Abstract
Background:
Obesity increases with age, is disproportionately prevalent in Black populations, and is associated with heart failure with preserved ejection fraction (HFpEF). An “obesity paradox”, or improved survival with obesity, has been reported in patients with HFpEF.
Objective:
To examine whether racial differences exist in the temporal trends and outcomes associated with obesity among older patients with HFpEF.
Design:
Community surveillance of acute decompensated heart failure (ADHF) hospitalizations, sampled by stratified design from 2005–2014.
Setting:
Atherosclerosis Risk in Communities Study (NC, MS, MD, MN)
Participants:
A total of 10,147 weighted hospitalizations for ADHF (64% female, 74% White, mean age 77 years), with ejection fraction ≥50%.
Measurements:
ADHF classified by physician review, HFpEF defined by ejection fraction ≥50%. Body mass index (BMI) calculated from weight at hospital discharge. Obesity defined by BMI ≥ 30 kg/m2, class III obesity by BMI ≥ 40 kg/m2.
Results:
When aggregated across 2005–2014, the mean BMI was higher for Black compared to White patients (34 vs. 30 kg/m2; P <0.0001), as was prevalence of obesity (56% vs. 43%; P <0.0001) and class III obesity (24% vs. 13%; P <0.0001). Over time, the annual mean BMI and prevalence of class III obesity remained stable for Black patients, but steadily increased for White patients, with annual rates statistically differing by race (P-interaction =0.04 and P=0.03, respectively). For both races, a U-shaped adjusted mortality risk was observed across BMI categories, with the highest risk among patients with a BMI ≥40 kg/m2.
Conclusion:
Black patients were disproportionately burdened by obesity in this decade-long community surveillance of older hospitalized patients with HFpEF. However, temporal increases in mean BMI and class III obesity prevalence among White patients narrowed the racial difference in recent years. For both races, the worst survival was observed with class III obesity. Effective strategies are needed to manage obesity in patients with HFpEF.
Keywords: obesity, HFpEF, race, epidemiology, surveillance
Introduction
Heart failure with preserved ejection fraction (HFpEF) typically presents in advanced age and is accompanied by multiple comorbidities and chronic, systemic inflammation.1–3 Recognized as a true geriatric syndrome2, HFpEF is now the most common form of heart failure (HF),4–6 yet remains refractory to available pharmacotherapies.7,8 Obesity is a unique, modifiable risk factor for the development of HFpEF in older individuals,9,10 and is associated with worse hemodynamics, poor quality of life and higher risk of hospitalization.11–14 Despite its prognostic utility, our understanding of the obesity burden in older patients with HFpEF is limited. While a disproportionately high prevalence of obesity has been reported in Black patients with HFpEF,6,13 race-specific temporal trends have not been examined, nor is it known whether race-specific obesity prevalence has changed over time in HFpEF populations.
The ideal management of obesity in older patients with HFpEF is also uncertain. Obesity was long thought to be protective in older individuals, a hypothesis which was debunked in clinical trials randomizing older subjects to intentional weight loss.15,16 Although intentional weight loss has been associated with better hemodynamics and quality of life for patients with HFpEF,17,18 an “obesity paradox”, or better survival with obesity, has also been reported for patients with HFpEF. To gain a better understanding of the burden, race-specific temporal trends, and outcomes of obesity in patients with HFpEF, we analyzed a decade-long community surveillance of Black and White patients with HFpEF who were hospitalized with acute decompensated heart failure (ADHF).
Methods
The Atherosclerosis Risk in Community (ARIC) Study’s data and materials are publicly available to qualified investigators with an approved manuscript proposal and data use agreement. The data and analytic methods may be requested from the ARIC coordinating center and made available upon approval by the ARIC Publications Committee.
The ARIC Study Community Surveillance
From 2005–2014, the ARIC study conducted community surveillance of hospitalized ADHF in Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and Minneapolis, Minnesota. As previously reported in literature,19 hospitalizations were randomly sampled within pre-specified strata based on the ARIC community, discharge code (428.x or all other eligible codes), age (55–74, 75–84, or ≥85 years), sex, and race (Black or White) and reviewed by a physician panel. ADHF was differentiated from stable, chronic HF by evidence of new onset or worsening signs or symptoms of HF. Surveillance activities were approved by local institutional review boards (IRBs). Patient consent was not required for surveillance because personal identifiers were redacted from the analytic dataset. The present investigation was considered a secondary analysis of a deidentified dataset and was exempt from IRB review.
Data Abstraction
Demographic, clinical, and echocardiographic data were obtained from the medical record by certified abstractors following a standardized protocol. We defined obesity by a body mass index (BMI) ≥ 30 kg/m2 and class III obesity by a BMI ≥ 40 kg/m2, based on the weight at hospital discharge. Natriuretic peptide levels were abstracted from the record, based on availability in the hospital record. Most hospitalizations (71%) assayed B-type natriuretic peptide (BNP) rather than N-terminal pro hormone natriuretic peptide (NT-proBNP). Comorbid conditions were classified by presence or absence in the medical record. Ejection fraction was abstracted from in-hospital echocardiography reports and considered indicative of HFpEF when ≥50%. Left ventricular hypertrophy and pulmonary hypertension were considered present if classified as “moderate” or “severe” on the echocardiography report, while diastolic dysfunction and right ventricular dilation were classified by presence or absence on the echocardiography report.
Outcomes
Our primary outcomes of interest were annual mean BMI, annual prevalence of obesity, and annual prevalence of class III obesity among race-specific subgroups of patients hospitalized with acute decompensated HFpEF from 2005–2014. As a secondary outcome we examined all-cause mortality within 1 year of the hospitalization event. Mortality outcomes were ascertained by the ARIC study, by linking hospital records with the National Death Index.20
Statistical Analysis
All analyses were performed using SAS Survey Procedures 9.4 (SAS Institute; Cary, NC). Statistical tests and models accounted for the stratified sampling design and were weighted by the inverse of the sampling probability.21 Continuous variables were assessed for normality and compared using the difference in least square means from weighted linear regression. Categorical variables were compared using Rao-Scott χ2 tests. The annual mean BMI, obesity prevalence, and class III obesity prevalence among Black and White patients were plotted and compared visually, with trend lines fit by 2nd order polynomials. Significance of annual trends across 2005–2014 were analyzed using linear and logistic regression, respectively, with year of admission as the regressor. Racial comparisons of annual trends were analyzed by testing the multiplicative interaction between race and year of admission. Mortality risks within various BMI cutpoints (18.5 to <25, 25 to <30, 30 to <35, 35 to <40, and ≥40 kg/m2) were plotted and compared visually, to examine the shape of the distribution and determine the lowest-risk BMI category. Overall and race-stratified multivariable Cox regression models adjusted for demographics (age, race, sex, year of admission, hospital of admission), length of stay (a surrogate of hospitalization acuity), and comorbidities (current smoking, chronic obstructive pulmonary disease, hypertension, atrial fibrillation, coronary artery disease, and diabetes mellitus) were constructed to analyze the relationship between BMI categories and mortality, using the lowest-risk BMI category as the reference group. Modification of the mortality association by race was tested by the multiplicative interaction between race and BMI categories. Patients with a BMI <18.5 kg/m2 were excluded from mortality models because underweight BMI in this population likely reflects severe cachexia from chronic illness.
Several sensitivity analyses were conducted to assess the robustness of our observations. In the first sensitivity analysis, race-stratified temporal trends in mean BMI, obesity prevalence, and class III obesity prevalence were examined within the ARIC communities contributing substantial sample sizes of Black patients (Forsyth, NC and Jackson, MS). Following this, we examined race-stratified temporal trends separately for women and men. We also examined race-specific mortality hazards ratios associated with BMI categories, by stratifying the primary analysis mortality model by race. Additionally, we examined mortality hazard ratios for the entire study population by comparing BMI categories to a normal BMI reference group of 18.5 to <25 kg/m2. Finally, we examined the mortality hazard ratios from the primary model with additional adjustment for B-type natriuretic peptide, among the subset with available biomarker abstractions.
Results
A total of 23,409 hospitalizations were sampled from 2005 – 2014. Of these, 8,914 were classified as definite or probable ADHF with race identified as Black or White. After excluding admissions lacking in-hospital echocardiography, 5,542 remained. Of these, 2,250 were classified as HFpEF. After excluding patients missing BMI abstractions (N = 41) or mortality outcomes (N = 76), 2,133 remained, corresponding to 10,147 weighted events (Supplemental Figure S1). The presentation of all subsequent results reflects the weighted population.
The overall study population was predominantly female (64%) and White (74%) and on average, 77 years old. Nearly half were classified as obese (47%). Patients with obesity were younger, more often women and Black, and had a higher prevalence of comorbidities than patients without obesity (Table 1). B-type natriuretic peptide levels declined with increasing BMI but tended to be higher among Black patients (Figure 1). When aggregated across 2005–2014 and stratified by race, Black patients had a higher mean BMI than White patients (34 vs. 30 kg/m2; P <0.0001), and a greater prevalence of obesity (56% vs. 43%; P <0.0001) and class III obesity (24% vs. 13%; P <0.0001). The racial differences in prevalence of obesity and class III obesity were observed in all 4 ARIC communities; Supplemental Figure S2. The mean BMI increased over time for White patients (29 kg/m2 [2005–2009] to 31 kg/m2 [2010–2014]; P for annual trend = 0.0003), as did prevalence of obesity (37% [2005–2009] to 46% [2010–2014]; P for annual trend = 0.0009) and class III obesity (10% [2005–2009] to 14% [2010–2014]; P for annual trend = 0.04), Figure 2. However, little temporal change was noted in mean BMI for Black patients (34 kg/m2 in both 2005–2009 and 2010–2014; P for annual trend across 2005–2014 = 0.6), or obesity prevalence (56% in both time intervals; P for annual trend = 1.0), while the prevalence of class III obesity declined slightly during the study period (26% [2005–2009] to 22% [2010–2014], P for annual trend = 0.3), Figure 2. The rate of temporal increase in average BMI differed for White vs. Black patients (P for interaction by race = 0.04), as did the annual rate of increase in obesity prevalence (P for interaction by race = 0.06) and class III obesity prevalence (P for interaction by race = 0.03), Figure 2. Similar trends were observed when limiting the analysis to the ARIC communities with substantial sample sizes of Black patients (Forsyth County, NC and Jackson, MS, contributing 2407 Black patients); Supplemental Figure S3. When stratified by sex, the accelerated increase in annual obesity prevalence for White relative to Black patients was largely observed among female patients (P for interaction by race = 0.08), with no apparent racial differences in the annual rate of increase among male patients (P for interaction by race = 0.3), Supplemental Figure S4.
Table 1:
Demographics and clinical characteristics of patients hospitalized with acute decompensated heart failure with preserved ejection fraction, stratified by obesity. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014
| Obese N = 5427 | Non-Obese N = 4720 | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 73 ± 10 | 80 ± 11 | <0.0001 |
| Female | 3130 (66%) | 3336 (61%) | 0.03 |
| Black | 1502 (32%) | 1182 (22%) | <0.0001 |
| Health Insurance | 4618 (98%) | 5275 (97%) | 0.3 |
| Non-Cardiovascular | |||
| Body mass index (kg/m2) | 39 ± 9 | 24 ± 4 | |
| Current smoking | 537 (11%) | 589 (11%) | 0.7 |
| Diabetes mellitus | 2938 (62%) | 1869 (34%) | <0.0001 |
| Chronic kidney disease* | 2235 (66%) | 2571 (63%) | 0.3 |
| Chronic bronchitis / COPD | 1919 (41%) | 1835 (34%) | 0.002 |
| Sleep apnea | 1272 (27%) | 269 (5%) | <0.0001 |
| Anemia† | 2517 (54%) | 2770 (51%) | 0.4 |
| Thyroid disease | 986 (21%) | 1316 (24%) | 0.1 |
| Depression | 1115 (24%) | 966 (18%) | 0.003 |
| Cardiovascular | |||
| Myocardial infarction | 818 (17%) | 986 (18%) | 0.7 |
| Coronary artery disease | 2152 (46%) | 2585 (48%) | 0.4 |
| Peripheral artery disease | 518 (11%) | 546 (10%) | 0.5 |
| Hypertension | 4488 (95%) | 5017 (92%) | 0.02 |
| Atrial fibrillation | 1736 (37%) | 2133 (39%) | 0.3 |
| Stroke / TIA | 890 (19%) | 1077 (20%) | 0.6 |
| Hospital Visit | |||
| Length of stay (days) | 8.1 ± 14 | 8.7 ± 15 | 0.2 |
Values expressed as number (percentage) or mean ± standard deviation
Abbreviations: COPD = chronic obstructive pulmonary disease, TIA = transient ischemic attack
Chronic kidney disease defined by receipt of hemodialysis or an estimated glomerular filtration rate <45 mL/min/1.73m2 in patients with available serum creatinine assessments (2654 missing, no creatinine abstractions performed in 2014).
Anemia defined by a hemoglobin value <11 g/dL in patients with available laboratory values (74 missing)
Figure 1:
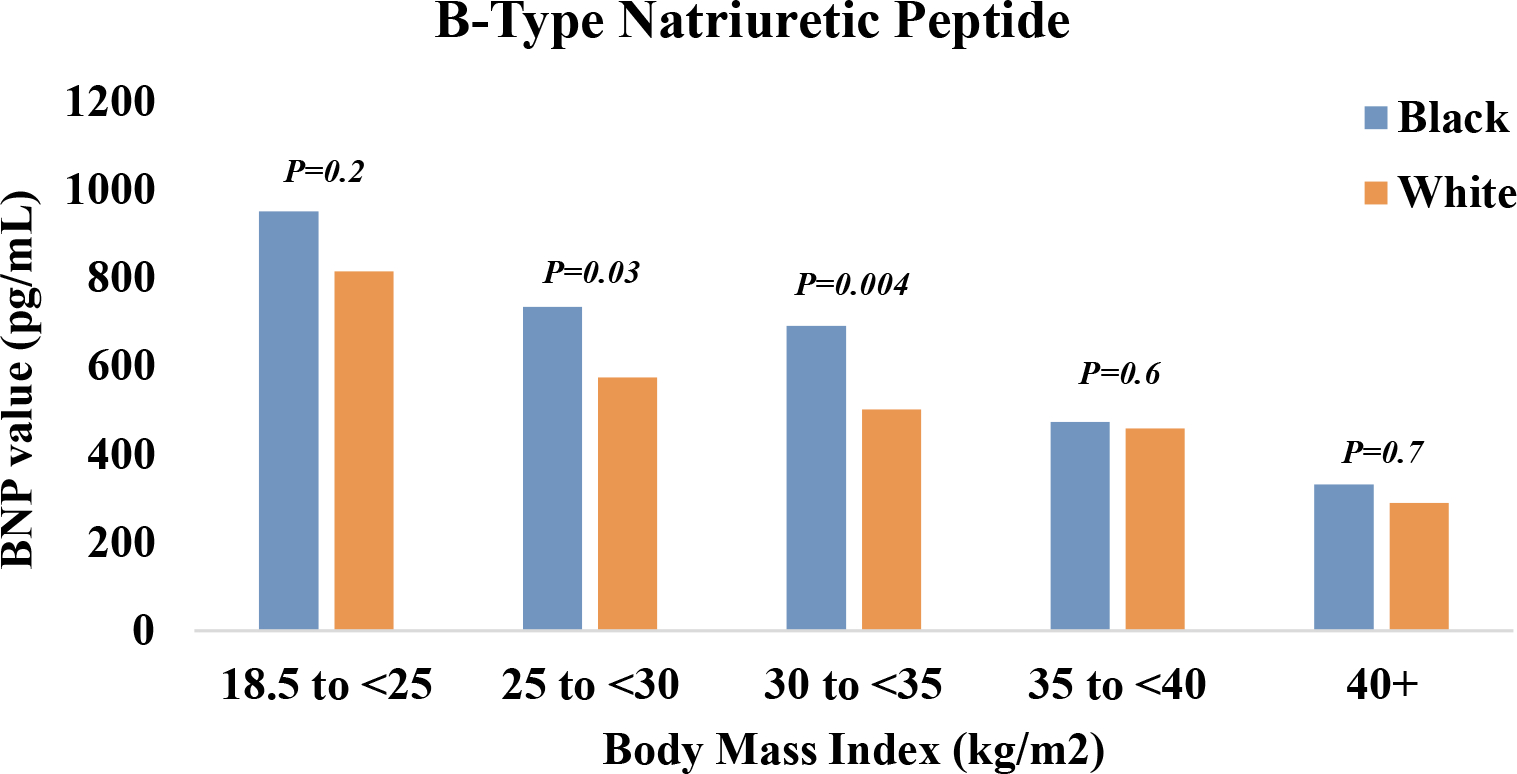
Mean B-type natriuretic peptide levels among Black and White patients hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014*
*Footnote: Analysis limited to a subset (N = 7188, 71%) with available B-type natriuretic peptide abstractions from the hospital record.
Figure 2:
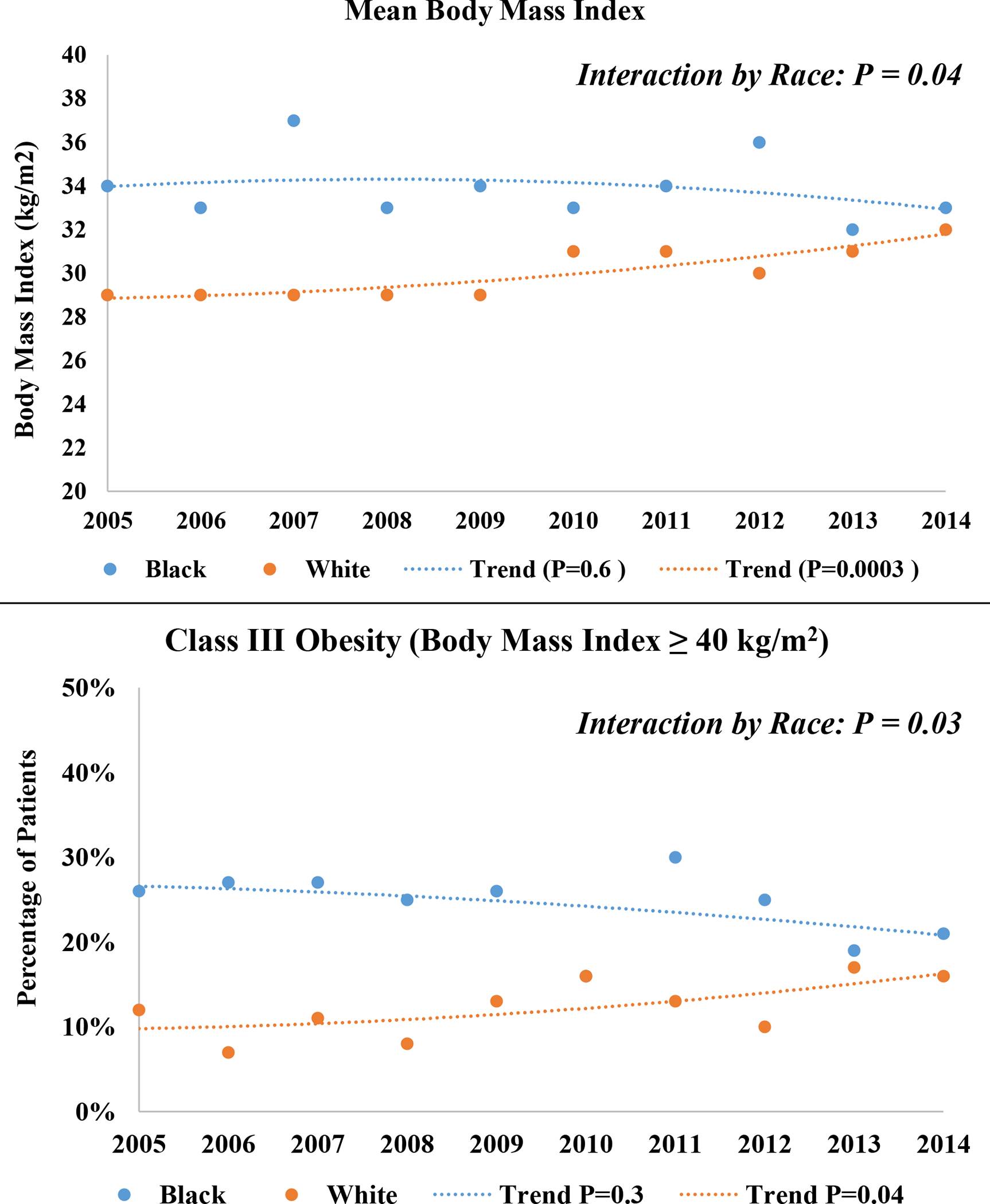
Temporal trends in mean body mass index, and annual prevalence of class III obesity (≥40 kg/m2) among Black and White patients hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
When aggregated across 2005–2014 and stratified by race, Black patients with obesity were younger on average than White patients with obesity (69 vs. 75 years) and more often women (71% vs. 64%). The mean age at admission was stable across 2005–2014 for patients with obesity of both races (Table 2). However, gender distributions changed over time, with an increasing percentage of women constituting both Black and White patients with obesity hospitalized with ADHF. The temporal increase in female patients did not differ by race, however (P for interaction by race = 0.5). Most patients with obesity had a BMI exceeding 35 kg/m2, both in 2005–2009 and 2010–2014 (Table 2). The mean BMI did not change substantially by for Black patients with obesity (41 kg/m2 both in 2005–2009 and 2010–2014; P for annual trend = 0.5) but increased among White patients with obesity (37 kg/m2 [2005–2009] to 39 kg/m2 [2010–2014]; P for annual trend = 0.06). Among the patients with obesity, there was an increase in the annual prevalence of sleep apnea and depression, for both races. The rate of increase did not differ by race (P for interaction by race = 0.6 and 0.4, respectively). The annual prevalence of atrial fibrillation increased only among Black patients with obesity, while the annual prevalence of chronic kidney disease decreased only among White patients with obesity. Interestingly, the prevalence of left ventricular hypertrophy declined substantially over time, both for Black patients with obesity (P for annual trend = 0.004) and White patients with obesity (P for annual trend = 0.02), but the rate of decline did not statistically differ by race (P for interaction by race = 0.6). Diastolic dysfunction was increasingly reported in White patients with obesity over time, with a temporal increase that statistically differed from Black patients (P for interaction by race = 0.01). Right ventricular dilation and pulmonary hypertension were increasingly reported over time for White but not Black patients with obesity; however, the annual rate of change did not statistically differ by race.
Table 2:
Demographics and clinical characteristics of patients with obesity who were hospitalized with acute decompensated heart failure with preserved ejection fraction, stratified by race. The Atherosclerosis Risk in Communities Study, 2005–2014.
| Characteristic | Black, Obese | White, Obese | Black vs. White | |||||
|---|---|---|---|---|---|---|---|---|
| 2004–2009 | 2010–2014 | P value* | 2004–2009 | 2010–2014 | P value* | P value† | P value‡ | |
| N=497 | N=1005 | Trend | N=1005 | N=2213 | Trend | Aggregate | Trends | |
| Demographics | ||||||||
| Age (years) | 69 ± 9 | 70 ± 9 | 0.4 | 75 ± 9 | 74 ± 10 | 0.7 | <0.0001 | 0.7 |
| Female | 346 (70%) | 727 (72%) | <0.0001 | 621 (62%) | 1435 (65%) | 0.003 | 0.006 | 0.5 |
| Health insurance | 484 (98%) | 975 (97%) | 0.06 | 970 (96%) | 2189 (99%) | 0.01 | 0.1 | 0.3 |
| Medical History | ||||||||
| Body mass index | 41 ± 9 | 41 ± 10 | 0.5 | 37 ± 7 | 39 ± 8 | 0.06 | <0.0001 | 0.2 |
| Class III obesity | 232 (47%) | 399 (40%) | 0.2 | 278 (28%) | 677 (31%) | 0.4 | <0.0001 | 0.1 |
| Current smoking | 66 (13%) | 144 (14%) | 0.2 | 130 (13%) | 197 (9%) | 0.009 | 0.06 | 0.009 |
| Hypertension | 474 (95%) | 941 (94%) | 0.8 | 913 (91%) | 1972 (89%) | 0.2 | 0.002 | 0.5 |
| Diabetes mellitus | 358 (72%) | 691 (69%) | 0.08 | 550 (55%) | 1339 (61%) | 0.9 | 0.003 | 0.2 |
| Chronic kidney disease§ | 307 (62%) | 350 (61%) | 0.6 | 723 (72%) | 856 (64%) | 0.01 | 0.07 | 0.04 |
| Coronary artery disease | 214 (43%) | 427 (43%) | 0.4 | 488 (49%) | 1023 (46%) | 0.3 | 0.2 | 0.9 |
| Atrial fibrillation | 92 (19%) | 240 (24%) | 0.01 | 396 (39%) | 1008 (46%) | 0.2 | <0.0001 | 0.1 |
| Stroke / TIA | 65 (13%) | 173 (17%) | 0.2 | 233 (23%) | 419 (19%) | 0.04 | 0.07 | 0.03 |
| Chronic bronchitis / COPD | 155 (31%) | 424 (42%) | 0.06 | 364 (36%) | 976 (44%) | 0.1 | 0.3 | 0.5 |
| Sleep apnea | 92 (18%) | 334 (33%) | 0.003 | 169 (17%) | 677 (31%) | 0.0002 | 0.5 | 0.6 |
| Thyroid disease | 62 (13%) | 127 (13%) | 1.0 | 198 (20%) | 599 (27%) | 0.04 | <0.0001 | 0.3 |
| Depression | 35 (7%) | 170 (17%) | 0.003 | 190 (19%) | 720 (33%) | 0.001 | <0.0001 | 0.4 |
| Echocardiography | ||||||||
| Left ventricular hypertrophy | 233 (47%) | 336 (33%) | 0.004 | 332 (33%) | 536 (24%) | 0.02 | 0.0002 | 0.6 |
| Diastolic dysfunction | 156 (31%) | 315 (31%) | 0.6 | 224 (22%) | 661 (30%) | 0.0004 | 0.2 | 0.01 |
| Right ventricular dilation | 96 (19%) | 224 (22%) | 0.3 | 164 (16%) | 617 (28%) | 0.007 | 0.3 | 0.4 |
| Pulmonary hypertension | 144 (29%) | 311 (31%) | 0.5 | 336 (33%) | 957 (43%) | 0.003 | 0.001 | 0.4 |
| Hospital Visit | ||||||||
| Length of stay (days) | 8.6 ± 9 | 7.5 ± 7 | 0.2 | 7.1 ± 7 | 8.6 ± 20 | 0.8 | 0.9 | 0.5 |
Values expressed as number (percentage) or mean ± standard deviation
Abbreviations: COPD = chronic obstructive pulmonary disease, TIA = transient ischemic attack
P values for temporal increase or decrease in annual prevalence (2005–2014) assessed by logistic regression, regressing on year of admission and using the Cochran-Armitage test for trend. P-values for temporal (2005–2014) increase or decrease in annual mean values assessed by linear regression, regressing on year of admission
P values for aggregate comparisons between Black vs. White patients analyzed by aggregating data across 2005–2014, with mean values tested by least square means from linear regression, and prevalence values tested by Rao-Scott χ2 tests.
P values comparing differences in temporal comorbidity or demographic trends between Black vs. White patients assessed by the linear or logistic regression, regressing on race and year of admission and testing the multiplicative interaction of race by year of admission
Chronic kidney disease defined by receipt of hemodialysis or an estimated glomerular filtration rate <45 mL/min/1.73m2 in patients with available serum creatinine assessments (1311 missing, no creatinine abstractions performed in 2014)
Across the 10-year surveillance, there were 3037 (30%) deaths within 1 year of hospitalization. When categorizing BMI into 5 distinct groups: (18.5 to <25; N=510), (25 to <30; N=544), (30 to <35; N=412), (35 to <40; N=250), and (≥40 kg/m2; N=342; median=46 kg/m2), the lowest absolute risk of mortality within 1 year was observed among patients with mild obesity (30 to <35 kg/m2). In various multivariable modeling approaches, a U-shaped 1-year mortality risk was observed across increasing BMI categories (Supplemental Table S1). After full adjustment, the highest mortality risk was observed with normal BMI (18.5 to <25 kg/m2), yielding a hazard ratio of 1.36 (1.03 – 1.80); and class III obesity (BMI ≥40 kg/m2), yielding a hazard ratio of 1.37 (1.01 – 1.86), when compared to a BMI of 30 to <35 kg/m2; Figure 3. A similar U-shaped distribution in adjusted 1-year mortality risk was observed when stratified by race (Supplemental Figure S5). When comparing obesity categories to a normal BMI reference group, the associated hazards presented in a J-shaped distribution (Supplemental Figure S6). The obesity mortality risk relative to the reference group of 30 to <35 kg/m2 was not modified by race, within any BMI category (P for interaction by race > 0.6 in each category). In the subset of patients with available BNP abstractions, a similar U-shaped distribution in 1-year mortality risk was observed with BMI categories, which was unaltered by additional adjustment for BNP (Supplemental Table S2).
Figure 3:
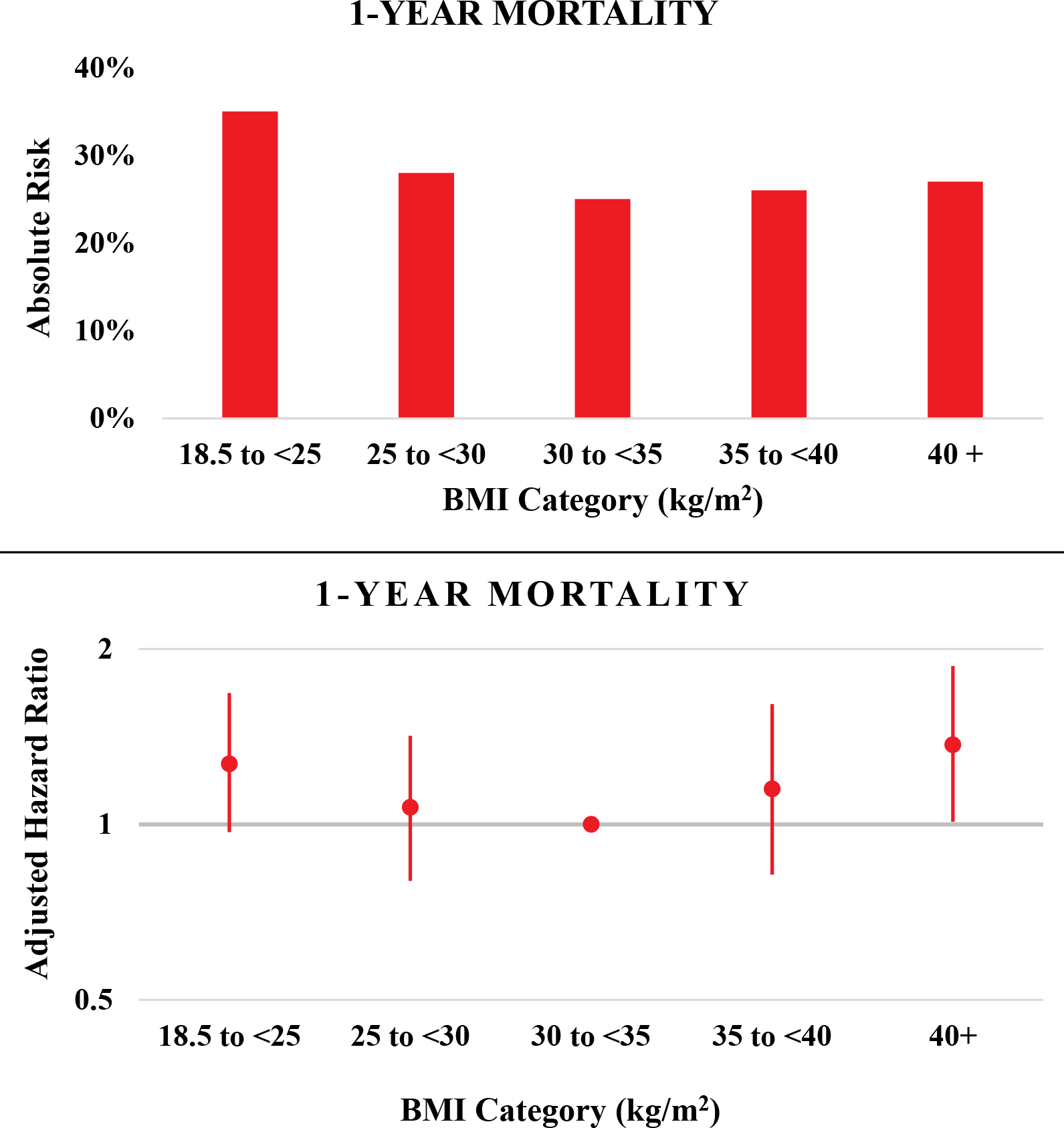
Absolute risk and adjusted* hazard ratios of 1-year all-cause mortality associated with various body mass index categories among patients hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
*Footnote: Mortality models adjusted for age, race, sex, year of admission, geographic location (Forsyth County NC, Jackson, MS, Minneapolis, MN, Washington County, MD), length of stay, current smoking, chronic obstructive pulmonary disease, hypertension, atrial fibrillation, coronary artery disease, and diabetes mellitus. Abbreviations: BMI = body mass index measured in kg/m2.
Discussion
In this decade-long community surveillance of older HFpEF patients hospitalized with ADHF, obesity was common and more prevalent among Black patients. Over the course of surveillance, the prevalence of obesity and class III obesity steadily increased among White patients but not Black patients, narrowing racial prevalence differences in more recent years. BMI was associated with a U-shaped distribution in adjusted 1-year mortality risk, with worse survival among patients with either normal body weight or class III obesity, with no effect modification by race.
Race-stratified temporal trends in obesity among HFpEF patients hospitalized with ADHF have not been previously reported. However, an overall increase in obesity among ADHF hospitalizations spanning 2005–2014 has been noted.22 During this same time frame, HFpEF became the predominant form of heart failure, accounting for the majority of ADHF hospitalizations.4–6 Obesity is more strongly associated with HFpEF as compared with heart failure with reduced ejection fraction (HFrEF),12,23 possibly explaining the reported increase in obesity prevalence among ADHF hospitalizations and patients with incident heart failure.4,22 On the other hand, it is also possible that demographic shifts have influenced obesity prevalence in ADHF hospitalizations in recent years. In support of this, a decreasing proportion of patients hospitalized with ADHF from 2005–2014 in the Get with the Guidelines (GWTG) registry was reported to be White (73% to 67%).22 In this analysis from the ARIC study community surveillance, obesity prevalence was higher among Black vs. White patients with HFpEF, irrespective of year of admission. Mean BMI and prevalence of obesity and class III obesity remained stable across 2005–2014 for Black patients, but increased significantly among White patients, with no indication of a plateau.
Consistent with other reports, the population of patients with HFpEF in the present analysis was predominantly female and over the age of 60. In the general Black and White US populations over the age of 60, the prevalence of obesity is estimated to be 48% and 39%, respectively. The racial difference becomes even more striking when considering Black and White women ≥60 years, who attain an obesity prevalence of 58% and 38%, respectively.24 In the present study, we observed a higher obesity prevalence among Black HFpEF patients hospitalized with ADHF than White patients (56% vs. 43%); distributions which largely parallel Black and White obesity estimates from the female general population. However, unlike in the general population, the prevalence of class III obesity (BMI ≥40 kg/m2) in our study population was 24% and 13%, respectively, for Black and White patients. By comparison, the prevalence of class III obesity among Black and White women ≥60 years in the general population is reported to be 14% and 6%, respectively.24 The overall mean BMI did not change over time in our study population of Black patients (34 kg/m2 in both the first and second half of the surveillance), but increased from 29 to 31 kg/m2 for White patients, shifting the average classification from “overweight” to “obese” and possibly increasing the risk of adverse outcomes such as heart failure readmissions.25A narrowing of racial disparities in poor cardiovascular health, as assessed by the “Life’s Simple 7” index, has previously been reported for the general adult population, with a disproportionate temporal increase in poor physical activity and diet among White Americans compared to other groups.26 This may be a plausible explanation for the increasing obesity prevalence among White, but not Black patients hospitalized with HFpEF in the ARIC study Community Surveillance. Alternatively, it is possible that obesity prevalence has reached a plateau among Black HFpEF patients, possibly reflecting selective survival and obesity-associated death prior to hospitalization for ADHF.
Previous investigations have reported an “obesity paradox”, or survival benefit associated with obesity among HFpEF populations. An early analysis (2001–2004) from the Acute Decompensated Heart Failure National Registry (ADHERE) reported an inverse, linear relationship between in-hospital mortality and BMI quartile, with the lowest risk observed in the highest BMI quartile.27 However, the median BMI in the highest quartile was only 39 kg/m2, and the mean ejection fraction was 42% (with >40% defined as HFpEF). In the present study, mortality risk was U-shaped rather than linear and increased with extreme BMI ≥40 kg/m2. A U-shaped distribution in long-term mortality risk was also reported by the VA Palo Alto Healthcare system and the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC), which analyzed chronic heart failure populations with HFpEF,28,29 as well the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial.30 Body composition may contribute to the improved survival of patients with HFpEF and mild obesity, particularly if sarcopenic obesity is more prevalent with a “normal” BMI and lean muscle mass is greater among those with an “obese” BMI.31,32 Cardiorespiratory fitness status may also influence outcomes of obesity,33 but preserved fitness is likely uncommon in patients hospitalized with ADHF. The GWTG registry, which analyzed patients hospitalized with ADHF, reported a stable or only minimal increase in 30-day and 1-year mortality hazard ratios as BMI increased from 30 to 45 kg/m2 among patients with HFpEF.13 Of note, the analysis from the GWTG did not include BMI values exceeding 45 kg/m2. In the present study, patients categorized with class III obesity (≥40 kg/m2) had a median BMI of 46 kg/m2 (25th to 75th percentiles: 43 – 52 kg/m2), and the highest risk of death.
The elevated 1-year mortality risk associated with class III obesity may be attributable to several factors. Patients with obesity had a greater burden of diabetes mellitus, hypertension, and sleep apnea; cardiometabolic conditions associated with poor prognosis. Obesity is also likely to exacerbate HFpEF.34 Adipose tissue upregulates aldosterone production, inducing sodium retention and plasma volume expansion, which contribute to elevated left ventricular filling pressure, increased pulmonary artery pressures, and right heart failure.23 The mechanical stress and pericardial restraint imposed by class III obesity likely contributes to diastolic dysfunction as well, by impairing left ventricular relaxation.35 Inflammatory cytokines released by the adipose tissue may diminish myocardial perfusion, by inducing endothelial dysfunction and rarefaction of the microvasculature.36 While diastolic dysfunction, pulmonary hypertension, and right ventricular dilation remained stable from 2005–2014 among Black patients with obesity in the present study, the prevalence increased significantly for White patients with obesity, possibly reflecting the increase in mean BMI and class III obesity prevalence. Though associated with obesity, a decreasing prevalence of left ventricular hypertrophy was noted across 2005–2014, both for Black and White patients with obesity, possibly reflecting better blood pressure control in recent years or the decline in chronic kidney disease among White patients with obesity over the surveillance period.
Our study findings have important clinical implications. In contrast to HFrEF, neurohumoral inhibition and other pharmacologic agents have not demonstrated efficacy in patients with HFpEF, leading to few strongly recommended therapies.7,8 Given the limited pharmacologic options, a phenotype-specific treatment approach has been proposed to manage HFpEF.37 Obesity represents a unique phenotype in HFpEF35 that appears to benefit from intentional weight loss, either by caloric restriction, structured exercise or bariatric surgery.17,18,38,39 In a single-center randomized controlled trial of patients with obesity with HFpEF, exercise capacity and quality of life were reported to improve for patients randomized to a combination of aerobic exercise training and caloric restriction, compared to patients randomized to either intervention alone.17 However, the long-term sustainability of weight loss with improved body composition is questionable.40 Weight loss induced by bariatric surgery has also been reported to decrease hospital admissions for ADHF, in a population-based analysis of records sourced from emergency departments in 3 states.38 While the majority of prior and ongoing HFpEF clinical trials have focused on drugs aimed directly at the cardiovascular system, pharmacological treatment of obesity—another potential strategy for the treatment of obesity in HFpEF—should also be explored further. Our analysis from the ARIC study community surveillance confirms worse 1-year survival among HFpEF patients with class III obesity who are hospitalized with acute decompensation. Notably, the mean BMI and annual prevalence of class III obesity increased from 2005–2014 among White patients and remained stable among Black patients, who remained disproportionately burdened by obesity throughout the study surveillance. In this context, it is noteworthy that HFpEF randomized controlled trials often exclude patients with a body mass index (BMI) exceeding upper limits, or require elevated natriuretic peptides levels for inclusion, which are known to be reduced by obesity.41,42 As examples of this, in 4 large trials performed over the past decade, the average BMI of patients with HFpEF ranged from 29–31 kg/m2, likely underrepresenting obese and Black patients with HFpEF.43–46
Our study has some limitations. This was an observational analysis, and data were limited to availability in the medical record. Temporal changes in diagnostic testing or documentation may have influenced the reporting of comorbidities or echocardiographic findings over time. However, obesity was defined by calculating BMI from the weight at discharge, which was routinely recorded by all hospitals throughout the surveillance period. Although annual trends in mean BMI and obesity prevalence did not significantly change among Black patients, the sample size was reduced compared to White patients, possibly limiting the statistical power. The ARIC Study is also limited to 4 geographic regions, which may limit the generalizability. Although obesity may increase the risk of HF readmissions,11,47 we were unable to consider longitudinal outcomes other than mortality because the community surveillance component of the ARIC Study does not track individual patients over time. Our analysis also has several noteworthy strengths. The ARIC Study provides a large, multiyear surveillance of 4 diverse US communities, allowing an analysis of contemporary trends spanning 10 years. Classifications of ADHF were determined by standardized physician review rather than by natriuretic peptide criteria, an important distinction from HFpEF clinical trials. Clinical and laboratory values were meticulously collected by certified abstractors following standardized protocols. ADHF was classified and adjudicated by physician review of the medical records, and mortality outcomes were verified by the National Death Index.
In conclusion, the obesity prevalence has steadily increased among White HFpEF patients hospitalized with ADHF, while remaining stable for Black patients. For both races, the mortality risk associated with obesity depends strongly upon the magnitude of BMI, with worse long-term survival among patients with class III obesity. This highlights the need to implement effective interventions for the treatment and prevention of class III obesity in patients with HFpEF.
Supplementary Material
Study population selection flowchart, race-specific obesity prevalence and trends stratified by ARIC community, race-specific trends stratified by sex, complete results for mortality models with minimal to full adjustment, mortality models stratified by race, mortality models using normal BMI as the reference, and mortality models with additional adjustment for BNP levels.
Supplemental Figure S1: Study population selection flowchart
Supplemental Figure S2: Prevalence of obesity (BMI ≥30 kg/m 2 ) and class III obesity (BMI ≥40 kg/m 2 ) among black and white patients hospitalized with acute decompensated heart failure with preserved ejection fraction, stratified by ARIC community. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S3: Temporal trends in mean body mass index, and annual prevalence of obesity (≥30 kg/m2 ) and class III obesity (≥40 kg/m2 ) among black and white patients hospitalized with acute decompensated heart failure with preserved ejection fraction in Forsyth County, NC and Jackson, MS. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S4: Sex stratified trends in annual prevalence of obesity among black and white patients hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S5: Adjusted* hazards of 1-year all-cause mortality among black and white patients of various body mass index categories who were hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S6: Adjusted* hazards of 1-year all-cause mortality among black and white patients of various body mass index categories who were hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Table S1: Adjusted* 1-year hazards of mortality among patients hospitalized with acute decompensated heart failure with preserved ejection fraction and various body mass index categories. The community surveillance component of the Atherosclerosis Risk in Communities Study, 2005–2014.
Supplemental Table S2: Adjusted* hazards of 1-year all-cause mortality among black and white patients of various body mass index categories who were hospitalized with acute decompensated heart failure with preserved ejection fraction and had available B-type natriuretic peptide abstractions (N=7,188). The Atherosclerosis Risk in Communities Surveillance Study, 2005– 2014.
Key Points.
Obesity increases with age, is disproportionately prevalent in Black populations, and is associated with heart failure with preserved ejection fraction (HFpEF).
In this 10-year surveillance of older adults hospitalized with HFpEF, mean BMI, prevalence of obesity, and prevalence of class III obesity were stable over time for Black patients, but steadily increased for White patients.
Despite the narrowing racial differences, Black patients remained disproportionately burdened by obesity throughout the surveillance period, and for both races, class III obesity was associated with the worst survival.
Why does this paper matter?
In this 10-year surveillance of hospitalized HFpEF, obesity was stable and disproportionately high for Black patients, but increased over time for White patients. Class III obesity was associated with poor survival, highlighting the need for its effective management in older patients with HFpEF.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sponsor Role
The sponsor had no role in the study conceptualization, statistical analysis, manuscript writing, interpretation, or presentation of results.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Disclosures
No authors report disclosures relevant to the contents of this paper. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. Dr. Qamar is supported by institutional grant support from the NorthShore Auxiliary research scholar fund and has received funding from Daiichi-Sankyo, American Heart Association and fees for educational activities from the American College of Cardiology, Society for Vascular Medicine, Society for Cardiovascular Angiography and Interventions, Janssen and Janssen, Pfizer, Medscape, and Clinical Exercise Physiology Association. Dr. Mentz receives research support from the National Institutes of Health (U01HL125511-01A1 and R01AG045551-01A1), Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis and Sanofi; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, Roche, Sanofi and Vifor; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. Dr. Shah has received research grants from the National Institutes of Health (R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423), the American Heart Association (#16SFRN28780016), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapeutics, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Eisai, GSK, Ionis, Ironwood, Merck, MyoKardia, Novartis, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest
References:
- 1.Pandey A, Vaduganathan M, Arora S, et al. Temporal Trends in Prevalence and Prognostic Implications of Comorbidities Among Patients With Acute Decompensated Heart Failure. Circulation. 2020;142(3):230–243. doi: 10.1161/CIRCULATIONAHA.120.047019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc. 2017;65(11):2431–2440. doi: 10.1111/jgs.15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 4.Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–580. doi: 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber Y, Weston SA, Redfield MM, et al. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996. doi: 10.1001/jamainternmed.2015.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014) ARIC study community surveillance. Circulation. 2018;138(1):12–24. doi: 10.1161/CIRCULATIONAHA.117.027551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019 [doi] [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. J Card Fail. 2017;23(8):628–651. doi: 10.1016/j.cardfail.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Cesari M, Penninx BWJH, et al. Abdominal Obesity Is an Independent Risk Factor for Chronic Heart Failure in Older People. J Am Geriatr Soc. 2006;54(3):413–420. doi: 10.1111/j.1532-5415.2005.00624.x [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Nicklas BJ. Pivotal Role of Excess Intra-Abdominal Adipose in the Pathogenesis of Metabolic/Obese HFpEF. JACC Hear Fail. 2018;6(12):1008–1010. doi: 10.1016/j.jchf.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Berry JD, Drazner MH, Fang JC, Tang WHW, Grodin JL. Body Mass Index, Natriuretic Peptides, and Risk of Adverse Outcomes in Patients With Heart Failure and Preserved Ejection Fraction: Analysis From the TOPCAT Trial. J Am Heart Assoc. 2018;7(21). doi: 10.1161/JAHA.118.009664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A, LaMonte M, Klein L, et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69(9):1129–1142. doi: 10.1016/j.jacc.2016.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell-Wiley TM, Ngwa J, Kebede S, et al. Impact of Body Mass Index on Heart Failure by Race/Ethnicity From the Get With The Guidelines–Heart Failure (GWTG–HF) Registry. JACC Hear Fail. 2018;6(3):233–242. doi: 10.1016/j.jchf.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy YNV, Rikhi A, Obokata M, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22(6):1009–1018. doi: 10.1002/ejhf.1788 [DOI] [PubMed] [Google Scholar]
- 15.Shea MK, Houston DK, Nicklas BJ, et al. The Effect of Randomization to Weight Loss on Total Mortality in Older Overweight and Obese Adults: The ADAPT Study. Journals Gerontol Ser A Biol Sci Med Sci. 2010;65A(5):519–525. doi: 10.1093/gerona/glp217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea MK, Nicklas BJ, Houston DK, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr. 2011;94(3):839–846. doi: 10.3945/ajcn.110.006379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA - J Am Med Assoc. 2016;315(1):36–46. doi: 10.1001/jama.2015.17346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy YNV, Anantha-Narayanan M, Obokata M, et al. Hemodynamic Effects of Weight Loss in Obesity. JACC Hear Fail. 2019;7(8):678–687. doi: 10.1016/j.jchf.2019.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Fail. 2012;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics NCHS Fact Sheet | August 2020. National Death Index. https://www.cdc.gov/nchs/data/factsheets/factsheet_ndi.pdf [Google Scholar]
- 21.Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352(January):1–2. doi: 10.1136/bmj.i189 [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Zhao X, Hammill BG, et al. Trends in Noncardiovascular Comorbidities Among Patients Hospitalized for Heart Failure: Insights From the Get With The Guidelines-Heart Failure Registry. Circ Hear Fail. 2018;11(6):1–10. doi: 10.1161/CIRCHEARTFAILURE.117.004646 [DOI] [PubMed] [Google Scholar]
- 23.Pandey A, Patel K V., Vaduganathan M, et al. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Hear Fail. 2018;6(12):975–982. doi: 10.1016/j.jchf.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA - J Am Med Assoc. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma A, Lavie CJ, Borer JS, et al. Meta-Analysis of the Relation of Body Mass Index to All-Cause and Cardiovascular Mortality and Hospitalization in Patients With Chronic Heart Failure. Am J Cardiol. 2015;115(10):1428–1434. doi: 10.1016/j.amjcard.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 26.Brown AF, Liang LJ, Vassar SD, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168(8):541–549. doi: 10.7326/M17-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108 927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153(1):74–81. doi: 10.1016/j.ahj.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 28.Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: A U-shaped relationship. Am Heart J. 2010;159(1):75–80. doi: 10.1016/j.ahj.2009.10.026 [DOI] [PubMed] [Google Scholar]
- 29.Padwal R, Mcalister FA, Mcmurray JJV, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: A meta-analysis of individual patient data. Int J Obes. 2014;38(8):1110–1114. doi: 10.1038/ijo.2013.203 [DOI] [PubMed] [Google Scholar]
- 30.Haass M, Kitzman DW, Anand IS, et al. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients With Preserved Ejection Fraction Results From the Irbesartan in Heart Failure With Preserved Ejection (I-PRESERVE) Trial. Published online 2011:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkman DL, Bohmke N, Billingsley HE, Carbone S. Sarcopenic Obesity in Heart Failure With Preserved Ejection Fraction. Front Endocrinol (Lausanne). 2020;11. doi: 10.3389/fendo.2020.558271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbone S, Billingsley HE, Rodriguez-Miguelez P, et al. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr Probl Cardiol. 2020;45(11):100417. doi: 10.1016/j.cpcardiol.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavie CJ, Cahalin LP, Chase P, et al. Impact of Cardiorespiratory Fitness on the Obesity Paradox in Patients With Heart Failure. Mayo Clin Proc. 2013;88(3):251–258. doi: 10.1016/j.mayocp.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalos D, Mascherbauer J, Zotter-Tufaro C, et al. Functional Status, Pulmonary Artery Pressure, and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68(2):189–199. doi: 10.1016/j.jacc.2016.04.052 [DOI] [PubMed] [Google Scholar]
- 35.Obokata M, Reddy YNV, Pislaru SV., Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136(1):6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammed SF, Hussain I, AbouEzzeddine OF, et al. Right Ventricular Function in Heart Failure With Preserved Ejection Fraction. Circulation. 2014;130(25):2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation. 2016;134(1):73–90. doi: 10.1161/CIRCULATIONAHA.116.021884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric Surgery and Emergency Department Visits and Hospitalizations for Heart Failure Exacerbation: Population-Based, Self-Controlled Series. J Am Coll Cardiol. 2016;67(8):895–903. doi: 10.1016/j.jacc.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 39.Mikhalkova D, Holman SR, Jiang H, et al. Bariatric Surgery-Induced Cardiac and Lipidomic Changes in Obesity-Related Heart Failure with Preserved Ejection Fraction. Obesity. 2018;26(2):284–290. doi: 10.1002/oby.22038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houston DK, Miller ME, Kitzman DW, et al. Long-Term Effects of Randomization to a Weight Loss Intervention in Older Adults: A Pilot Study. J Nutr Gerontol Geriatr. 2019;38(1):83–99. doi: 10.1080/21551197.2019.1572570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitzman DW, Lam CSP. Obese Heart Failure with Preserved Ejection Fraction Phenotype: From Pariah to Central Player. Circulation. 2017;136(1):20–23. doi: 10.1161/CIRCULATIONAHA.117.028365 [DOI] [PubMed] [Google Scholar]
- 42.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68(2):200–203. doi: 10.1016/j.jacc.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 43.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450 [DOI] [PubMed] [Google Scholar]
- 44.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 45.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 46.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 47.Carbone S, Lavie CJ. Disparate effects of obesity on survival and hospitalizations in heart failure with preserved ejection fraction. Int J Obes. 2020;44(7):1543–1545. doi: 10.1038/s41366-020-0579-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study population selection flowchart, race-specific obesity prevalence and trends stratified by ARIC community, race-specific trends stratified by sex, complete results for mortality models with minimal to full adjustment, mortality models stratified by race, mortality models using normal BMI as the reference, and mortality models with additional adjustment for BNP levels.
Supplemental Figure S1: Study population selection flowchart
Supplemental Figure S2: Prevalence of obesity (BMI ≥30 kg/m 2 ) and class III obesity (BMI ≥40 kg/m 2 ) among black and white patients hospitalized with acute decompensated heart failure with preserved ejection fraction, stratified by ARIC community. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S3: Temporal trends in mean body mass index, and annual prevalence of obesity (≥30 kg/m2 ) and class III obesity (≥40 kg/m2 ) among black and white patients hospitalized with acute decompensated heart failure with preserved ejection fraction in Forsyth County, NC and Jackson, MS. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S4: Sex stratified trends in annual prevalence of obesity among black and white patients hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S5: Adjusted* hazards of 1-year all-cause mortality among black and white patients of various body mass index categories who were hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Figure S6: Adjusted* hazards of 1-year all-cause mortality among black and white patients of various body mass index categories who were hospitalized with acute decompensated heart failure with preserved ejection fraction. The Atherosclerosis Risk in Communities Surveillance Study, 2005–2014.
Supplemental Table S1: Adjusted* 1-year hazards of mortality among patients hospitalized with acute decompensated heart failure with preserved ejection fraction and various body mass index categories. The community surveillance component of the Atherosclerosis Risk in Communities Study, 2005–2014.
Supplemental Table S2: Adjusted* hazards of 1-year all-cause mortality among black and white patients of various body mass index categories who were hospitalized with acute decompensated heart failure with preserved ejection fraction and had available B-type natriuretic peptide abstractions (N=7,188). The Atherosclerosis Risk in Communities Surveillance Study, 2005– 2014.


