Abstract
Background
The use of patient-reported outcome measures (PROMs) is becoming increasingly important in healthcare. However, incorporation of PROMs into routine nephrological care is challenging. This study describes the first experience with PROMs in Dutch routine dialysis care.
Methods
A pilot study was conducted in dialysis patients in 16 centres. Patients were invited to complete PROMs at baseline and 3 and 6 months. PROMs consisted of the 12-item short-form and Dialysis Symptom Index to assess health-related quality of life (HRQoL) and symptom burden. Response rates, HRQoL and symptom burden scores were analysed. Qualitative research methods were used to gain insight into patients’ views on using PROMs in clinical practice.
Results
In total, 512 patients (36%) completed 908 PROMs (24%) across three time points. Response rates varied from 6 to 70% among centres. Mean scores for physical and mental HRQoL were 35.6 [standard deviation (SD) 10.2] and 47.7 (SD 10.6), respectively. Patients experienced on average 10.8 (SD 6.1) symptoms with a symptom burden score of 30.7 (SD 22.0). Only 1–3% of the variation in PROM scores can be explained by differences between centres. Patients perceived discussing their HRQoL and symptom scores as insightful and valuable. Individual feedback on results was considered crucial.
Conclusions
The first results show low average response rates with high variability among centres. Dialysis patients experienced a high symptom burden and poor HRQoL. Using PROMs at the individual patient level is suitable and may improve patient–professional communication and shared decision making. Further research is needed to investigate how the collection and the use of PROMs can be successfully integrated into routine care to improve healthcare quality and outcomes.
Keywords: chronic kidney disease, dialysis, health-related quality of life, patient-reported outcome measures, symptom burden
INTRODUCTION
Patients with advanced chronic kidney disease (CKD) experience numerous physical and emotional disease-related symptoms and a poor health-related quality of life (HRQoL) [1–3]. In daily healthcare, these patient-reported outcomes (PROs) are frequently underrecognized and underestimated [2, 4] and consequently may remain unattended [5]. The under-identification may be partly explained by patients not sharing their symptoms and needs easily [6, 7] and by difficulties for clinicians to identify the full spectrum and severity of patients’ symptoms and needs [4, 7, 8].
The use of patient-reported outcome measures (PROMs) may facilitate communication about symptoms and needs and may provide insights into PROs both at the individual and the centre or national level [9–13]. Although the importance of PROs is recognized [14–16] and the use of PROMs in routine care is highly supported [9, 16], PROMs are often not yet part of standard nephrological care [9, 13, 17]. In Europe, few renal registries have initiated the routine collection of PROMs [18–20]. The Scottish Renal Registry recently described their first experience with collecting PROMs, encountering challenges that included a low response rate, selective response, organizational struggles and low commitment from centres [18]. Literature also corroborates that it is challenging to incorporate PROMs into routine care [9–11, 17, 20–22]. A major challenge is to incorporate PROMs in such a way that they can be used for different purposes at different levels, to evaluate healthcare quality at the aggregated level and, perhaps even more important for patients, to support patient–professional communication and decision making at the individual patient level [9–11, 13]. Using PROMs for different purposes requires engagement at all levels, high response rates and feedback on outcomes tailored to the context and the purpose [9–13].
Currently PROMs are being implemented into Dutch nephrological care to provide insights into PROs of individual patients and at the centre and national level. PROMs will be collected in Renine, the Dutch Renal Registry (www.nefrovisie.nl/renine), in which all patients on renal replacement therapy (RRT) are registered. This study describes the first experience with PROMs in Dutch routine dialysis care. We aim to evaluate the introduction of the national registry of PROMs by answering the following research questions:
What is the response rate and how does the response rate vary among centres? Which differences in characteristics are observed between responders and non-responders?
What is the HRQoL and symptom burden of patients receiving dialysis, what variation in scores is observed among centres and to what extent can variation in scores be explained by differences in the patient population?
What are patients’ experiences and views on the use of PROMs in clinical practice?
MATERIALS AND METHODS
Study design and patients
The registry of PROMs was introduced in routine nephrological care through a pilot study among adult patients on dialysis in 16 Dutch centres from September 2016 to April 2017. These centres treat 26% of all Dutch patients receiving dialysis. Patients undergoing any type of dialysis were included. Clinicians invited their patients to complete the online PROMs at three time points: at baseline and 3 and 6 months after the study start. This frequency was considered suitable by patients [23] and is expected to be sufficient for centres to become familiar with PROMs. Aiming at optimal incorporation of PROMs in routine care, centres were free to develop the process of inviting and motivating patients that fit their workflow [24, 25]. Clinicians could decide not to invite a patient, for example, because of the patient’s work schedule or medical condition. At 6 months, PROMs were available to complete in the following languages: Dutch, English, Turkish and Arabic.
PROMs: HRQoL and symptom burden questionnaires
The PROMs consist of two questionnaires: the 12-item short-form (SF-12) health survey to assess HRQoL and the Dialysis Symptom Index (DSI) to assess symptom burden. These questionnaires were carefully selected in close collaboration with patients, professionals, the Dutch Kidney Patients Association (Nierpatiënten Vereniging Nederland; NVN) and the quality institution Nefrovisie [23]. The literature also recommends the SF-12 as an appropriate questionnaire to assess HRQoL in routine care, but no recommendation is provided for the assessment of symptom burden [20]. Therefore a four-phase mixed methods study was conducted to select the best suitable symptom questionnaire, in collaboration with patients and experts, and by using existing symptom questionnaires and literature. In this study, the DSI was found to be the most relevant, complete and comprehensible symptom questionnaire for routine assessment in patients with advanced CKD. The details of this selection process have been described elsewhere [23].
The SF-12 is a generic questionnaire consisting of 12 questions regarding HRQoL on a physical component scale and a mental component scale, especially developed for large-scale monitoring [26]. Within the dialysis population, the SF-12 is frequently used and has been shown to be a preferred and valid questionnaire [20, 27]. Norm-based scoring algorithms were used to calculate physical and mental scores (PCS and MCS, respectively). Component scores range from 0 to 100, with higher scores indicating better HRQoL. The PCS and MCS scales are standardized to the US population with a mean of 50 and a standard deviation (SD) of 10 [26, 28].
The DSI is a 30-item disease-specific symptom questionnaire to assess physical and emotional symptom burden [29]. To ensure comprehensiveness for individual patients, an open-ended question was added to report three additional symptoms [23]. Patients indicate for each symptom if it was present (yes/no) during the past week and, if so, how much it bothered them (5-point scale ranging from 1 ‘not at all’ to 5 ‘very much’). The number (0–30 symptoms) and burden [score ranging from 0 (no symptoms) to 150 (all 30 symptoms are present and are very burdensome)] of symptoms were calculated, with higher scores indicating higher symptom burden [30]. Scores were calculated for patients who completed ≥28 questions, whereby missing symptoms were assumed absent (burden score 0) [30].
Potentially explanatory factors
From Renine, we obtained patient, disease and treatment characteristics describing the study population: age, sex, primary kidney disease (according to European Renal Association–European Dialysis and Transplantation Association codes [31]), socio-economic status (SES, using zip code [32]), dialysis modality and time on RRT (using date of RRT initiation).
Statistical analysis
Statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA). P-values <0.05 were considered statistically significant. Variables are shown as mean (SD) or percentages. Non-normally distributed variables were log-transformed and presented as the geometric mean (SD). Missing values for patient, disease and treatment characteristics (Table 1) were assumed ‘missing at random’ and estimated using multiple imputation [33, 34]. Ten imputed datasets were created [34]. The imputation model included all patient, disease and treatment characteristics (see potentially explanatory factors), centre, response, if patients received support completing PROMs, death during follow-up, cause of death and all outcomes (PCS, MCS, symptom number and burden score) [34, 35].
Table 1.
Characteristics of responders and non-responders (N = 1440)
| Characteristics | Respondera (n = 512) | Non-respondera (n = 928) | P-value |
|---|---|---|---|
| Sex (male)b, n (%) | 342 (67.9) | 484 (57.1) | <0.001 |
| Age (years)c, mean (SD) | 66.6 (13.8) | 64.7 (16.0) | 0.022 |
| SESd, mean (SD) | <0.001 | ||
| Low | 119 (24.1) | 305 (36.5) | |
| Middle | 309 (62.6) | 430 (51.5) | |
| High | 66 (13.4) | 100 (12.0) | |
| Primary kidney diseasee, mean (SD) | 0.005 | ||
| Glomerulonephritis/sclerosis | 55 (12.5) | 98 (12.8) | |
| Pyelonephritis | 23 (5.2) | 40 (5.2) | |
| Polycystic kidney disease | 42 (9.5) | 40 (5.2) | |
| Hypertension | 72 (16.4) | 158 (20.6) | |
| Renal vascular disease | 67 (15.2) | 96 (12.5) | |
| Diabetes mellitus | 84 (19.1) | 194 (25.3) | |
| Miscellaneous | 97 (22.0) | 142 (18.5) | |
| Dialysis modalityf, mean (SD) | 0.121 | ||
| HD centre | 407 (82.6) | 695 (82.3) | |
| HD home | 18 (3.7) | 50 (5.9) | |
| PD | 68 (13.8) | 99 (11.7) | |
| Time on RRT (years)g, geometric mean (SD) | 2.5 (3.8) | 3.1 (3.4) | 0.005 |
SES, social economic status; HD, haemodialysis; PD, peritoneal dialysis; RRT, renal replacement therapy.
Patients are considered responders if they participated at least once. Non-responders were invited at least once, but never participated.
Sex is available for 504 (98.4%) responders and 847 (91.3%) non-responders.
Age is available for 504 (98.4%) responders and 846 (91.2%) non-responders.
SES is available for 494 (96.5%) responders and 835 (90.0%) non-responders.
Primary kidney disease is available for 440 (85.9%) responders and 768 (82.8%) non-responders.
Dialysis modality is available for 493 (96.3%) responders and 844 (90.9%) non-responders.
Time on RRT is available for 497 (97.1%) responders and 847 (91.3%) non-responders.
Patients who died or the centre indicated that they did not invite the patient were excluded from analyses for relevant time points (Figure 1). Patients were considered a responder if they participated at least once. Student’s t-test and chi-squared tests were used to compare the characteristics of responders and non-responders. To compare response rates between time points and centre volume (number of dialysis patients), chi-squared test and linear regression analysis were performed, respectively. For patients who participated at multiple time points, their first measurement was used in HRQoL and symptoms analyses (Figure 1).
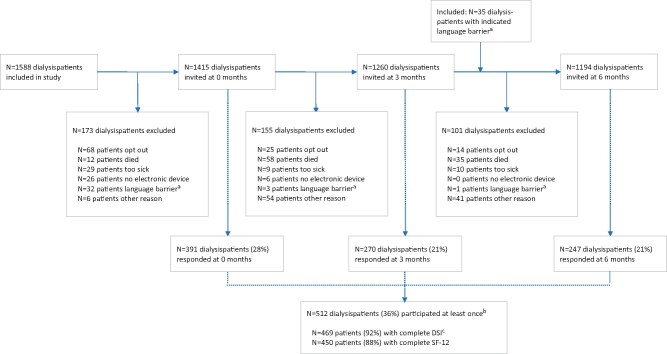
FIGURE 1.
Flow chart for the number of patients included, invited and participating at each time point. aPatients that were excluded because of a language barrier at 0 or 3 months were again included at 6 months: PROMs were also available in English, Turkish and Arabic at 6 months. bIn total, 1440 patients were invited at atleast one time point. cThe DSI was considered complete if ≥28 questions were answered.
MCS, PCS, symptom number and burden scores were calculated for responders who completed the full questionnaire (Figure 1). To explore variation among centres, MCS, PCS and symptom burden scores were assessed per centre and compared with the overall study population through indirect standardization. To that end, the following steps were taken. First, an expected score was calculated per patient for each outcome separately by using a multivariable linear regression model including patient, disease and treatment characteristics as predictors. Second, the mean expected and observed scores were calculated for each centre. Third, adjusted PROM scores per centre were calculated as follows: (observed centre mean − expected centre mean)+ overall mean, thereby creating centre scores comparable with the overall study population. Crude and adjusted PROM scores are shown in funnel plots [36]. Funnel plots were created in R version 3.4.2 (R Foundation, Vienna, Austria).
To examine to what extent variation among centres can be explained by differences in the patient population, intraclass correlation (ICC)—also referred to as ‘rankability’—was calculated using multilevel regression analysis (MLRA) [37–39]. ICC is the proportion of variance in MCS, PCS or symptom burden scores that occurs at the centre level. This variance may be attributable to centre factors or to the patient population (responders) of each centre [37, 38]. Patient, disease and treatment characteristics were included as fixed effects and centres as a random effect in the MLRA model. Comparison of the ICC before and after including characteristics in the model (i.e. comparing crude and adjusted ICC) shows to what extent centre variation can be explained by differences in characteristics of the centre populations [38].
Patients’ experiences and preferences
As PROMs are intended to become part of regular care, we wanted to know more about patients’ experiences with and preferences for discussing PROM scores with their healthcare professional. At 3 months, all patients were asked if they would like to share and discuss their PROM scores with their healthcare professional. Hereafter, in each centre, professionals received an individual digital report from five randomly selected patients who gave consent. Professionals were invited to discuss the report with these patients at their next consultation visit. This report contained the patient’s responses and PROM scores and a comparison with all responders and—for MCS and PCS—the general Dutch population. At 6 months, patients and professionals were asked how they experienced the conversation about PROM scores. Patients also reported which professional discussed the PROM scores with them and how satisfied they were with the conversation (5-point scale: 1, poor–5, excellent).
Additionally, the use of PROMs was evaluated in a focus group with patients receiving dialysis. Patients were recruited by the NVN via e-mail and social media. During the focus group, patients’ views and preferences concerning the use of PROMs in clinical practice were discussed. The focus group lasted 2.5 hrs and was chaired by a health educator (K. Prantl). The patients’ discussions were recorded in detail by handwritten field notes and, when possible, verbatim by the chair and two observers (H.A.J.B. and F.W.D.). All written information was analysed using Atlas.ti. Statements was analysed by a researcher trained in qualitative research (E.M.v.d.W.) and discussed with an experienced qualitative researcher (Y.M.).
RESULTS
Response rate
Figure 1 shows the number of patients who were invited and responded across the time points. In total, 1440 patients were invited at least once. The main reasons not to invite a patient were their medical condition and patients indicated that they did not want to be invited. In total, 512 patients (36%) responded at least once and altogether completed 908 PROMs (24%) across the three time points.
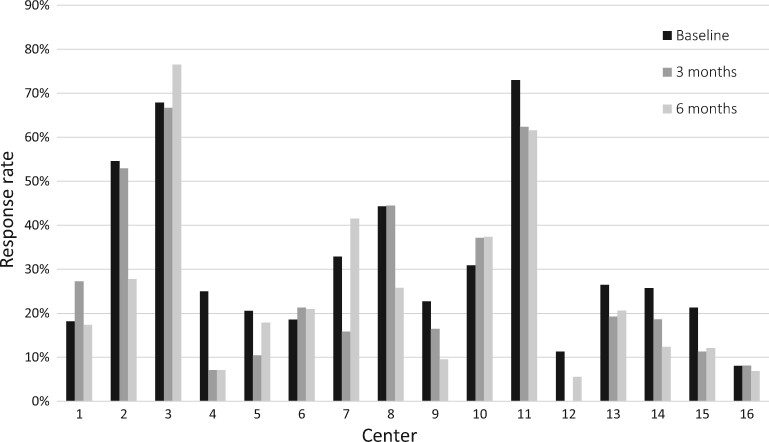
The response rate was higher at baseline, with 28%, compared with 21% at 3 and 6 months (P < 0.001). Figure 2 presents response rates per centre at all time points. A large variation among centres was found, with response rates ranging from 6 to 70%.
FIGURE 2.
Response rates per time point in 16 pilot centres. Centres are ranked (low to high) according to the number of patients on dialysis included at baseline. Larger centres (i.e. higher number of patients included at baseline) had a slightly lower response rate compared with smaller centres: the response rate decreases by 2% per 10 additional patients (P < 0.001).
Responders
Table 1 presents the characteristics of responders (n = 512) compared with non-responders (n = 928). Responders were more frequently male, older, had a higher SES and started RRT more recently. Responders’ primary kidney disease was more frequently polycystic kidney disease and less frequently hypertension or diabetes.
Responders needed on average 12.2 (SD 6.1) min to complete the PROMs. In total, 211 of 512 patients (41%) received some support to complete the PROMs, ranging from 7 to 65% across centres. When support was provided, the support mainly consisted of reading questions aloud (81%), filling in patients’ answers (79%), translating questions (6%) and completing the questionnaire on their behalf (e.g. their partner, 8%). Eleven patients (5%) indicated that another support was provided, such as assistance in using an electronic device or discussing questions with relatives to remember their experiences. Furthermore, some centres with high response rates indicated that they provided tablets so that patients could complete the PROMs while receiving dialysis treatment. The non-Dutch questionnaires that were available at 6 months were used twice: once in English and once in Arabic.
PROs
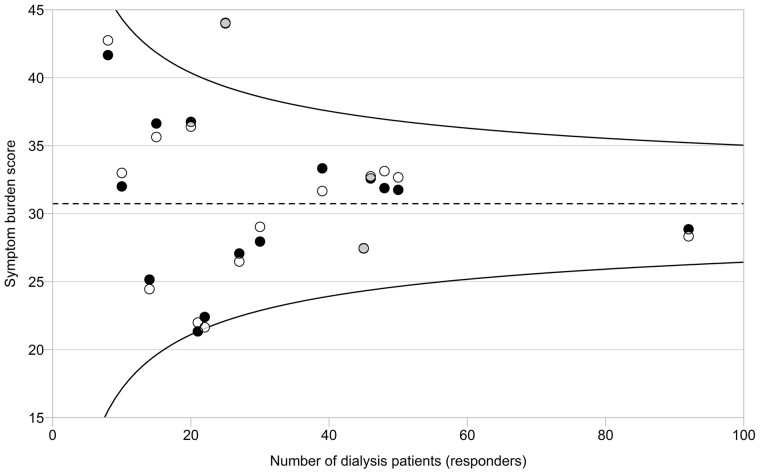
Patients experienced on average 10.8 (SD 6.1) of 30 symptoms, ranging from 8.0 and 14.8 symptoms across centres. The overall mean symptom burden score was 30.7 (SD 22.0) on a scale ranging from 0 (no symptoms) to 150 (all 30 symptoms bother ‘very much’). Table 2 presents the 10 most frequently experienced symptoms and 10 most burdensome symptoms. The most common symptom was fatigue, which was experienced by 76% of the patients. ‘Difficulty becoming sexually aroused’ was—if present—reported as the most bothersome symptom, with a mean score of 3.4 on the 5-point scale. Figure 3 presents the variation among centres in symptom burden score compared with the overall mean score.
Table 2.
Top 10 most frequent and most burdensome symptomsa
| Rank | Symptom frequency | n (%) | Symptom burdenb | Mean (SD) |
|---|---|---|---|---|
| 1 | Feeling tired or lack of energy | 366 (76.4) | Difficulty becoming sexually aroused | 3.42 (1.4) |
| 2 | Dry skin | 283 (58.7) | Trouble falling asleep | 3.26 (1.1) |
| 3 | Trouble staying asleep | 260 (54.3) | Decreased interest in sex | 3.25 (1.5) |
| 4 | Muscle cramps | 246 (51.0) | Feeling tired of lack of energy | 3.24 (1.0) |
| 5 | Itching | 240 (50.0) | Bone or joint pain | 3.23 (1.1) |
| 6 | Bone or joint pain | 225 (47.0) | Trouble staying asleep | 3.18 (1.1) |
| 7 | Dry mouth | 223 (46.8) | Dry skin | 3.04 (1.2) |
| 8 | Trouble falling asleep | 206 (43.2) | Numbness or tingling in feet | 2.99 (1.0) |
| 9 | Shortness of breath | 207 (43.1) | Restless legs or difficulty keeping legs still | 2.94 (1.0) |
| 10 | Decreased interest in sex | 193 (41.8) | Itching | 2.88 (1.0) |
Symptom frequency and burden reported using the DSI: top 10 of 30 symptoms. Symptoms were available for 459–484 patients (90–95%).
Average burden score (range: 1–5) reported when the symptom was present.
FIGURE 3.
Observed and adjusted mean symptom burden score in 16 pilot centres. Circles represent the mean observed (white circles) and adjusted (adjusted for sex, age, SES, primary kidney disease, dialysis modality and time on RRT; black circles) symptom burden score for each centre. Overlapping part of circles is depicted in grey. The overall mean (dotted line) is used as a reference in the comparison with each centre. The 95% confidence interval (CI; curved lines) is provided around the overall mean. The mean score of one centre is outside the 95% CI, indicating a statistically significant higher symptom burden score compared with the overall mean.
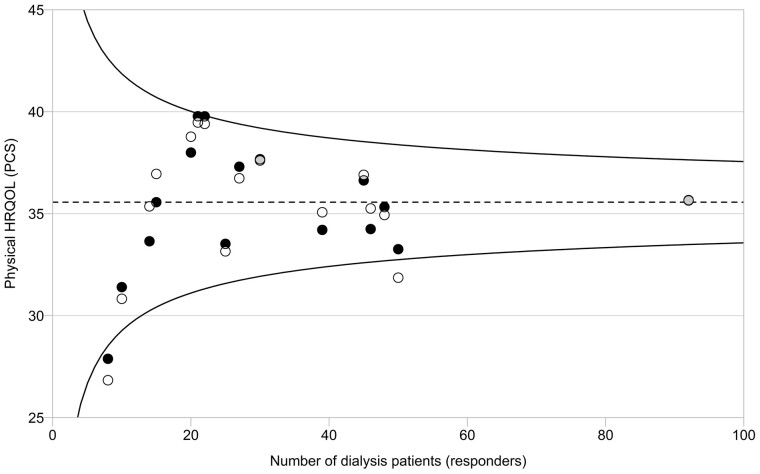
The mean scores for HRQoL were 35.6 (SD 10.2) and 47.7 (SD 10.6) on PCS and MCS, respectively. Figures 4 and 5 present the variation among centres in PCS and MCS compared with the overall mean scores.
FIGURE 4.
Observed and adjusted mean physical HRQoL (PCS) in 16 pilot centres. Circles represent the mean observed (white circles) and adjusted (adjusted for sex, age, SES, primary kidney disease, dialysis modality and time on RRT; black circles) score for physical HRQoL per centre. Overlapping part of the circles is depicted in grey. The overall mean PCS (dotted line) is used as a reference in the comparison with each centre. The 95% confidence interval (CI; curved lines) is provided around the overall mean PCS. The adjusted mean score of one centre is outside the 95% CI, indicating a statistically significant lower PCS compared with the overall mean PCS.
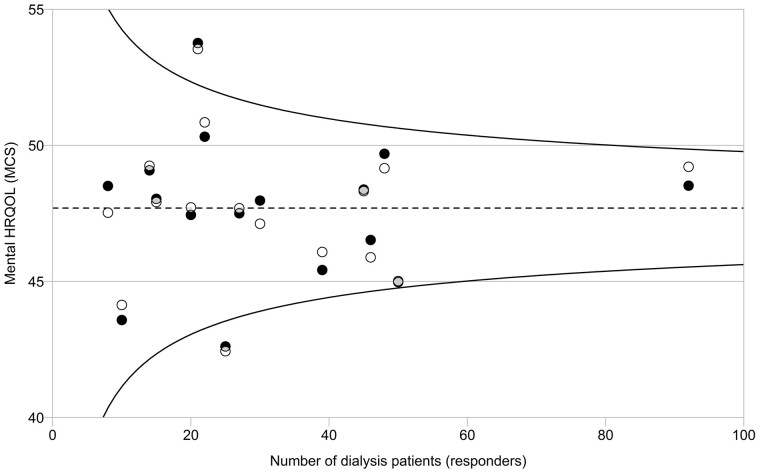
FIGURE 5.
Observed and adjusted mean mental HRQoL (MCS) in 16 pilot centres. Circles represent the mean observed (white circles) and adjusted (adjusted for sex, age, SES, primary kidney disease, dialysis modality and time on RRT; black circles) score for mental HRQoL per centre. Overlapping part of the circles is depicted in grey. The overall mean MCS (dotted line) is used as a reference in the comparison with each centre. The 95% confidence interval (CI; curved lines) is provided around the overall mean MCS. The mean scores of two centres are outside the 95% CI, one above and one below the funnel, indicating a statistically significant higher and lower MCS compared with the overall mean MCS, respectively.
Variance at the centre level
The part of the observed variance in symptom burden, PCS and MCS scores explained by differences among centres was 2.6% (P = 0.34), 1.0% (P = 0.64) and 1.5% (P = 0.45), respectively. The adjusted ICC was 3.1% (P = 0.32), 0.6% (P = 0.80) and 2.0% (P = 0.41), respectively.
Patient experiences and preferences
At 3 months, 214 patients (79%) indicated that they wanted to share and discuss their results on HRQoL and symptom burden with their clinician. In total, 71 individual reports were sent to professionals: five patients in each centre, unless fewer patients gave their consent at 3 months (e.g. one centre had no responders at 3 months). At 6 months, 16 patients from 10 different centres indicated that they had discussed the PROM scores and gave feedback on how they experienced the conversation. Patients discussed the results with a nephrologist (n = 11), a nurse (n = 8) and/or a social worker (n = 2). Patients rated the way in which results were discussed with a mean score of 3.8 (SD 0.8; score range: 1, poor 5, excellent). Professionals also appreciated discussing patients’ PROM scores and experienced it as insightful. Additionally, professionals indicated that their involvement is important for implementing PROMs into routine care. Moreover, response rates were highest in centres where professionals indicated that they had put a lot of effort into informing and inviting patients.
Eight patients participated in the focus group: seven patients were male, ages 33–78 years, six patients received haemodialysis and two patients received peritoneal dialysis. Five themes were discussed: ‘online tool’, ‘communication about content and purpose’, ‘benefits of using PROMs’, ‘feedback is crucial’ and ‘interpreting PROM scores’. Examples of corresponding quotations by patients are presented in Table 3. Overall, patients were satisfied with the content, length and structure of the PROMs and the online completion was mentioned as an advantage. Communication about the content and the purpose of PROMs was not always clear for patients. Additional information is needed when receiving the invitation and when completing PROMs. Furthermore, patients indicated that the use of PROMs can contribute to their treatment by providing insights for both the clinician and the patient. Additionally, it may enhance patient–clinician communication, as it offers guidance during and in preparation for the conversation. Patients indicated that the provision of individual feedback, written and oral, is crucial and that clinicians play an important role in this. Patients mentioned that individual feedback should be presented in a relevant context. They stressed the need for a reference score (e.g. average score of similar patients) to interpret their own results, not to compare their results.
Table 3.
Examples of corresponding quotations by eight patients receiving dialysis for the identified themes
| Themes | Illustrative quotations |
|---|---|
| Online tool | ‘When filling it [the questionnaire] in online, you can also save and keep track of changes [in PROM scores over time] yourself. This can be an advantage’. |
| Communication about content and purpose | ‘Titles like PROMs, DSI and SF-12 make no sense. Use clear terms that appeal to the patients, such as “symptom questionnaire” or “quality of life questionnaire”’. |
| Benefits of using PROMs | ‘The questionnaires can be used as a kind of checklist. To help you remember things … The questionnaires help to come up with ideas’. |
| ‘Questionnaires help patients in initiating conversations. Some subjects are difficult to discuss’. | |
| ‘You can adjust your treatment goal and plan according to these changes [in PROM scores] over time, and this can be discussed with your healthcare professional’. | |
| Feedback is crucial | ‘Getting feedback on the results [PROM scores] should be the basis of each PROMs measurement. After all, it is about your treatment’. |
| ‘Healthcare professionals have the important task to conduct the conversation well. Not every patient is outspoken and active enough [to express needs and experiences]’. | |
| Interpreting PROM scores | ‘It is nice to know what other kidney patients score, this gives some context…You want to know if a score of 46 is high, low or average’. |
| ‘I am not very interested in the average [PROM] score in my hospital… Hospital scores should be available for patients … and local patients advocate to address quality improvement’. |
DISCUSSION
This study describes the first experience with PROMs in Dutch routine dialysis care. Overall, response rates were low with high variability among centres. Patients receiving dialysis experienced a high symptom burden and a decreased HRQoL. With regard to these PROM scores, no centre effect could be observed. Patients believed that discussing HRQoL and symptom burden scores with their healthcare professional was highly insightful and valuable. Individual feedback on PROM scores was considered crucial.
This is the first study presenting results on HRQoL and symptom burden in the Dutch routine dialysis care setting. Patients receiving dialysis experienced a decreased physical HRQoL, with an average score of 36 compared with 51 in the general Dutch population (ages 60–69 years) [40]. The Mental HRQoL was comparable to that of the general Dutch population [40]. The substantial symptom burden found is comparable to the literature as well [3, 23, 30]. In line with a recent study [41], this study shows that the most common symptoms are not necessarily the ones that bother patients the most. The importance of certain symptoms may be different for each patient. Therefore it is important to monitor and discuss the presence and burden of symptoms to understand what is most important to each patient. Further research is needed on how individual PROM scores can best be used to address their needs.
Patients and professionals were very positive about the use of PROMs and they considered the provision of individual feedback to be crucial. These first results are promising and imply that PROMs are suitable for use at the individual level. The number of patients [n = 16 (23%)] who indicated they discussed their PROM scores seems low, but is proportionally similar compared with the response rate at 6 months. Moreover, the real number of patients who discussed their PROM scores is probably higher, for instance, because they discussed their report after the third time point. Since all patients and professionals who discussed the PROM scores highly appreciated the conversation, we decided to send the individual reports of the remaining patients (who gave consent) to their professionals and to include individual PROM reports in the electronic registration system.
Results from the focus group suggest that PROMs can provide insights into a patient's health and needs, improve patient–professional communication and increase shared decision making. Similar potential benefits of PROMs are described in the literature [9–13]; however, there is a paucity of evidence on whether and how the use of PROMs actually leads to improvements in patients’ outcomes. There are some studies suggesting that using PROMs will lead to better outcomes; for example, a randomized controlled trial in routine cancer care showed that web-based symptom monitoring resulted in improved HRQoL after 6 months, fewer hospital admissions and better 1-year survival even though no specific guidance was provided to professionals on how to respond to reported symptoms [42]. Scholars also argue that patients receiving dialysis expect improvements when using PROMs—for instance, improved symptom experience as a consequence of improved patient–professional communication about symptoms [41]. However, further research is needed to investigate whether and how the use of PROMs leads to long-term improvements in healthcare quality and outcomes in patients receiving dialysis.
The low response rate in this study is similar to the response rate (31%) of the Scottish Renal Registry when first introducing PROMs, confirming that it is challenging to incorporate PROMs into routine care [18]. Several factors may explain our results. First, professionals play an important role in informing and motivating patients. The highest response rates were observed in centres where professionals were highly engaged in the process. Therefore interventions to increase professionals’ engagement may be beneficial. Previous studies show that training and guidance on why and how to use PROMs and how to act in response to individual PROM scores may facilitate the uptake of PROMs by professionals [24, 43, 44]. In the Scottish registry, interventions to improve patient information letters and staff awareness resulted in an increase in their response rate to 48% [18]. Second, the process of inviting patients was regulated by each centre independently to promote incorporation into their workflow. A drawback of this approach may be that not every centre organized this in a structured way or had the desired facilities (e.g. availability of resources such as a process coordinator, printer and Internet access) and consequently some patients may not have been invited. Moreover, differences across centres existed with regard to the type and amount of support that patients received when filling in PROMs (e.g. availability of electronic devices in centres), who provided support (e.g. medical staff or partner) and at which location (home or medical centre). It is possible that the centres’ support and the completing PROMs on site contributed to higher response rates. On the other hand, the availability or lack of support in centres could also have influenced patients’ responses. However, we did not observe differences among centres with regard to PROs. Third, some patients are more likely to participate than others. In line with the literature, we found that older patients with a higher SES [18] and male [45] patients were more likely to participate. Further research focussing on non-responders is needed to gain more insights into barriers and potential facilitators for participation to implement recruitment strategies tailored to these more difficult-to-reach patients [46, 47].
Higher response rates are needed for optimal use of PROMs at the patient level (e.g. individualized prognosis) and aggregated level (e.g. evaluation of healthcare quality) [9]. Based on this study and the literature, we provide the following recommendations to increase the response rate. First, provide additional training and support to increase the engagement of healthcare professionals and to reinforce the professionals’ feeling of being comfortable and able to handle PROM scores [48, 49]. Second, recruitment strategies should be improved and, given that dialysis patients regularly encounter healthcare professionals, recruitment strategies should particularly focus on tailored communication (e.g. on personal relevance and confidentiality) and support (e.g. completing PROMs online) [25]. Third, communication between stakeholders should be improved, for instance, by supportive resources such as the provision of material to inform patients, individualized reports on PROM scores and updates on centres’ experiences (best practices), response rates and outcomes [25, 48]. Fourth, logistics should be further developed to improve response rates and to support professionals and patients in using PROMs in clinical practice; for example, provide individual reports directly after PROMs completion, incorporate PROMs into the electronic health record and send automated invitations (e.g. prior to patient’s upcoming consultation visit [24]) and reminders to complete PROMs [50]. Finally, we propose to assess and discuss PROM scores twice per year, as we believe this provides insight into the patient’s outcomes over time with minimal burden to patients and professionals. Some centres suggested using PROMs during a more extended consultation, such as an annual check-up, to discuss PRO progression over time, patient needs and treatment goals.
The low response rate and selective response are important results but also limitations in this study. For instance, our results suggest that there is no relevant centre effect on patients’ HRQoL and symptom burden; however, possibly real centre effects could not be detected due to low and selective responses. Furthermore, responders are likely to be more health conscious and involved in healthcare compared with non-responders (i.e. healthy responder bias). For example, the patients who shared their experiences about discussing PROM scores may be more involved and may have a more positive attitude towards using PROMs in clinical practice, which should be taken into account when interpreting the results. Additionally, the selective response may have led to effect underestimation of patients’ outcomes: symptom burden is likely to be higher and HRQoL lower in the total dialysis population. However, information about non-responders was also presented and can therefore be taken into account when interpreting the results.
Although current data may be insufficient to evaluate healthcare quality, the electronic registration of PROMs as part of Renine is designed in such a way that future data may be used for this purpose [9]. We believe that it is a major strength that PROMs can be used both at the individual level in clinical practice and at the aggregated level to evaluate healthcare quality. Possibly this combination is crucial, as the use of PROMs for individual patient’s treatment may be the most important factor in reaching sufficient response rates to enable evaluation of healthcare quality.
Another strength is the multicentre study design and methods used in this study. With 16 participating centres, a substantial sample of all Dutch dialysis patients was included. Additionally, by leaving centres free to incorporate PROMs into their workflow, a broad variation of in-centre processes was included, which may provide valuable information (e.g. insights into best practices for using PROMs in clinical settings) and may eventually promote adaptation and implementation of PROMs into clinical practice (e.g. due to limited workflow disruptions and research processes that are in line with the priorities of patients and professionals) [13, 24, 25]. Moreover, all relevant stakeholders (e.g. patients, healthcare professionals and researchers) were involved from the start, resulting in widely supported PROMs that fit clinical practice and research [13, 24, 25]. Also, during the developmental phase, much attention was paid to the electronic registration system and the selection of valid questionnaires [23, 24]. The pilot study confirms that the questionnaires were suitable and feasible, with only minor suggestions for improvement. Finally, by making use of both quantitative and qualitative methods, we obtained a broad picture of perceived benefits and barriers for implementing PROMs into nephrological care and possibilities for improvement.
In conclusion, the first results from the Dutch registry of PROMs in patients receiving dialysis showed low response rates with high centre variability. Achieving higher response rates is challenging and requires extra encouragement of patients and professionals. Patients experienced a high symptom burden and a decreased physical HRQoL. Discussing symptom and HRQoL results was greatly appreciated and is considered crucial for the use of PROMs in routine care. Further research is needed to investigate how the collection and use of PROMs can be successfully integrated into routine care to improve healthcare quality and outcomes.
ACKNOWLEDGEMENTS
The authors thank K. Prantl, the NVN and all patients for their contributions to and participation in the focus group. The authors are grateful to the centres, patients and healthcare professionals for their contributions. We also thank B.B.L. Penning de Vries for statistical support. Finally, the authors gratefully acknowledge Nefrovisie for the facilitation and data management of this study. Ethics approval and consent to participate was according to national guidelines and permission of the Medical Ethics Committee was not required since participants were not subjected to procedures or required to follow rules or behaviour [51]. Data handling was performed by Nefrovisie. All patients registered in Renine gave written permission to use their data for research purposes and a statement on privacy and confidentiality was included in the PROMs invitation letter.
FUNDING
This study was supported by unrestricted grants from Nierstichting Nederland (A1D1P04), Patiëntenfederatie Nederland and Zorgverzekeraars Nederland. The funding organizations had no role in the study design; collection, analysis and interpretation of the data; writing the report and the decision to submit the report for publication.
AUTHORS’ CONTRIBUTIONS
E.M.v.d.W., M.H.H., H.A.J.B., F.J.v.I., F.W.D. and Y.M. were responsible for the research idea and study design. M.H.H., H.A.J.B., J.M.H.-v.d.A. and F.W.D. were responsible for data acquisition. E.M.v.d.W., F.W.D. and Y.M. were responsible for data analysis. All authors were responsible for data interpretation. W.J.W.B., F.W.D. and Y.M. provided supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
There are no financial or other conflicts of interest to declare. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Mittal SK, Ahern L, Flaster E et al. Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant 2001; 16: 1387–1394 [DOI] [PubMed] [Google Scholar]
- 2. Raj R, Ahuja KD, Frandsen M et al. Symptoms and their recognition in adult haemodialysis patients: interactions with quality of life. Nephrology (Carlton ) 2017; 22: 228–233 [DOI] [PubMed] [Google Scholar]
- 3. Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 2013; 39: 140–150 [DOI] [PubMed] [Google Scholar]
- 4. Weisbord SD, Fried LF, Mor MK et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 2007; 2: 960–967 [DOI] [PubMed] [Google Scholar]
- 5. Claxton RN, Blackhall L, Weisbord SD et al. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage 2010; 39: 211–218 [DOI] [PubMed] [Google Scholar]
- 6. Pugh-Clarke K, Read SC, Sim J. Symptom experience in non-dialysis-dependent chronic kidney disease: a qualitative descriptive study. J Ren Care 2017; 43: 197–208 [DOI] [PubMed] [Google Scholar]
- 7. Feldman R, Berman N, Reid MC et al. Improving symptom management in hemodialysis patients: identifying barriers and future directions. J Palliat Med 2013; 16: 1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stephens RJ, Hopwood P, Girling DJ et al. Randomized trials with quality of life endpoints: are doctors' ratings of patients' physical symptoms interchangeable with patients' self-ratings? Qual Life Res 1997; 6: 225–236 [DOI] [PubMed] [Google Scholar]
- 9. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ et al. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q 2014; 92: 754–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bingham CO 3rd, Noonan VK, Auger C et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 4: patient-reported outcomes can inform clinical decision making in chronic care. J Clin Epidemiol 2017; 89: 136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Catarinella FS, Peters D, de Roos K-P et al. Praktische handvatten voor het gebruik van patient-reported outcome measures (PROMs). Nederlands Tijdschrift Voor Heelkunde 2017; 26: 29–33 [Google Scholar]
- 12. Calvert M, Kyte D, Price G et al. Maximising the impact of patient reported outcome assessment for patients and society. BMJ 2019; 364: k5267. [DOI] [PubMed] [Google Scholar]
- 13. Anderson NE, Calvert M, Cockwell P et al. The use of patient-reported outcomes in patients treated with maintenance hemodialysis: a perspective. Am J Kidney Dis 2019; 74: 399–406 [DOI] [PubMed] [Google Scholar]
- 14. Urquhart-Secord R, Craig JC, Hemmelgarn B et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis 2016; 68: 444–454 [DOI] [PubMed] [Google Scholar]
- 15. Manns B, Hemmelgarn B, Lillie E et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014; 9: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verberne WR, Das-Gupta Z, Allegretti AS et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis 2019; 73: 372–384 [DOI] [PubMed] [Google Scholar]
- 17. van der Veer SN, Aresi G, Gair R. Incorporating patient-reported symptom assessments into routine care for people with chronic kidney disease. Clin Kidney J 2017; 10: 783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nimmo A, Bell S, Brunton C et al. Collection and determinants of patient reported outcome measures in haemodialysis patients in Scotland. QJM 2018; 111: 15–21 [DOI] [PubMed] [Google Scholar]
- 19. Evans K, Pyart R, Steenkamp R et al. UK Renal Registry 20th Annual Report: introduction. Nephron 2018; 139(Suppl 1): 1–12 [DOI] [PubMed] [Google Scholar]
- 20. Breckenridge K, Bekker HL, Gibbons E et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant 2015; 30: 1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biber J, Ose D, Reese J et al. Patient reported outcomes – experiences with implementation in a University Health Care setting. J Patient Rep Outcomes 2018; 2: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noonan VK, Lyddiatt A, Ware P et al. Montreal Accord on patient-reported outcomes (PROs) use series – paper 3: patient-reported outcomes can facilitate shared decision-making and guide self-management. J Clin Epidemiol 2017; 89: 125–135 [DOI] [PubMed] [Google Scholar]
- 23. van der Willik EM, Meuleman Y, Prantl K et al. Patient-reported outcome measures: selection of a valid questionnaire for routine symptom assessment in patients with advanced chronic kidney disease – a four-phase mixed methods study. BMC Nephrol 2019; 20: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foster A, Croot L, Brazier J et al. The facilitators and barriers to implementing patient reported outcome measures in organisations delivering health related services: a systematic review of reviews. J Patient Rep Outcomes 2018; 2: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flythe JE, Narendra JH, Hilliard T et al. Cultivating a research-ready dialysis community. J Am Soc Nephrol 2019; 30: 375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ware JE Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233 [DOI] [PubMed] [Google Scholar]
- 27. Loosman WL, Hoekstra T, van Dijk S et al. Short-Form 12 or Short-Form 36 to measure quality-of-life changes in dialysis patients? Nephrol Dial Transplant 2015; 30: 1170–1176 [DOI] [PubMed] [Google Scholar]
- 28. Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales, Boston: Health Institute, New England Medical Center, 1995 [Google Scholar]
- 29. Weisbord SD, Fried LF, Arnold RM et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage 2004; 27: 226–240 [DOI] [PubMed] [Google Scholar]
- 30. Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2016. Amsterdam: Amsterdam University Medical Center, 2018.
- 32.Statusscores 2016. 2018. https://www.scp.nl/Onderzoek/Lopend_onderzoek/A_Z_alle_lopende_onderzoeken/Statusscores/statusscores_2016 (10 December 2018, date last accessed).
- 33. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30: 377–399 [DOI] [PubMed] [Google Scholar]
- 34. Sterne JA, White IR, Carlin JB et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: b2393–b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moons KG, Donders RA, Stijnen T et al. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006; 59: 1092–1101 [DOI] [PubMed] [Google Scholar]
- 36. Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med 2005; 24: 1185–1202 [DOI] [PubMed] [Google Scholar]
- 37. Merlo J, Chaix B, Yang M et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: linking the statistical concept of clustering to the idea of contextual phenomenon. J Epidemiol Community Health 2005; 59: 443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merlo J, Yang M, Chaix B et al. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health 2005; 59: 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Dishoeck AM, Lingsma HF, Mackenbach JP et al. Random variation and rankability of hospitals using outcome indicators. BMJ Qual Saf 2011; 20: 869–874 [DOI] [PubMed] [Google Scholar]
- 40. Mols F, Pelle AJ, Kupper N. Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Qual Life Res 2009; 18: 403–414 [DOI] [PubMed] [Google Scholar]
- 41. Flythe JE, Hilliard T, Castillo G et al. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin J Am Soc Nephrol 2018; 13: 735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34: 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bausewein C, Simon ST, Benalia H et al. Implementing patient reported outcome measures (PROMs) in palliative care – users' cry for help. Health Qual Life Outcomes 2011; 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf 2014; 23: 508–518 [DOI] [PubMed] [Google Scholar]
- 45. Stengel B, Metzger M, Combe C et al. Risk profile, quality of life and care of patients with moderate and advanced CKD: the French CKD-REIN Cohort Study. Nephrol Dial Transplant 2019; 34: 277–286 [DOI] [PubMed] [Google Scholar]
- 46. Thurman WA, Harrison TC. Reaching the “hard-to-reach”: recruitment of rural-dwelling adults with disabilities. J Transcult Nurs 2019; doi:10.1177/1043659619856667 [DOI] [PubMed] [Google Scholar]
- 47. van der Waerden JE, Hoefnagels C, Jansen MW et al. Exploring recruitment, willingness to participate, and retention of low-SES women in stress and depression prevention. BMC Public Health 2010; 10: 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang R, Burgess ER, Reddy MC et al. Provider perspectives on the integration of patient-reported outcomes in an electronic health record. JAMIA Open 2019; 2: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hardee JT, Rehring TF, Cassara JE et al. Effect and durability of an in-depth training course on physician communication skills. Perm J 2019; 23: 18–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nakash RA, Hutton JL, Jorstad-Stein EC et al. Maximising response to postal questionnaires – a systematic review of randomised trials in health research. BMC Med Res Methodol 2006; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Central Committee on Research Involving Human Subjects. Behavioural research and the WMO. In: Manual for the review of medical research involving human subjects. The Hague: Central Committee on Research Involving Human Subjects, 2002. https://english.ccmo.nl/investigators/publications/publications/2002/01/01/ccmo-memorandum-behavioural-research (June 2019, date last accessed).