Abstract
Background
We previously reported elsewhere of a follicular lymphoma patient suffering from persistent COVID-19 pneumonia that was still ongoing at 2 months after onset.
Materials and Methods
We provide a follow-up report of the case along with a literature review of immunocompromised lymphoma patients experiencing prolonged COVID-19 infections.
Results
Although requiring a full 1 year, the presented case eventually achieved spontaneous resolution of COVID-19 pneumonia. Anti-SARS-CoV-2 antibodies could not be detected throughout the disease course, but COVID-19-directed T-cell response was found to be intact. The patient also developed secondary immune thrombocytopenia subsequent to COVID-19 pneumonia. We found 19 case reports of immunocompromised lymphoma patients with prolonged COVID-19 infections in the literature. All 5 patients who died did not receive convalescent plasma therapy, whereas resolution of COVID-19 infection was achieved in 8 out of 9 patients who received convalescent plasma therapy.
Conclusions
We demonstrate through the presented case that while time-consuming, resolution of COVID-19 infections may be achieved without aid from humoral immunity if cellular immunity is intact. Immunocompromised lymphoma patients are at risk of a prolonged disease course of COVID-19, and convalescent plasma therapy may be a promising approach in such patients.
Keywords: Anti-sars-cov-2 antibodies, Chemotherapy, Coronavirus, Convalescent plasma, Itp
Microabstract
Persisting clinical infections and prolonged viral shedding of COVID-19 in immunocompromised patients is a rapidly emerging issue of concern. Persisting COVID-19 infections not only threaten the well-being of the patient, but may also be a potential long-term contagious threat to the environment, and special care must be provided concerning in-hospital isolation and self-quarantine periods. We report an immunocompromised lymphoma patient recovering from a 1-year disease course of COVID-19 pneumonia along with a review of the literature.
Introduction
We previously reported a follicular lymphoma patient on rituximab maintenance therapy suffering from persistent COVID-19 pneumonia that was still active at 2 months after disease onset.1 This is a follow-up report describing the resolution after a 1 year disease course of COVID-19 pneumonia and subsequent development of COVID-19-associated secondary immune thrombocytopenia (ITP). A literature review of 19 case reports concerning prolonged COVID-19 infections in immunocompromised lymphoma patients is also provided.
Materials and methods
This study was conducted in accordance with the Declaration of Helsinki, and the patient gave written informed consent. CD4+ and CD8+ T-cell responses against SARS-CoV-2 antigens were assessed by using an activation-induced marker assay. Peripheral blood mononuclear cells (PBMCs) were cultured in the presence of peptide pools of spike protein (mixture of S, S+, and S1), membrane protein, and nucleocapsid of SARS-CoV-2, as well as cytomegalovirus pp65 protein (all from Miltenyi Biotec, Bergisch Gladbach, Germany). Twenty two hours later, PBMCs were stained with the Zombie Yellow Fixable Viability Kit (Biolegend, San Diego, CA, USA) and monoclonal antibodies against human cell-surface antigens including CD3, CD4, CD8, CD137 (4–1BB), OX40, and CD69 (all from Biolegend). Data were acquired on a FACS LSR Fortessa (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using Flowjo software (TreeStar Inc., Ashland, OR, USA). Antigen-reactive cells were identified based on the co-expression of two activation markers: OX40 and CD137 for CD4+ T-cells and CD69 and CD137 for CD8+ T-cells.
For the literature review, a MEDLINE database search through PubMed was carried out using key words “lymphoma” or “lymphocytic” in combination with either “prolonged COVID-19″ or “persisting COVID-19″. Only English reports were considered. The most recent PubMed search was conducted in May 2021. A total of 19 case reports including the current case of prolonged COVID-19 infections were extracted and summarized in Table 1 . Days, weeks, and months were calculated from day of COVID-19 symptom onset. Persistence of COVID-19 infection was defined if the patient had ongoing COVID-19-related symptoms or lung lesions. A prolonged COVID-19 infection was defined if COVID-19 symptoms or lung lesions persisted for more than 20 days.
Table 1.
Cases of persistent and prolonged COVID-19 infections occurring in lymphoma patients.
| Case Number | Reporter, Year | Sex | Age | Lymphoma type | Disease Status of Lymphoma | Type of Most Recent Chemotherapy | Time From Last Therapy to COVID-19 Infection | Persistence of COVID-19 Infection | Treatment for COVID-19 | Development of anti-SARS-CoV-2 Antibodies | Outcome / Last Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tepasse et al., 2020 | M | 65 | DLBCL | N/A | R-DeVIC | 2 weeks | 23 days from onset | invasive ventilation | N/A | died of COVID-19 at 23 days from onset |
| 2 | Tepasse et al., 2020 | M | 66 | MCL | CR | maintenance rituximab + ibrutinib | 2 weeks | 30 days from onset | invasive ventilation | N/A | died of COVID-19 at 30 days from onset |
| 3 | Karataş et al., 2020 | M | 61 | PTCL | PR | ASCT with BEAM conditioning | 100 days from ASCT | > 85 days from onset | hydroxychloroquine, azithromycin, convalescent plasma | N/A | alive with active COVID-19 symptoms for >85 days |
| 4 | Clark et al., 2020 | F | 76 | MZL | N/A | BR | 10 days | 86 days from onset | lopinavir/ritonavir, steroids, convalescent plasma | no | complete recovery at 86 days from onset |
| 5 | Helleberg et al., 2020 | M | 50s | CLL | CR | R-FC | 3 months | 60 days from onset | remdesivir, convalescent plasma | no | alive in good general health 65 days from onset |
| 6 | Kos et al., 2020 | M | 72 | NMZL | CR | maintenance rituximab | 8 months | 31 days from onset | IVIG 25 g/day for 5 days | N/A | improved clinical condition at 31 days from onset |
| 7 | Moore et al., 2020 | F | 63 | NHL | remission | maintenance obinutuzumab | 37 days | approximately 90 days from onset | convalescent plasma | no | asymptomatic at approximately 97 days from onset |
| 8 | Baang et al., 2020 | M | 60 | MCL | refractory | CD20 bispecific antibody + another B-cell directed antibody + CPA + DXR + PSL |
during chemotherapy | 131 days from onset | remdesivir, convalescent plasma | probably no | afebrile with improved chest radiograph at 156 days from onset |
| 9 | Malsy et al., 2020 | F | 53 | FL | N/A | maintenance obinutuzumab | approximately 2 months | approximately 130 days from onset | remdesivir, convalescent plasma | no | asymptomatic at approximately 140 days from onset |
| 10 | Otsuka et al., 2020 | M | 56 | MCL | N/A | BR | 17 days | 42 days from onset | favipiravir, ciclesonide, hydroxychloroquine, IVIG | N/A | died of COVID-19 at 42 days from onset |
| 11 | Nakajima et al., 2020 | M | 47 | FL | CR | maintenance obinutuzumab | 2 months | approximately 65 days from onset | favipiravir, ciclesonide, lopinavir/ritonavir | no | nasopharyngeal swab PCR remained negative on day 82 from onset |
| 12 | Camprubí et al., 2021 | F | 37 | FL | PR | R-ESHAP | approximately 1 month | 63 days from onset | lopinavir/ritonavir, hydroxychloroquine, azithromycin, anakinra, remdesivir, steroids, darunavir/cobicistat, IVIG | no | resolution of fever at 63 days from onset |
| 13 | Honjo et al., 2021 | F | 72 | CLL | N/A | obinutuzumab | 23 days | 59 days from onset | hydroxychloroquine, convalescent plasma | no | asymptomatic at 119 days from onset |
| 14 | Puzyrenko et al., 2021 | M | 49 | CLL | N/A | N/A | N/A | 3 months from onset | steroids, ruxolitinib | N/A | died of COVID-19 at 3 months from onset |
| 15 | Reuken et al., 2021 | F | 56 | FL | CR | maintenance rituximab | 12 days | 6 months from onset | invasive ventilation, remdesivir, convalescent plasma, infliximab | no | good general condition and resolution of pulmonary lesions at 7.5 months from onset |
| 16 | Rnjak et al., 2021 | M | 53 | DLBCL | N/A | maintenance rituximab | 5 weeks | 115 days from onset | convalescent plasma, remdesivir, steroids | no | afebrile with regression of pneumonia at 129 days from onset |
| 17 | Hoffmann et al., 2021 | M | 75 | DLBCL | N/A | R-CHOP | during chemotherapy | 3 weeks from onset | N/A | N/A | died of COVID-19 at 3 weeks from onset |
| 18 | Fujii et al., 2021 | M | 43 | cHL | favorable | A+AVD | 17 days | 28 days from onset | favipiravir, ciclesonide, remdesivir | N/A | no recurrence of symptoms after 29 days from onset |
| 19 | presented case | F | 61 | FL | CR | maintenance rituximab | 11 weeks | 12 months from onset | clinical trial | no | asymptomatic after 10 months and resolution of lung lesions at 12 months |
Abbreviations: A + AVD = brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine; ASCT = autologous stem cell transplantation; BEAM = carmustine, etoposide, cytarabine, and melphalan; BR = bendamustine and rituximab; cHL = classic Hodgkin lymphoma; CLL = chronic lymphocytic leukemia; CPA = cyclophosphamide; CR = complete response; DLBCL = diffuse large B-cell lymphoma; DXR = doxorubicin; F = female; FL = follicular lymphoma; IVIG = intravenous immunoglobulin; M = male; MCL = mantle cell lymphoma; MZL = marginal zone lymphoma; N/A = not available; NHL = non-Hodgkin lymphoma; NMZL = nodal marginal zone lymphoma; PR = partial response; PSL = prednisolone; PTCL = peripheral T-cell lymphoma; R-CHOP = rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone; R-DeVIC = rituximab, dexamethasone, etoposide, ifosfamide, and carboplatin; R-ESHAP = rituximab, etoposide, cisplatin, cytarabine, and methylprednisolone; R-FC = rituximab, fludarabine, and cyclophosphamide.
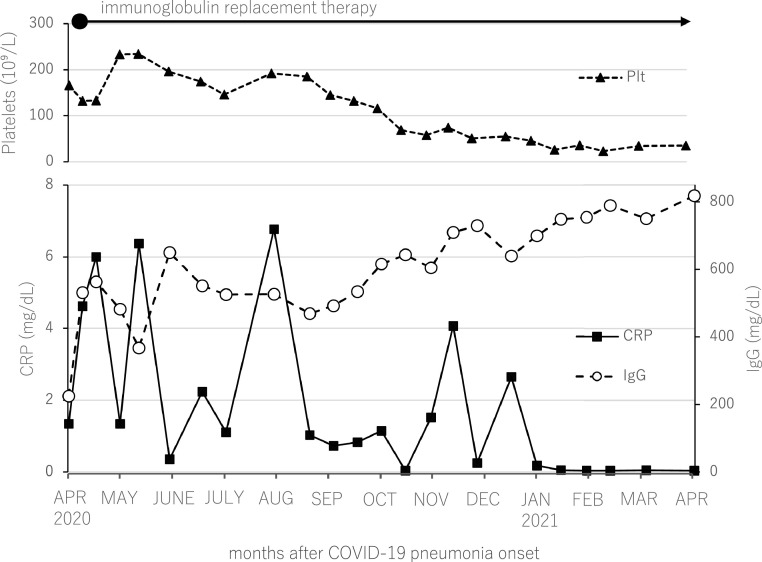
Case report
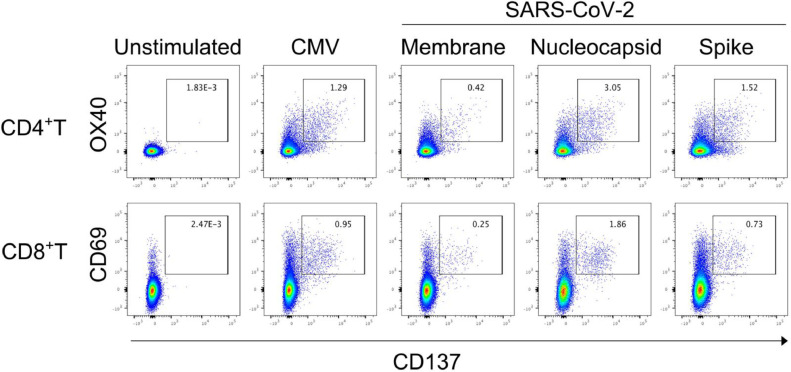
A 61 year-old woman developed COVID-19 pneumonia during rituximab maintenance therapy for follicular lymphoma in April 2020. She suffered from an abnormally prolonged COVID-19 disease course, which was thought to be the result of failure to develop anti-SARS-CoV-2 antibodies as previously reported.1 She was discharged on day 66 of hospitalization, but the lung lesions persisted and continued to migrate on CT scans, and waxing and waning of clinical manifestations such as fever and cough continued. CRP levels also fluctuated in parallel with the clinical manifestations. Because of ongoing COVID-19 symptoms, self-quarantine was continued. It was not until February 2021 that her clinical manifestations finally resolved and CRP negativity became sustained, approximately 10 months after onset of COVID-19 pneumonia (Figure 1 ). Lung lesions on CT scans were still apparent in February 2021, but almost completely diminished upon May 2021 CT scans, approximately one year from COVID-19 pneumonia onset. Periodic blood tests for anti-SARS-CoV-2 IgG and IgM antibodies performed in April, May, July, and September of 2020, and blood tests for anti-SARS-CoV-2 IgG antibodies performed in October and December of 2020, and January and May of 2021, all resulted negative (Elecsys Anti-SARS-CoV-2 immunoassay, Roche Diagnostics GmbH, Mannheim). CD4+ and CD8+ T-cell responses against SARS-CoV-2 antigens were assessed with an activation-induced marker assay using patient PBMCs collected in April 2021. Upon stimulation with CMV pp65, a positive control of the assay, 1.29% of CD4+ and 0.95% of CD8+ T-cells were double-positive for activation markers CD137 and OX40, and CD137 and CD69, respectively (Figure 2 ). When stimulated with structural proteins of SARS-CoV-2 (membrane, nucleocapsid, and spike), robust antigen-specific T-cell responses were observed among both CD4+ and CD8+ T-cells at similar or higher levels compared to previous reports of COVID-19 convalescent individuals (Figure 2).2 , 3 These results indicated that T-cell responses against SARS-CoV-2 antigens were intact in the patient.
Figure 1.
Clinical course of prolonged COVID-19 infection and development of secondary immune thrombocytopenia in an immunocompromised lymphoma patient.
Figure 2.
SARS-CoV-2-reactive T-cell responses
Peripheral blood mononuclear cells were stimulated with peptide pools of cytomegalovirus pp65(CMV), structural proteins of SARS-CoV-2 (membrane, nucleocapsid, and spike). Twenty-two hours later, antigen-specific T-cell activation was analyzed by flow cytometry. Dot plots show activated CD4+ T and CD8+ T-cells identified as CD137+OX40+and CD137+CD69+ cells, respectively.
Repetitive immunoglobulin replacement therapy was continued in the patient, and IgG levels were maintained at around 500–800 mg/dl. Although the COVID-19 pneumonia resolved, the patient's platelet counts started to decrease around September 2020, and subsequently plateaued at around 30 × 109/L as of February 2021. Bone marrow evaluation along with other screening tests were carried out in February 2021, but causes of thrombocytopenia including recurrence of follicular lymphoma could not be detected, and she was diagnosed with COVID-19-associated secondary ITP. Urea breath test was negative, and thus Helicobacter pylori infection could not be confirmed. She is currently doing well as an outpatient, and the ITP is being observed with no treatment.
Discussion and literature review
We describe the disease course of COVID-19 pneumonia requiring an astonishing 1 year for recovery in a follicular lymphoma patient undergoing preceding anti-CD20 antibody therapy. IgG levels continued to rise with time despite that the dose and frequency of immunoglobulin replacement therapy was fixed as of September 2020, implying a recovering humoral immunity. However, the patient failed to develop anti-SARS-CoV-2 IgG and IgM antibodies throughout the disease course. Furthermore, up until March 2021, we tested for anti-SARS-CoV-2 IgG and IgM antibodies within the immunoglobulin preparations used for immunoglobulin replacement therapy whenever we encountered a new product lot, but all lots were found to be negative. Thus, the eventual recovery from COVID-19 pneumonia was most likely accomplished without aid from external anti-SARS-CoV-2 antibody supplementation and patient-derived humoral immunity. Also, COVID-19-directed treatment was carried out only for the first 2 weeks according to a clinical trial to which she failed to respond, and thus the eventual recovery 1 year later was accomplished spontaneously.
We observed robust SARS-CoV-2-specific T-cell responses in PBMCs of the patient collected 1 year after diagnosis of COVID-19. The percentages of spike or nucleocapsid-specific CD4+ T-cells were even higher than that of CMV-specific CD4+ T-cells (positive control). It can be speculated that strong SARS-CoV-2-specific T-cell responses were generated because of the chronic exposure to the virus, and that cellular immunity most probably played an important role in the clearance of the virus.
Persisting clinical infection and prolonged viral shedding of COVID-19 in immunocompromised patients is a rapidly emerging issue of concern. Including the presented case, 19 case reports of prolonged COVID-19 infections occurring in immunocompromised lymphoma patients have been reported and are summarized in Table 1.1 , 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Median persistence of COVID-19 infections was 65 days (range: 3 weeks to 12 months), and median time from last lymphoma therapy to COVID-19 onset was 26.5 days (range: during chemotherapy to 8 months). Anti-CD20 antibody therapy was included in most recent lymphoma therapy in 16 out of 19 patients. 5 patients died, 1 patient was alive with active COVID-19 symptoms, and 13 patients eventually recovered from COVID-19 infections. All 11 patients studied failed to develop anti-SARS-CoV-2 antibodies. Although it was argued by Baang et al. whether seroconversion occurred in case 8 because transient positivity for anti-SARS-CoV-2 antibodies was observed, the patient was only positive after convalescent plasma administration and negative on other occasions, and therefore these observations most likely do not imply seroconversion but a decay of antibodies administered in the convalescent plasma. A total of 9 patients were treated with convalescent plasma, of which subsequent resolution of COVID-19 infection was observed in 8 patients. Convalescent plasma therapy was not described in all 5 patients that died (case 1, 2, 10, 14, and 17).
Besides the 19 case reports studied in this report, there are multiple case series reporting on immunocompromised lymphoma patients suffering from prolonged COVID-19 symptoms.21, 22, 23 Duléry et al. reported that 32 out of 111 lymphoma patients admitted for COVID-19 underwent prolonged in-hospital stay, with a median length of stay of 58 days (range: 31–235 days). Within 12 months prior to hospitalization for COVID-19, 26 patients (81%) had received treatment for lymphoma, and all patients treated received anti-CD20 monoclonal antibody therapy. Only 2 out of 19 patients studied attained anti-SARS-CoV-2 IgG-IgM positive serology. Convalescent plasma was administered to 9 patients, and 8 patients subsequently recovered from COVID-19 symptoms.21 Hueso et al. also reported 17 B-cell depleted patients including 15 lymphoma patients who presented with prolonged COVID-19 symptoms lasting for a median of 56 days. All 17 patients failed to develop anti-SARS-CoV-2 antibodies. All 17 patients received convalescent plasma therapy, and all but 1 patient recovered with improvement of fever within 48 hours.22 Betrains et al. reported 5 lymphoma patients with previous rituximab therapy suffering from prolonged COVID-19 symptoms. All 5 patients failed to develop anti-SARS-CoV-2 antibodies. All 5 patients were treated with convalescent plasma therapy (median time to convalescent plasma therapy was 56 days), and a clinical response was seen in 4 patients.23
In the general population, seroconversion occurs in the majority of COVID-19 infected patients, and anti-SARS-CoV-2 IgG are detected in more than 90% of patients after day 15.24 The vast majority of immunocompromised lymphoma patients found in our literature review of case reports and case series could not mount a COVID-19-targeted humoral immune response and failed to develop anti-SARS-CoV-2 antibodies. In such patients, it is rational to speculate that convalescent plasma therapy providing the neutralizing anti-SARS-CoV-2 antibodies necessary for viral clearance is an extremely efficacious treatment approach, and the positive outcomes of reported cases support this theory. Although our case report demonstrated that recovery from COVID-19 is possible without aid from humoral immunity, the patient suffered from a 1 year disease course of COVID-19 pneumonia, and convalescent plasma therapy should be vigorously sought in such patients.
Another interesting aspect of the presented case is the development of ITP. ITP is a disorder in which platelet destruction and impaired platelet production occurs due to pathogenic autoantibodies directed towards platelets and megakaryocytes. As is the case with many viral infections, COVID-19 has also been reported to trigger ITP, and one case has been reported to develop persistent ITP ongoing for more than 7 months.25, 26, 27 The situation found in our patient is perplexing, because despite failure to develop anti-SARS-CoV-2 antibodies, in theory, the patient managed to develop pathogenic autoantibodies causing ITP.
Conclusion
In conclusion, we report an immunocompromised lymphoma patient suffering from a prolonged COVID-19 infection that required a full 1 year for recovery. The patient failed to develop anti-SARS-CoV-2 antibodies throughout the disease course, but a robust SARS-CoV-2-specific T-cell response was confirmable. Thus, we demonstrate that even with an impaired humoral immunity, an intact cellular immunity alone was capable of overcoming COVID-19 infection. However, cases failing to accomplish seroconversion are at risk of persistent COVID-19 infections, and as our literature review shows, administration of convalescent plasma early in the disease course may be a rational and effective treatment approach for such patients. Prolonged COVID-19 infections occurring in the immunocompromised not only threaten the well-being of the patient, but may also be a potential long-term contagious threat to others, and special care must be provided concerning in-hospital isolation and self-quarantine periods.
Clinical practice points
-
•
Immunocompromised lymphoma patients are at risk of abnormally prolonged COVID-19 infections.
-
•
Anti-SARS-CoV-2 antibodies are most often absent in such patients, and convalescent plasma therapy may be an effective treatment approach.
-
•
Persisting COVID-19 infections not only threaten the well-being of the patient, but may also be a potential long-term contagious threat to the environment, and special care must be provided concerning in-hospital isolation and self-quarantine periods.
Disclosure
The authors have stated that they have no conflicts of interest.
Acknowledgments
Writing the manuscript: HY, YM (author #2), AC
Data collection, analysis, and interpretation: JB, GM, and YM (author #6)
Literature research: HY, AC
Revising the manuscript: SM and NK
We also thank Takamasa Yamamoto (Department of Clinical Laboratory, Juntendo University Hospital, Tokyo, Japan) for carrying out anti-SARS-CoV-2 immunoassays.
References
- 1.Yasuda H., Tsukune Y., Watanabe N., et al. Persistent COVID-19 Pneumonia and Failure to Develop Anti-SARS-CoV-2 Antibodies During Rituximab Maintenance Therapy for Follicular Lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20:774–776. doi: 10.1016/j.clml.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.015. 1489–501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateus J., Grifoni A., Tarke A., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepasse P.R., Hafezi W., Lutz M., et al. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. 2020;190:185–188. doi: 10.1111/bjh.16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karataş A., İnkaya A., Demiroğlu H., et al. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apher Sci. 2020;59 doi: 10.1016/j.transci.2020.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark E., Guilpain P., Filip I.L., et al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol. 2020;190:e154–e1e6. doi: 10.1111/bjh.16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helleberg M., Niemann C.U., Moestrup K.S., et al. Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy. J Infect Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kos I., Balensiefer B., Roth S., et al. Prolonged Course of COVID-19-Associated Pneumonia in a B-Cell Depleted Patient After Rituximab. Front Oncol. 2020;10:1578. doi: 10.3389/fonc.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore J.L., Ganapathiraju P.V., Kurtz C.P., Wainscoat B. A 63-Year-Old Woman with a History of Non-Hodgkin Lymphoma with Persistent SARS-CoV-2 Infection Who Was Seronegative and Treated with Convalescent Plasma. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baang J.H., Smith C., Mirabelli C., et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis. 2021;223:23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malsy J., Veletzky L., Heide J., et al. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19. Clin Infect Dis. Oct 27 2020 doi: 10.1093/cid/ciaa1637. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otsuka Y., Kobayashi T. Case Report: a Patient with COVID-19 under Myelosuppression Induced by Chemotherapy. Am J Trop Med Hyg. 2020;103:1983–1985. doi: 10.4269/ajtmh.20-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima Y., Ogai A., Furukawa K., et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27:387–389. doi: 10.1016/j.jiac.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camprubí D., Gaya A., Marcos M.A., et al. Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir. Int J Infect Dis. 2021;104:379–381. doi: 10.1016/j.ijid.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honjo K., Russell R.M., Li R., et al. Convalescent plasma-mediated resolution of COVID-19 in a patient with humoral immunodeficiency. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2020.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puzyrenko A., Felix J.C., Sun Y., Rui H., Sheinin Y. Acute SARS-CoV-2 pneumonitis with cytotoxic CD8 positive T-lymphocytes: case report and review of the literature. Pathol Res Pract. 2021;220 doi: 10.1016/j.prp.2021.153380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuken P.A., Stallmach A., Pletz M.W., et al. Severe clinical relapse in an immunocompromised host with persistent SARS-CoV-2 infection. Leukemia. 2021;35:920–923. doi: 10.1038/s41375-021-01175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rnjak D., Ravlić S., Šola A.M., et al. COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus Clin Biol. 2021;28(3):264–270. doi: 10.1016/j.tracli.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M.S., Ganguly S. Delayed COVID-19 Respiratory Failure in Patients with Lymphoma on Rituximab-based Chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2021;21:e548–ee50. doi: 10.1016/j.clml.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii H., Tsuji T., Sugitani M., et al. Prolonged persistence of SARS-CoV-2 infection during A+AVD therapy for classical Hodgkin’s lymphoma: a case report. Curr Probl Cancer. Mar 24 2021 doi: 10.1016/j.currproblcancer.2021.100739. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duléry R., Lamure S., Delord M., et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am. J. Hematol. 2021;96(8):933–944. doi: 10.1002/ajh.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hueso T., Pouderoux C., Péré H., et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136:2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betrains A., Godinas L., Woei A.J.F., et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2021;192:1100–1105. doi: 10.1111/bjh.17266. [DOI] [PubMed] [Google Scholar]
- 24.Tuaillon E., Bollore K., Pisoni A., et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J Infect. 2020;81:e39–e45. doi: 10.1016/j.jinf.2020.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharjee S., Banerjee M. Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr Clin Med. Sep 19 2020:1–11. doi: 10.1007/s42399-020-00521-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kewan T., Gunaratne T.N., Mushtaq K., Alayan D., Daw H., Haddad A. Outcomes and management of immune thrombocytopenia secondary to COVID-19: cleveland clinic experience. Transfusion. 2021;61(7):2014–2018. doi: 10.1111/trf.16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa Y., Ando M., Azusawa Y., et al. Persistent immune thrombocytopaenic purpura associated with SARS-CoV-2 infection. eJHaem. May 4 2021 doi: 10.1002/jha2.201. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]