Google Scholar]
- 18.Evison M, Edwards J, McDonald F, et al. Stage III non-small cell lung cancer: a UK national survey of practice. Clin Oncol 2020;32:527–36. 10.1016/j.clon.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 19.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S–40. 10.1378/chest.12-2360 [DOI] [PubMed] [Google Scholar]
- 20.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1–21. 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 21.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307–12. 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- 22.Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the quintet recruitment intervention (QRI). Trials 2016;17:283. 10.1186/s13063-016-1391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee EC, Whitehead AL, Jacques RM, et al. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol 2014;14:41. 10.1186/1471-2288-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philipson P, Diggle P, Sousa I. joineR: Joint modelling of repeated measurements and time-to-event data. undefined Published Online First:, 2012. Available: https://www.semanticscholar.org/paper/joineR%3A-Joint-modelling-of-repeated-measurements-Philipson-Diggle/0506a41b4b641b40f03c8fc5006a8c991cacc376 [Accessed 19 Nov 2020].
- 25.Ritchie J, Spencer L. Qualitative Data Analysis for Applied Social Policy Research. In: Bryman A, Burgess B, eds. Analysing qualitative data. Routledge, 1994. [Google Scholar]
- 26.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004;24:105–12. 10.1016/j.nedt.2003.10.001 [DOI] [PubMed] [Google Scholar]


