Abstract
Cyclic peptides are widespread throughout the plant kingdom, and display diverse sequences, structures and bioactivities. The potential applications attributed to these peptides and their unusual biosynthesis has captivated the attention of researchers for many years. Several gene sequences for plant cyclic peptides have been discovered over the last two decades but it is only recently that we are beginning to understand the intricacies associated with their biosynthesis. Recent studies have focussed on three main classes of plant derived cyclic peptides, namely orbitides, SFTI related peptides and cyclotides. In this mini-review, we discuss the expansion of the known sequence and structural diversity in these families, insights into the enzymes involved in the biosynthesis, the exciting applications which includes a cyclotide currently in clinical trials for the treatment of multiple sclerosis, and new production methods that are being developed to realise the potential of plant cyclic peptides as pharmaceutical or agricultural agents.
Keywords: cyclotide, orbitide, sunflower trypsin inhbitor
Introduction
Cyclic peptides have received significant attention in the literature having been found in all domains of life [1]. In addition to deciphering how these unusual peptides are produced in vivo, developing approaches for producing them in vitro, and determination of their structures, bioactivities and potential applications have been key areas of interest. Here, we refer to cyclic peptides as those with a head-to-tail cyclic backbone, rather than cyclized through disulfide bonds or side chain linkages. A disproportionally large number of head-to-tail backbone cyclic peptides have been characterised from plants relative to other organisms. These peptides have been identified in a range of plant families as previously outlined in a comprehensive review published in 2006 by Tan and Zhou [2]. As to why higher numbers of plant derived cyclic peptides have been characterised remains to be seen, but in the meantime, a wealth of information is being gathered on this intriguing and potentially valuable class of molecules.
Recent studies in the field of plant derived cyclic peptides have focussed heavily on three classes, namely orbitides, cyclotides and SFTI-1 related peptides. Orbitides are plant cyclic peptides that do not contain any disulfide bonds or non-natural amino acids. The first orbitide, evolidine, was isolated in the 1950s from the rainforest tree Melicope xanthoxyloides and subsequent studies have confirmed its sequence, as summarised by Fisher et al. [3]. These peptides, which contain between 5 and 16 residues, were originally referred to as Caryophyllaceae-type cyclic peptides, but have since been termed orbitides [4–6]. In the 1970s evidence of a new class of plant derived cyclic peptides was emerging based on analysis of an indigenous medicine used in Africa [7]. However, it was more than 20 years later that the sequence and structure of the cyclic peptide kalata B1, originally isolated from Oldenlandia affinis, was characterised [8]. Subsequent studies made it clear that kalata B1 was a member of a large family of related peptides and the term cyclotide was coined in 1999 [9]. Similar to orbitides, cyclotides generally do not contain non-native amino acids, but they contain approximately 30 residues including six cysteine residues, which form a cystine knot motif. At the same time as cyclotides were being classified, the first example of another class of plant cyclic peptides was reported from sunflower seeds [10]. This peptide was termed sunflower trypsin inhibitor-1 (SFTI-1); it inhibits trypsin in the sub-nanomolar range, is similar to the orbitides in size, but contains a single disulfide bond and subsequent studies have found several related peptides [11,12].
There have been several reviews relating to plant derived cyclic peptides, but studies in the last few years have been particularly exciting and have provided key advances relating to the sequence diversity of plant derived cyclic peptides, their biosynthesis and potential applications. This mini-review provides and overview of these recent findings relating to both fundamental and applied aspects of plant derived cyclic peptides.
Discovery and characterisation
The early discoveries of plant cyclic peptide sequences were based primarily on tedious chemical/proteomic analyses, but not surprisingly transcriptomic/genomic approaches now facilitate their discovery. A combination of transcriptomic and proteomic approaches has been particularly useful in recent studies on orbitides, cyclotides and SFTI-1-like peptides as outlined below.
Orbitides
The number of orbitide sequences characterised has significantly increased in recent years. A range of species from the Asteraceae family have been analysed and 46 orbitides identified [6,13]. These peptides are referred to as PLP-1 to PLP-46, with PLP referring to PawL-derived Peptides [12]. It has been estimated that Asteraceae orbitides could number in the thousands based on the abundance within some species and the hypervariability of the sequences [6]. The structures of four of the PLP peptides have been determined using NMR spectroscopy and reveal relatively well-defined structures for such small peptides (6–8 residues in length). PLP-10 contains a single proline residue and the NMR data allowed determination of both the cis and trans isomers. Although there are examples of orbitides with antimicrobial activity, the bioactivity of the PLP peptides is unclear, as several were tested in antibacterial and antifungal assays but did not display activity.
Orbitides from other plant families have also been found recently, including a cyclic peptide from Pseudostellaria heterophylla [14] and a novel orbitide derived from a genetic deletion was detected from flaxseed [15]. Furthermore, a study that revisited the discovery of evolidine from the Rutaceae species M. xanthoxyloides also resulted in the discovery of six novel orbitides (xanthoxycyclin A–F) encoded by similar transcripts [3]. The structures of synthetic versions of two of these orbitides were studied using NMR spectroscopy, with the 8-residue peptide xanthoxycyclin D showing a well-defined structure in contrast with the 9-residue peptide xanthoxycyclin F, which appeared to have conformational heterogeneity [3]. The sequences of these peptides differ significantly with the latter containing two proline residues, which might be responsible for the structural distinctions. A major finding from this study was that the new orbitides had diverse C-terminal residues, in contrast with the PLP orbitides, which has significant implications for the biosynthesis of these sub-families of cyclic peptides.
Cyclotides
Cyclotides represent one of the largest families of cysteine-containing cyclic peptides, and they have been discovered in a range of plant families, including Rubiaceae, Violaceae, Fabaceae, Solanaceae and Cucurbitaceae [16]. Not all species within these families have been found to contain cyclotides, but the Violaceae family appears to be unique in that all species studied so far have contained cyclotides. However, two recent studies on Violaceae species have highlighted some striking differences. Analysis of the medicinal herb Hybanthus enneaspermus using RNAseq data revealed the presence of 93 putative cyclotide sequences, and 16 acyclic cyclotides (i.e. cyclotide related peptides that do not contain a cyclic backbone, which have been referred to as acyclotides) [17]. In contrast, analysis of another Violaceae plant species, Rinorea bengalensis, showed a large number of acyclotides and only one cyclotide sequence [18]. Despite the lack of the cyclic backbone, several of these peptides have been shown to have cytotoxic activities similar to their cyclic counterparts [18]. New cyclotide sequences have also recently been found in Palicourea sessilis, that have potential as immunosuppressants [19].
SFTI-like peptides
Following on from the original study which identified SFTI-1 [10], it has become clear that SFTI-1 is the prototypic member of a family of related peptides found in the Asteraceae family [20]. These peptides have been termed PDPs (PawS-derived peptides), based on the name of the precursor protein for SFTI-1 [21]. The sequences and structures of this family of peptides has been reviewed in Franke et al. [20]. The discovery of PDPs is primarily based on similarity with the gene sequence encoding SFTI-1, but significant sequence and structural diversity has been found. Interestingly, not all PDPs are cyclic. An Asp-Gly cyclisation motif is present in all cyclic PDPs stemming from an N-terminal glycine and C-terminal aspartic acid in the precursor protein. When the C-terminal is an Asn rather than an Asp the resulting mature peptides are acyclic [11,12,22]. The original discovery identified SFTI-1 as a potent trypsin inhibitor, but it has not been as straightforward to elucidate the bioactivity of the related peptides, even for peptides such as PDP-20, which has significant similarity to SFTI-1 but does not inhibit trypsin [11].
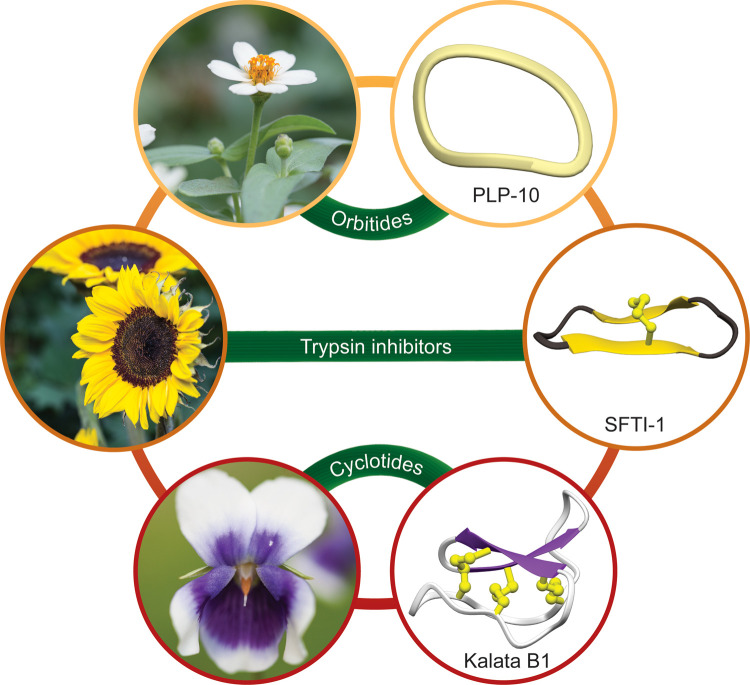
To highlight the diversity of the structures and plants from which the cyclic peptides are derived, examples from each of the three main families discussed here are given in Figure 1. The single disulfide bond of SFTI-1 braces the β-strands, whereas the three disulfide bonds in the prototypic cyclotide, kalata B1, form a cystine knot motif whereby two of the bonds with the connecting backbones form a ring through which the third bond threads.
Figure 1. Representative structures of plant cyclic peptides from the orbitide, PLP and cyclotide families.
SFTI-1 (PDB code: 1JBL) from the sunflower Helianthus annus, PLP-10 (PDB code: 6AZF) trans-isomer from Zinnia sp., Kalata B1 (PDB code: 1NB1) from Viola hederacea.
Biosynthesis
There is still a lot to learn about the biosynthesis of plant derived cyclic peptides, but significant advances have been made within the last decade and remarkable similarities and links have been observed amongst the different classes. The genes for the precursor proteins for several plant cyclic peptides have been characterised and we are beginning to understand how the mature peptides are released.
The first precursor genes of orbitides were cloned from Vaccaria hispanica (Saponaria vaccaria) [23] for the subfamily referred to as segetalins, and a subsequent study revealed two enzymes involved in their biosynthesis. These enzymes were termed oligopeptidase 1, which catalyses the cleavage of intermediates at the N-terminus, and peptide cyclase 1 (PCY1), a serine protease which is involved in the subsequent step of backbone cyclisation and removal of the C-terminal flanking sequence [24]. However, the mechanisms/processes involved in cyclisation do not appear to be conserved, at least not entirely, as studies on other plant cyclic peptides have identified different classes of enzymes involved in the cyclisation process.
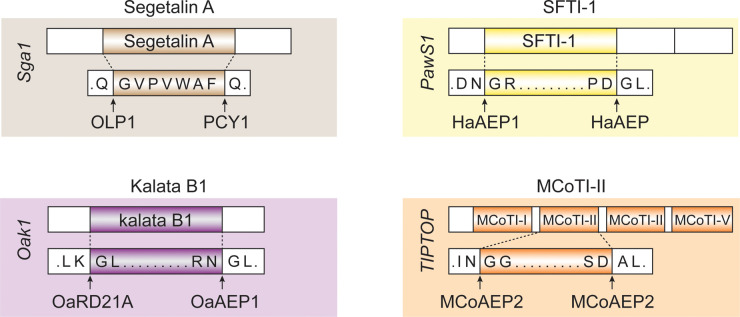
Cyclisation of SFTI-like peptides (PDPs), orbitides from the Asteraceae family (PLPs) and cyclotides all appear to involve asparaginyl endopeptidases (AEP) proteins, despite different precursor protein structures. AEPs recognise a conserved Asp/Asn at the C-terminal processing site, and can facilitate cyclisation via transpeptidation [25–27]. The AEPs characterised from cyclic peptide producing plants include butelase-1, OaAEP1b, VyPALs and HaAEP1 [28–31]. These enzymes are involved in the C-terminal cleavage event, but there is variation in the N-terminal processing site. For kalata-like peptides, a papain-like cysteine protease, termed kalatase, has been shown to be involved in the N-terminal processing [32]. In contrast, the AEP (MCoAEP2) involved in the C-terminal cleavage of the subfamily of cyclotides that inhibit trypsin is involved in both the N-terminal and C-terminal processing [33]. SFTI-1 and related PDP peptides are characteristically buried within a precursor protein that also encodes a napin-type seed storage albumin [20,21]. Despite this unusual ‘hijacking’ of the albumin gene to encode PDPs, a conserved Asn/Asp at the C-terminus of the mature cyclic peptide and prior to the N-terminal residue make it likely that AEPs are also involved in the maturation of PDPs. A summary of the known enzymes involved in processing plant derived cyclic peptides is given in Figure 2.
Figure 2. Overview of the known enzymes involved in plant cyclic peptide processing.
Schematic presentation of the precursor proteins from the different classes of plant derived cyclic peptides. OLP1 and PCY1 have been identified in orbitide processing, HaAEP for SFTI processing, kalatase and OaAEP for cyclotide processing, MCoAEP2 for trypsin inhibitor cyclotide processing [24,32–34]. The precursor proteins are not drawn to scale; the orbitides and SFTI-1 are much smaller than the cyclotides.
The three-dimensional structure of the SFTI-1 precursor has been determined using NMR spectroscopy and revealed that the SFTI-1 and the albumin domains are both well folded and separated by a flexible linker [34]. Interestingly, when the recombinant precursor was incubated with a recombinant sunflower AEP (HaAEP), the formation of the cyclic peptide was inefficient [34]. This observation coupled with the range of C-terminal residues found in the orbitides from M. xanthoxyloides, which are not consistent with the known plant cyclizing enzymes, suggests that there are cyclizing enzymes yet to be discovered [3].
Potential applications and production methods
One of the drivers behind many of the studies related to plant cyclic peptides has been exploring the potential of these peptides in agricultural or pharmaceutical applications [35–38]. Orbitides have been shown to have a range of bioactivities including anti-cancer and immunomodulating properties [39], and similarly, cyclotides also have a range of bioactivities including insecticidal and antimicrobial activity [40]. At this stage, perhaps the most exciting is a cyclotide analogue that results in diminished symptoms in an experimental autoimmune encephalomyelitis multiple sclerosis mouse model following oral administration. There was no report of adverse effects [41] and this analogue is currently in clinical trials [42]. Furthermore, a cyclotide extract from Clitoria ternatea is approved as an insecticide [43].
There have also been numerous studies on using the cyclic peptides as scaffolds for the design of pharmaceutical lead molecules as reviewed previously [16,44]. These studies have mainly utilised cyclotides and SFTI-1 as the scaffolds, with small bioactive sequences grafted into one of the inter-cysteine loops of the cyclic peptide. Such studies have generally relied on synthetic methods such as native chemical ligation to produce sufficient quantities for structural and functional studies. However, a recent study involved grafting the cystatin first hairpin sequence into the trypsin inhibitor cyclotide MCoTI-II [45] and utilised recombinant expression of the grafted peptide. Although the grafted peptide was able to bind to papain, a property thought to have significant potential in translational medicine, the grafted peptide did not contain the cyclic backbone, highlighting one of the major limitations of this approach for cyclic peptide production.
For cyclic peptides to be useful as pharmaceutical or agricultural agents it is imperative that they can be produced in sufficient quantities using economical processes. The insecticide from C. ternatea is directly obtained from a plant extract and, although this is proving effective in this instance, it has been suggested that limitations of plant extraction, including the developmental stage of a plant, and seasonal fluctuations, could have implications for the viability of this approach [46]. One approach that is being explored for the continuous and uniform production of cyclotides is the use of in vitro cultures. A somatic embryo culture of Viola odorata has been developed whereby the relative abundance of cyclotides was higher in the somatic embryo extract compared with the natural plant extract [46]. The use of a yeast-based production method has also been used in conjunction with in vitro cyclisation using recombinant AEPs [47]. This approach was used to produce a range of cyclic peptides, including an SFTI analogue and cyclotide. The yields were significantly improved over other methods, boding well for future studies in this area.
Given how widespread cyclic peptides are throughout nature, and the complexities associated with the formation of the cyclic backbone, it appears likely that there are significant advantages to compensate for the extra effort involved in producing them. Based on this thought process the efforts going into establishing efficient methods for the production of cyclic peptides are worthwhile. However, the counter argument is that in some cases acyclotides are no less stable than cyclotides because of the stability conferred by the cystine knot motif, and therefore might have less complicated production processes without the cyclic backbone [48]. It appears likely that the formation of three disulfide bonds alone is likely to add complexities to large scale production methods and therefore the differences in the production of cyclotides relative to acyclotides might end up being minimal.
Conclusions
The peptides mentioned in this mini-review by no means represent the full breadth of cyclic peptides identified in plants, but rather highlight the major classes that have been studied recently. There is exciting potential in the field with a plant cyclic peptide currently in clinical trials and methods being developed for large scale production. The academic curiosity related to the discovery of novel cyclic peptides and their biosynthetic mechanisms it not likely to abate soon, but likely to increase. This curiosity is likely to result in significantly more diverse cyclic peptides being discovered and the potential applications expanding. The cyclotides represent one of the largest families of plant cyclic peptides, not only in terms of number of peptides characterised but also molecular mass. The smallest backbone cyclic peptides have already been discovered in plants, including the recent discovery of the dipeptide cyclo(Arg-Trp) from the Chilean hazelnut cotyledons [49], but it remains to be seen whether the cyclotides represent the largest plant cyclic peptides or whether higher molecular mass peptides/proteins await discovery.
Perspective
Backbone cyclisation is a key post-translational modification found predominately in plants.
Understanding the biosynthesis of plant cyclic peptides is likely to facilitate their production and potential applications.
Plant cyclic peptides hold promise for the development of new pharmaceutical and agricultural agents.
Abbreviations
- AEP
asparaginyl endopeptidases
- PCY1
peptide cyclase 1
- PDPs
PawS-derived peptides
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
No specific funding was received for this manuscript.
Open Access
Open access for this article was enabled by the participation of James Cook University in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
Author Contributions
N.L.D. and D.W. wrote the manuscript.
References
- 1.Wang, C.K., Kaas, Q., Chiche, L. and Craik, D.J. (2008) Cybase: a database of cyclic protein sequences and structures, with applications in protein discovery and engineering. Nucleic Acids Res. 36, D206–D210 10.1093/nar/gkm953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan, N.H. and Zhou, J. (2006) Plant cyclopeptides. Chem. Rev. 106, 840–895 10.1021/cr040699h [DOI] [PubMed] [Google Scholar]
- 3.Fisher, M.F., Payne, C.D., Chetty, T., Crayn, D., Berkowitz, O., Whelan, J.et al. (2020) The genetic origin of evolidine, the first cyclopeptide discovered in plants, and related orbitides. J. Biol. Chem. 295, 14510–14521 10.1074/jbc.RA120.014781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher, M.F., Payne, C.D., Rosengren, K.J. and Mylne, J.S. (2019) An orbitide from Ratibida columnifera seed containing 16 amino acid residues. J. Nat. Prod. 82, 2152–2158 10.1021/acs.jnatprod.9b00111 [DOI] [PubMed] [Google Scholar]
- 5.Arnison, P.G., Bibb, M.J., Bierbaum, G., Bowers, A.A., Bugni, T.S., Bulaj, G.et al. (2013) Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 10.1039/C2NP20085F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher, M.F., Zhang, J., Taylor, N.L., Howard, M.J., Berkowitz, O., Debowski, A.W.et al. (2018) A family of small, cyclic peptides buried in preproalbumin since the Eocene epoch. Plant Direct. 2, e00042 10.1002/pld3.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gran, L., Sandberg, F. and Sletten, K. (2000) Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine. J. Ethnopharmacol. 70, 197–203 10.1016/S0378-8741(99)00175-0 [DOI] [PubMed] [Google Scholar]
- 8.Saether, O., Craik, D.J., Campbell, I.D., Sletten, K., Juul, J. and Norman, D.G. (1995) Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry 34, 4147–4158 10.1021/bi00013a002 [DOI] [PubMed] [Google Scholar]
- 9.Craik, D.J., Daly, N.L., Bond, T. and Waine, C. (1999) Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 294, 1327–1336 10.1006/jmbi.1999.3383 [DOI] [PubMed] [Google Scholar]
- 10.Luckett, S., Garcia, R.S., Barker, J.J., Konarev, A.V., Shewry, P.R., Clarke, A.R.et al. (1999) High-resolution structure of a potent, cyclic proteinase inhibitor from sunflower seeds. J. Mol. Biol. 290, 525–533 10.1006/jmbi.1999.2891 [DOI] [PubMed] [Google Scholar]
- 11.Franke, B., Jayasena, A.S., Fisher, M.F., Swedberg, J.E., Taylor, N.L., Mylne, J.S.et al. (2016) Diverse cyclic seed peptides in the Mexican zinnia (Zinnia haageana). Biopolymers 106, 806–817 10.1002/bip.22901 [DOI] [PubMed] [Google Scholar]
- 12.Elliott, A.G., Delay, C., Liu, H., Phua, Z., Rosengren, K.J., Benfield, A.H.et al. (2014) Evolutionary origins of a bioactive peptide buried within Preproalbumin. Plant Cell 26, 981–995 10.1105/tpc.114.123620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayasena, A.S., Fisher, M.F., Panero, J.L., Secco, D., Bernath-Levin, K., Berkowitz, O.et al. (2017) Stepwise evolution of a buried inhibitor peptide over 45 My. Mol. Biol. Evol. 34, 1505–1516 10.1093/molbev/msx104 [DOI] [PubMed] [Google Scholar]
- 14.Zhao, X.F., Zhang, Q., Zhao, H.T., Zhang, Y.D., Liu, H.R., Yuan, L.J.et al. (2020) A new cyclic peptide from the fibrous root of Pseudostellaria heterophylla. Nat. Prod. Res. 1–7 10.1080/14786419.2020.1858413 [DOI] [PubMed] [Google Scholar]
- 15.Burnett, P.G., Young, L.W., Olivia, C.M., Jadhav, P.D., Okinyo-Owiti, D.P. and Reaney, M.J.T. (2018) Novel flax orbitide derived from genetic deletion. BMC Plant Biol. 18, 90 10.1186/s12870-018-1303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Y.H., Du, Q. and Craik, D.J. (2019) Cyclotides: disulfide-rich peptide toxins in plants. Toxicon 172, 33–44 10.1016/j.toxicon.2019.10.244 [DOI] [PubMed] [Google Scholar]
- 17.Du, Q., Chan, L.Y., Gilding, E.K., Henriques, S.T., Condon, N.D., Ravipati, A.S.et al. (2020) Discovery and mechanistic studies of cytotoxic cyclotides from the medicinal herb Hybanthus enneaspermus. J. Biol. Chem. 295, 10911–10925 10.1074/jbc.RA120.012627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang, T.T., Chan, L.Y., Tombling, B.J., Harvey, P.J., Gilding, E.K. and Craik, D.J. (2021) In planta discovery and chemical synthesis of bracelet cystine knot peptides from Rinorea bengalensis. J. Nat. Prod. 84, 395–407 10.1021/acs.jnatprod.0c01065 [DOI] [PubMed] [Google Scholar]
- 19.Pinto, M.E.F., Chan, L.Y., Koehbach, J., Devi, S., Grundemann, C., Gruber, C.W.et al. (2021) Cyclotides from Brazilian Palicourea sessilis and their effects on human lymphocytes. J. Nat. Prod. 84, 81–90 10.1021/acs.jnatprod.0c01069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke, B., Mylne, J.S. and Rosengren, K.J. (2018) Buried treasure: biosynthesis, structures and applications of cyclic peptides hidden in seed storage albumins. Nat. Prod. Rep. 35, 137–146 10.1039/C7NP00066A [DOI] [PubMed] [Google Scholar]
- 21.Mylne, J.S., Colgrave, M.L., Daly, N.L., Chanson, A.H., Elliott, A.G., McCallum, E.J.et al. (2011) Albumins and their processing machinery are hijacked for cyclic peptides in sunflower. Nat. Chem. Biol. 7, 257–259 10.1038/nchembio.542 [DOI] [PubMed] [Google Scholar]
- 22.Jayasena, A.S., Secco, D., Bernath-Levin, K., Berkowitz, O., Whelan, J. and Mylne, J.S. (2014) Next generation sequencing and de novo transcriptomics to study gene evolution. Plant Methods 10, 34 10.1186/1746-4811-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condie, J.A., Nowak, G., Reed, D.W., Balsevich, J.J., Reaney, M.J., Arnison, P.G.et al. (2011) The biosynthesis of Caryophyllaceae-like cyclic peptides in Saponaria vaccaria L. from DNA-encoded precursors. Plant J. 67, 682–690 10.1111/j.1365-313X.2011.04626.x [DOI] [PubMed] [Google Scholar]
- 24.Barber, C.J., Pujara, P.T., Reed, D.W., Chiwocha, S., Zhang, H. and Covello, P.S. (2013) The two-step biosynthesis of cyclic peptides from linear precursors in a member of the plant family Caryophyllaceae involves cyclization by a serine protease-like enzyme. J. Biol. Chem. 288, 12500–12510 10.1074/jbc.M112.437947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saska, I., Gillon, A.D., Hatsugai, N., Dietzgen, R.G., Hara-Nishimura, I., Anderson, M.A.et al. (2007) An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J. Biol. Chem. 282, 29721–8 10.1074/jbc.M705185200 [DOI] [PubMed] [Google Scholar]
- 26.Gillon, A.D., Saska, I., Jennings, C.V., Guarino, R.F., Craik, D.J. and Anderson, M.A. (2008) Biosynthesis of circular proteins in plants. Plant J. 53, 505–515 10.1111/j.1365-313X.2007.03357.x [DOI] [PubMed] [Google Scholar]
- 27.Conlan, B.F., Colgrave, M.L., Gillon, A.D., Guarino, R., Craik, D.J. and Anderson, M.A. (2012) Insights into processing and cyclization events associated with biosynthesis of the cyclic peptide Kalata B1. J. Biol. Chem. 287, 28037–28046 10.1074/jbc.M112.347823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen, G.K., Wang, S., Qiu, Y., Hemu, X., Lian, Y. and Tam, J.P. (2014) Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat. Chem. Biol. 10, 732–738 10.1038/nchembio.1586 [DOI] [PubMed] [Google Scholar]
- 29.Harris, K.S., Durek, T., Kaas, Q., Poth, A.G., Gilding, E.K., Conlan, B.F.et al. (2015) Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase. Nat. Commun. 6, 10199 10.1038/ncomms10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemu, X., El Sahili, A., Hu, S., Wong, K., Chen, Y., Wong, Y.H.et al. (2019) Structural determinants for peptide-bond formation by asparaginyl ligases. Proc. Natl Acad. Sci. U.S.A. 116, 11737–11746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haywood, J., Schmidberger, J.W., James, A.M., Nonis, S.G., Sukhoverkov, K.V., Elias, M.et al. (2018) Structural basis of ribosomal peptide macrocyclization in plants. eLife 7, e32955 10.7554/eLife.32955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehm, F.B.H., Jackson, M.A., De Geyter, E., Yap, K., Gilding, E.K., Durek, T.et al. (2019) Papain-like cysteine proteases prepare plant cyclic peptide precursors for cyclization. Proc. Natl Acad. Sci. U.S.A. 116, 7831–7836 10.1073/pnas.1901807116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du, J., Yap, K., Chan, L.Y., Rehm, F.B.H., Looi, F.Y., Poth, A.G.et al. (2020) A bifunctional asparaginyl endopeptidase efficiently catalyzes both cleavage and cyclization of cyclic trypsin inhibitors. Nat. Commun. 11, 1575 10.1038/s41467-020-15418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franke, B., James, A.M., Mobli, M., Colgrave, M.L., Mylne, J.S. and Rosengren, K.J. (2017) Two proteins for the price of one: structural studies of the dual-destiny protein preproalbumin with sunflower trypsin inhibitor-1. J. Biol. Chem. 292, 12398–12411 10.1074/jbc.M117.776955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camarero, J.A. (2017) Cyclotides, a versatile ultrastable micro-protein scaffold for biotechnological applications. Bioorg. Med. Chem. Lett. 27, 5089–5099 10.1016/j.bmcl.2017.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhuri, D., Aboye, T. and Camarero, J.A. (2019) Using backbone-cyclized Cys-rich polypeptides as molecular scaffolds to target protein-protein interactions. Biochem. J. 476, 67–83 10.1042/BCJ20180792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camarero, J.A. and Campbell, M.J. (2019) The potential of the cyclotide scaffold for drug development. Biomedicines 7, 31 10.3390/biomedicines7020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojeda, P.G., Cardoso, M.H. and Franco, O.L. (2019) Pharmaceutical applications of cyclotides. Drug Discov. Today 24, 2152–2161 10.1016/j.drudis.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 39.Zou, X.G., Li, J., Sun, P.L., Fan, Y.W., Yang, J.Y. and Deng, Z.Y. (2020) Orbitides isolated from flaxseed induce apoptosis against SGC-7901 adenocarcinoma cells. Int. J. Food Sci. Nutr. 71, 929–939 10.1080/09637486.2020.1750573 [DOI] [PubMed] [Google Scholar]
- 40.Grover, T., Mishra, R., Bushra, G.P. and Mohanty, A. (2021) An insight into biological activities of native cyclotides for potential applications in agriculture and pharmaceutics. Peptides 135, 170430 10.1016/j.peptides.2020.170430 [DOI] [PubMed] [Google Scholar]
- 41.Thell, K., Hellinger, R., Sahin, E., Michenthaler, P., Gold-Binder, M., Haider, T.et al. (2016) Oral activity of a nature-derived cyclic peptide for the treatment of multiple sclerosis. Proc. Natl Acad. Sci. U.S.A. 113, 3960–3965 10.1073/pnas.1519960113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gründemann, C., Stenberg, K.G. and Gruber, C.W. (2019) T20k: an immunomodulatory cyclotide on its way to the clinid. Int. J. Pept. Res. Ther. 25, 9–13 10.1007/s10989-018-9701-1 [DOI] [Google Scholar]
- 43.Oguis, G.K., Gilding, E.K., Jackson, M.A. and Craik, D.J. (2019) Butterfly Pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front Plant Sci. 10, 645 10.3389/fpls.2019.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta, L., Dhankhar, R., Gulati, P., Kapoor, R.K., Mohanty, A. and Kumar, S. (2020) Natural and grafted cyclotides in cancer therapy: an insight. J. Pept. Sci. 26, e3246 10.1002/psc.3246 [DOI] [PubMed] [Google Scholar]
- 45.Mishra, M., Singh, V., Tellis, M.B., Joshi, R.S. and Singh, S. (2020) Repurposing the McoTI-II rigid molecular scaffold in to inhibitor of ‘Papain superfamily’ cysteine proteases. Pharmaceuticals (Basel) 14, 7 10.3390/ph14010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayani, M., Sai Varsha, M.K.N., Potunuru, U.R., Sofi Beaula, W., Rayala, S.K., Dixit, M.et al. (2018) Production of bioactive cyclotides in somatic embryos of Viola odorata. Phytochemistry 156, 135–141 10.1016/j.phytochem.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 47.Yap, K., Du, J., Rehm, F.B.H., Tang, S.R., Zhou, Y., Xie, J.et al. (2021) Yeast-based bioproduction of disulfide-rich peptides and their cyclization via asparaginyl endopeptidases. Nat. Protoc. 16, 1740–1760 10.1038/s41596-020-00483-0 [DOI] [PubMed] [Google Scholar]
- 48.Tammineni, R., Gulati, P., Kumar, S. and Mohanty, A. (2020) An overview of acyclotides: past, present and future. Phytochemistry 170, 112215 10.1016/j.phytochem.2019.112215 [DOI] [PubMed] [Google Scholar]
- 49.Schmeda-Hirschmann, G., de Andrade, J.P., Jimenez-Aspee, F. and Mieres-Castro, D. (2020) A cyclic dipeptide from the Chilean hazelnut cotyledons (Gevuina avellana Mol. Proteaceae). Sci. Rep. 10, 7070 10.1038/s41598-020-63983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]