Abstract
Introduction
Gram-negative nosocomial pneumonia (NP), including hospital-acquired bacterial pneumonia (HABP), ventilated HABP (vHABP), and ventilator-associated bacterial pneumonia (VABP), is a significant cause of morbidity and mortality. Common pathogens, including Enterobacterales and Pseudomonas aeruginosa, are prevalent in healthcare settings and have few effective treatment options due to high rates of antibacterial resistance. Resistant pathogens are associated with significantly worse outcomes, relative to patients with susceptible infections. Ceftolozane/tazobactam (C/T) has established efficacy in clinical trials of patients with NP. This review aims to collate data on C/T use for HABP/vHABP/VABP infections in real-world clinical practice.
Methods
This systematic literature review searched online biomedical databases for real-world studies of C/T used to treat Gram-negative respiratory tract infections (RTIs) between January 2009 and June 2020.
Results
Thirty-three studies comprising 658 patients were identified. Pneumonia was the most common infection treated with C/T (85%), with a smaller number of unspecified RTIs (9%) and tracheobronchitis (5%) reported. The majority of patients had respiratory infections caused by P. aeruginosa (92.8%), of which 88.1% were multidrug-resistant. Examination of these studies demonstrated an increase in the percentage of patients receiving the recommended dose of C/T for respiratory infections (3 g q8h or renal impairment-adjusted) over time (36.8% of patients in 2017 to 71.5% in 2020). Clinical success rates ranged from 51.4 to 100%, with 10 studies (55.6% of studies reporting clinical success) reporting clinical success rates of > 70%; microbiological success rates ranged from 57.0 to 100.0%, with three studies (60.0% of studies reporting microbiological success) reporting microbiological success rates of > 70%. Thirty-day mortality ranged from 0.0 to 33.0%, with nine studies (90% of studies reporting mortality) reporting 30-day mortality of < 30%.
Conclusions
The studies identified in this review demonstrate that C/T shows similar outcomes as those seen in clinical trials, despite the higher frequency of multidrug-resistant pathogens, and comorbidities that may have been excluded from the trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-021-00491-x.
Keywords: Ceftolozane, Hospital-acquired bacterial pneumonia, Pseudomonas aeruginosa, Real-world evidence, Respiratory tract infection, Tazobactam, Ventilator-associated bacterial pneumonia
Key Summary Points
| Gram-negative nosocomial pneumonia, including hospital-acquired bacterial pneumonia (HABP), ventilated HABP (vHABP), and ventilator-associated bacterial pneumonia (VABP), is associated with significant morbidity and mortality. |
| Common causative pathogens, including Enterobacterales and Pseudomonas aeruginosa, are prevalent in healthcare settings and there are few effective treatment options due to high rates of antimicrobial resistance. |
| This review aimed to collate data on the use of ceftolozane/tazobactam (C/T) for the treatment of patients with Gram-negative respiratory tract infections in real-world clinical practice. From the 33 studies identified (n = 658 patients), clinical success rates ranged from 51.4 to 100%, microbiological success rates ranged from 57.0 to 100%, while 30-day mortality ranged from 0.0 to 33.0%. |
| This review demonstrated significant evidence pertaining to the effectiveness of C/T for the treatment of patients with HABP/vHABP/VABP and showed similar outcomes in the real-world setting to those seen in clinical trials, despite the higher frequency of multidrug-resistant pathogens and comorbidities which may have been excluded from the trials. |
Introduction
Gram-negative nosocomial pneumonia (NP) is a significant cause of morbidity and mortality. NP includes hospital-acquired bacterial pneumonia (HABP), when the infection occurs after 48 h in hospital, or ventilator-associated bacterial pneumonia (VABP), when the infection develops following 48 h of ventilation [1]. Ventilated HABP (vHABP) occurs when patients with HABP require ventilation due to declining health [2]. HABP represents the most common cause of death in critically ill patients, and VABP is the most frequently reported healthcare-acquired infection in intensive care units (ICUs) [3]. Patients with vHABP tend to suffer higher mortality than patients with VABP, whereas patients with VABP tend to suffer higher mortality than patients with HABP [4] .
Gram-negative HABP/VABP/vHABP are commonly caused by Enterobacterales and Pseudomonas aeruginosa [5]. There are limited treatment options because of the growing rates of resistance of these pathogens to available therapy. Multidrug-resistant (MDR) pathogens are resistant to antibacterial agents in three or more classes and are prevalent in the United States (US) and Europe [6, 7]. MDR P. aeruginosa is associated with higher mortality, longer length of stay, excess costs, higher readmission rates, and > US$10,000 excess net loss per case for the hospital relative to those with non-MDR P. aeruginosa infections [8]. Resistant pathogens also increase the likelihood of initial inappropriate antibacterial therapy (IIAT). This is when the initial treatment is ineffective, which results in diminished clinical outcomes and increased health care costs [9, 10].
The burden is such that the World Health Organization has named these common pathogens of NP a critical priority for the development of new antibacterials [11]. Ceftolozane/tazobactam (C/T) is a β-lactam/β-lactamase inhibitor antibacterial agent, consisting of a fixed (2:1) combination of an antipseudomonal cephalosporin, ceftolozane, and a well-established β-lactamase inhibitor, tazobactam [12]. C/T is approved in the US and Europe for clinical use in adults with HABP/VABP. The approval of C/T for HABP/VABP was supported by a multinational, randomized, double-blind, active comparator-controlled trial: ASEPCT-NP [13]. Since launch in 2014, the use of C/T in clinical practice has been accumulating. The purpose of this systematic literature review (SLR) was to identify and collate published evidence of C/T used in clinical practice to better understand the outcomes in patients with RTIs treated with C/T.
Methods
Literature Search
The full methodology is described in Puzniak et al. 2021 [14]. Briefly, a search of the literature for C/T used in clinical practice published between 1 January 2009 and 3 June 2020, was conducted in the following biomedical and economic databases via the OVID® platform: Embase®, MEDLINE®, PsycInfo, Econlit, and EBM Reviews (ACP Journal Club, Cochrane Database of Systematic Reviews, Cochrane Methodology Register, Database of Abstracts of Reviews of Effects, Health Technology Assessment, NHS Economic Evaluation Database, Cochrane Clinical Answers) [14]. Table 1 describes the search strategy. A further search of conference proceedings [Infectious Disease Week (IDWeek) and European Congress of Clinical Microbiology & Infectious Diseases (ECCMID)] from 2018, 2019, and 2020, was also conducted [14].
Table 1.
OVID search strategy
| # | Search terms |
|---|---|
| 1 | Ceftolozane/ OR Ceftolozane plus tazobactam/ |
| 2 | ((Ceftolozane adj1 tazobactam) OR ZERBAXA OR MK-7625A).ti,ab |
| 3 | 1 OR 2 |
| 4 | (exp animals/ OR nonhuman/) NOT exp human/ |
| 5 | exp controlled clinical trial/ |
| 6 | 4 OR 5 |
| 7 | 3 NOT 6 |
| OVID subtotal (deduplicated and limitsa applied) | |
| TOTAL (EndNote deduplication) | |
aEnglish and 2014–current
Study Selection
Two reviewers screened all records on the basis of title and abstract, with inclusions then screened on the basis of the full-text. Predetermined inclusion and exclusion criteria were used to assess the eligibility of identified abstracts and full-texts for inclusion. PICOS eligibility criteria included observational and non-controlled studies reporting on the use of C/T to treat adult patients (≥ 18 years of age) with Gram-negative infections in real-world clinical practice. This review includes data identified on the use of C/T to treat RTIs. Only studies in English were included. Studies were excluded if they did not meet the PICOS criteria, such as randomized controlled trials or other randomized or controlled experimental studies. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. A complete description of the PICOS criteria is provided in Table S1.
Data Extraction and Analysis
For studies that included patients with RTIs, and presented outcomes of interest in these patients, relevant study, patient, and treatment characteristics, microbiology, and efficacy outcomes were extracted by one reviewer and checked by a senior reviewer. Efficacy outcomes included clinical cure (typically defined as the resolution of signs or symptoms of RTI following therapy and survival), microbiological cure (typically defined as large reduction or eradication in the number of pathogens following therapy), and mortality (e.g., all-cause, inpatient, infection-related). Upon identifying such outputs contained in the SLR, the authors identified RTIs as a common indication for C/T and so conducted a sub-group analysis of these studies. Given the heterogenous nature of the data, a descriptive, qualitative analysis of a sub-group of comparable studies was conducted in order to shed light on understanding the outcomes of treatment with C/T in patients with RTIs.
Results
SLR Results
As reported in Puzniak et al. 2021, a total of 1222 records were identified from the database searches, with 23 records identified from the gray literature, which included a search of key conferences to identify any evidence not reported in the published literature [14]. Then, 874 non-duplicate records were screening by title and abstract; 730 records were excluded according to the PICOS criteria and 144 were included for full-text review [14]. Eighty-three studies were determined to be eligible for data extraction and qualitative synthesis; of these, 33 included patients with RTIs and presented outcomes of interest for these patients. As two-fifths (33/83) of studies represented a certain degree of consistency (e.g., focusing on RTIs), a descriptive analysis was conducted to describe the outcomes of treatment with C/T in this patient population. The results of the SLR and study selection processes are presented in Fig. 1.
Fig. 1.
PRISMA flow diagram for study selection. *‘Other’ includes duplicate records identified at the full-text stage and records that were identified as either conference proceedings or pre-publication manuscripts in the initial or November 2019 search, and then identified again as full-text publications in either the November 2019 or June 2020 search
Study Characteristics
Of the 33 studies included in the SLR that included data on patients with RTIs (and presented outcomes for these patients), 28 were published as peer-reviewed publications [15–42], and 5 were available as conference proceedings (either as abstracts or posters) [43–47]. Including studies that included patients from multiple different countries, the most common study locations were the US (23 studies) [15, 21, 24–33, 35–37, 39–41, 43–47], Spain (5) [20, 22, 23, 36, 42], and Italy (3) [15, 18, 19]. There was a mix of study designs included: of nine non-comparative studies, eight were retrospective [18, 19, 23, 24, 31, 36, 43, 46], and one was prospective [22]. There were nine case series identified [16, 20, 25, 27, 28, 30, 42, 44, 47] and one comparative cohort study [45]. A total of 14 single-patient case reports were screened [15, 17, 21, 26, 29, 32–35, 37–41]. Table 2 summarizes the characteristics of included studies.
Table 2.
Summary of respiratory studies
| Citation, study design, location | n C/T (Resp.) | Respiratory patient/infection description (n) | Respiratory patient disease severity | Respiratory C/T treatment | Respiratory outcome, % (n/N) | |||
|---|---|---|---|---|---|---|---|---|
| Clinical | Micro | Mortality | ||||||
| 2020 studies | ||||||||
| Peer-reviewed literature | ||||||||
|
Bassetti et al. (2020) [19] Retrospective, multicentre Italy |
153 (47) | ESBL-producing Enterobacterales infections, including NP (46) and CABP (1). Of patients with NP, 32 had HABP and 14 had VABP | – | Dose C/T: 38 patients (of all infection types included in the study—including NP and non-NP infections) received 3 g q8ha |
NP: 78.3 (36/46) CABP: 100 (1/1) |
– | – | |
|
Jones et al. (2020) [30] Retrospective single center, case series US |
7 (3) | PsA (2 non-MDR; 1 MDR) pneumonia | – |
Dose C/T: 2 patients received 4.5 g qd continuous infusion (CI), 1 patient received 9 g qd CI Duration: 1 patient who received 9 g qd: 6 days, 2 patients who received 4.5 g qd: 14–17 days |
100 (3/3) | – | – | |
|
Jorgensen et al. (2020) [31] Retrospective, multicentre US |
259 (163) MDR PsA: 226 (149) |
Patients had MDR Gram-negative infections, 163 of which had RTIs, of which 96 were VABP. Patients with MDR PsA infections (n = 226) were used as the primary analysis set. Of these 226, 149 had infections from a respiratory source, of which 89 were VABP | – |
Dose C/T: Overall RTI population: 116/163 patients received 3 g q8h (71.2%). 48 patients received a creatinine clearance adjusted dose: 19 (39.6%) received an adjusted dose based on 3 g q8h MDR PsA RTI population: 105/149 patients received 3 g q8h (70.5%) Duration: med. (IQR): 10 (6–15) days |
– | – |
30-day: MDR PsA Resp. 24.2 (NR) |
|
|
Mahmoud et al. (2020) [35] Case report US |
1 (1) | MDR PsA RTI (1) | ICU n = 1 |
Dose C/T: 3 g q8h initially, then 9 g qd CI Empiric/confirmed C/T: Confirmed Duration: 7 days |
– | 100 (1/1) | – | |
|
Romano et al. (2020) [37] Case report US |
1 (1) | MDR PsA pulmonary exacerbation of cystic fibrosis (1) | – |
Dose C/T: 3 g q8h Empiric/confirmed C/T: Confirmed Duration: 14 days |
100 (1/1) | – | Unclear timeframe: 0 (0/1) | |
| 2019 studies | ||||||||
| Peer-reviewed literature | ||||||||
|
Arena et al. (2019) [17] Case report Italy |
1 (1) | CR PsA pulmonary infection in a lung transplant recipient (1) | IMC n = 1 |
Dose C/T: 3 g q8h Empiric/confirmed C/T: Confirmed Duration: 15 days |
100 (1/1) | 100 (1/1) | Unclear timeframe: 0 (0/1) | |
|
Bassetti et al. (2019) [18] Retrospective, multicentre Italy |
101 (33) | PsA infections, including NP (32) and CABP (1) | – | Dose C/T: 21 patients with NP received 3 g q8h (65.6%), 11 received 1.5 g q8h (34.6%). 20 patients of all infection types received a creatinine clearance adjusted dose (the number of patients with NP who received this is NR) | NP: 75.0 (24/32) | – | – | |
|
Davis et al. (2019) [21] Case report US |
1 (1) | MDR PsA and ESBL-producing E. coli pulmonary exacerbation of cystic fibrosis (1) | ICU n = 1 |
Dose C/T: 6 g CI qd Empiric/confirmed C/T: Confirmed |
100 (1/1) | – | Unclear timeframe: 0 (0/1) | |
|
Gonzales Zamora et al. (2019) [26] Case report US |
1 (1) | MDR PsA and Curvularia spp. (a species of fungus) pneumonia and bacteremia, then Curvularia spp. brain abscess (1) | IMC n = 1 | Duration: 14 days | – | – | Unclear timeframe: 100b (1/1) | |
|
Maddocks et al. (2019) [34] Case report Australia |
1 (1) | PsA VABP (1) | ICU n = 1 |
Dose C/T: 1.5 g q8h, creatinine clearance adjusted Empiric/confirmed C/T: Confirmed, as desensitization therapy Duration: 42 days |
100c (1/1) |
100c (1/1) |
30-day: 0c (0/1) | |
|
Rodriguez-Nunez et al. (2019) [36] Retrospective, multicentre International |
90 (90) | Drug-resistant PsA RTIs (76.7% XDR; 23.3% MDR), including pneumonia (63) and purulent tracheobronchitis (27) | CCI med. = 5 |
Dose C/T: 1.5 g q8h or creatinine clearance adjusted; 40%, 3 g q8h or double creatinine clearance 60% Duration: med. (IQR): 14 (10–16) days |
Overall: 56.7 (51/90) | – |
30-day: Overall: 27.8 (25/90) 3 g q8h: 24.1 (13/54) 1.5 g q8h: 33.3 (12/36) |
|
| Conference proceedings | ||||||||
|
Hart et al. (2019) [43] Retrospective, multicentre US |
70 (39) | MDR PsA infections, including pneumonia (39), in IMC patients | IMC n = 39 | – | 61.5 (24/39) | – | 30-day: 20.5 (8/39) | |
|
Mills et al. (2019) [45] Retrospective, multicenter cohort US |
62 (62) | MDR PsA pneumonia (62) |
ICU n = 49 IMC n = 13 |
Duration mean: 16.1 days | 72.6 (45/62) | – | 30-day: 29.0 (18/62) | |
|
Sheffield et al. (2019) [47] Retrospective, case series US |
4 (1) | PsA or ESBL-producing E. coli infections, including RTI (1) | – | – | – | – |
Unclear timeframe: 0 (0/1) |
|
| 2018 studies | ||||||||
| Peer-reviewed literature | ||||||||
|
Alessa et al. (2018) [15] Case report US |
1 (1) | MDR PsA NP in a patient receiving hemodialysis (1) | – |
Dose C/T: 1.5 g loading dose then 0.3 g q8h (patient had ESRD) Empiric/confirmed C/T: Confirmed Duration: 13 days |
100 (1/1) | 100 (1/1) |
Unclear timeframe: 0 (0/1) |
|
|
Diaz-Cañestro et al. (2018) [22] Prospective, single center Spain |
58 (35) | PsA infections, including RTIs (35) | – |
Dose C/T: Of 25 patients with RTIs without renal insufficiency, 3 received 1.5 g q8h, and 22 received 3 g q8h Of 9 patients with RTIs on CRRT, 7 received 1.5 g q8h and 2 received 0.75 g q8h 1 patient with an RTI and moderate renal insufficiency (creatinine clearance 30–50 mL/min) received 1.5 g |
54.5 (18/33) | – | – | |
|
Escolà-Vergé et al. (2018) [23] Retrospective, single center Spain |
38 (14) | XDR PsA infections, including RTIs (14) | – | Dose C/T: 9 patients received 3 g q8h (or creatinine clearance adjusted equivalent), 5 received 1.5 g q8h (or creatinine clearance adjusted equivalent) | 78.6 (11/14) | – | – | |
|
Gallagher et al. (2018) [24] Retrospective, multicentre US |
205 (121) | MDR PsA infections, including pneumonia (121), of which 58 patients had VABP and 63 patients had non-VABP | – | Dose C/T: 97 patients (of all infection types included in the study) received 3 g q8ha |
Overall: 66.1 (80/121) VABP: 50.0 (29/58) Non-VABP: 81.0 (51/63) |
Overall: 57.0 (69/121) VABP: 53.4 (31/58) Non-VABP: 60.3 (38/63) |
30-day or inpatient: Overall: 25.6 (31/121) VABP: 37.9 (22/58) Non-VABP: 14.2 (9/63) |
|
|
Hakki et al. (2018) [28] Retrospective, single center, case series US |
6 (3) | MDR PsA infections, including pneumonia (3) in patients with hematological malignancy or hematopoietic stem cell transplant | IMC n = 3 |
Dose C/T: All patients received 3 g q8h Duration med. (range): 31 (14–103) days |
66.7 (2/3) | – |
30-day: 0 (0/3) |
|
|
Lewis et al. (2018) [33] Case report US |
1 (1) | MDR PsA HCAP complicated by lung abscess (1) | – |
Dose C/T: 1.5 g q8h Empiric/confirmed C/T: Empiric Duration: 12 days |
0 (0/1) | 0 (0/1) |
Unclear timeframe: 100 (1/1) |
|
|
Stewart et al. (2018) [39] Case report Australia |
1 (1) | MDR PsA pulmonary infection in kidney transplant (1) |
ICU n = 1 IMC n = 1 |
Dose C/T: 4.5 g qd continuous infusion Empiric/confirmed C/T: Confirmed Duration: 42 days |
100 (1/1) |
– |
30-day: 0 (0/1) |
|
|
Stokem et al. (2018) [40] Case report US |
1 (1) | MDR PsA pulmonary exacerbation of cystic fibrosis (1) | IMC n = 1 |
Dose C/T: 3 g q12h Duration: 14 days |
100 (1/1) | – |
30-day: 0 (0/1) |
|
|
Xipell et al. (2018) [42] Retrospective, single center, case series Spain |
23 (8) | MDR PsA infections, including pneumonia (4) and tracheobronchitis (4) | IMC n = 2 |
Dose C/T: 3 patients received 3 g q8h (with 2 then receiving 1.5 g q8h), 5 patients received 1.5 g q8h Empiric C/T: 12.5% Confirmed C/T: 87.5% Duration median (range): 7.5 (3–15) days |
Overall: 87.5 (7/8) 3 g q8h: 100.0 (3/3) 1.5 g q8h: 80.0 (4/5) |
Overall: 60.0 (3/5) 3 g q8h: 100.0 (1/1) 1.5 g q8h: 50.0 (2/4) |
30-day: Overall: 12.5d (1/8) 3 g q8h: 0 (0/3) 1.5 g q8h: 20.0 (1/5) |
|
| 2017 studies | ||||||||
| Peer-reviewed literature | ||||||||
|
Álvarez Lerma et al. 2017 [16] Retrospective, single center, case series Spain |
2 (2) | PDR PsA ventilation-associated RTIs (2) |
ICU n = 2 APACHE II mean = 25.5 |
Dose C/T: 1 patient received 1.5 g q8h then 0.75 g q8h, 1 patient received 0.75 g q8h (both patients had renal impairment, but the degree of this was NR) Empiric C/T: 0% Confirmed C/T: 100% Duration: mean = 15.5 days |
100 (2/2) |
100 (2/2) |
30-day: 0e (0/2) |
|
|
Castón et al. (2017) [20] Retrospective, multicenter, case series Spain |
12 (6) | MDR PsA infections, including RTIs (6), patients either had severe sepsis or septic shock | IMC n = 2 |
Dose C/T: 3 patients received 3 g q8h, 3 received 1.5 g q8h (it was unclear whether patients had renal impairment) Empiric C/T: 0% Confirmed C/T: 100% Duration med. (range): 12 (3–21) days |
Overall: 66.7 (4/6) 3 g q8h: 66.7 (2/3) 1.5 g q8h: 66.7 (2/3) |
Overall: 60.0 (3/5) 3 g q8h: 100.0 (3/3) 1.5 g q8h: 0 (0/2) |
30-day: Overall: 33.3 (2/6) 3 g q8h: 33.3 (1/3) 1.5 g q8h: 33.3 (1/3) |
|
|
Haidar et al. (2017) [27] Retrospective, single center, case series US |
21 (18) | MDR PsA infections, including pneumonia (16) and purulent tracheobronchitis (2) | IMC n = 8 |
Dose C/T: 5 patients received 3 g q8h (or creatinine clearance adjusted), 9 received 1.5 g q8h (or creatinine clearance adjusted), 2 on CRRT received 1.5 g q8h and 2 on iHD received 0.15 g q8h Duration med. (range): 14 (3–52) days |
Overall: 66.7 (12/18) 3 g q8h: 80.0 (4/5) 1.5 g q8h: 66.7 (6/9) |
- |
30-day: Overall:f 11.1 (2/18) 3 g q8h: 0 (0/5) 1.5 g q8h: 11.1 (1/9) |
|
|
Hernández-Tejedor et al. (2017) [29] Case report US |
1 (1) | MDR PsA ventilator-associated tracheobronchitis (1) |
ICU n = 1 IMC n = 1 |
Dose C/T: 1.5 g q8h Empiric/confirmed C/T: Confirmed Duration: 10 days |
100 (1/1) |
100 (1/1) |
Unclear timeframe: 0 (0/1) |
|
| 2016 studies | ||||||||
| Peer-reviewed literature | ||||||||
|
Kuti et al. (2016) [32] Case report US |
1 (1) | MDR PsA VABP (1) | ICU n = 1 |
Dose C/T: 3 g q8h Empiric/confirmed C/T: Confirmed Duration: 10 days |
100 (1/1) |
100 (1/1) |
Unclear timeframe: 0 (0/1) |
|
|
Vickery et al. 2016 [41] Case report US |
1 (1) | MDR PsA pulmonary exacerbation of cystic fibrosis (1) | - |
Dose C/T: 3 g q8h Empiric/confirmed C/T: Confirmed Duration: 12 days |
100 (1/1) |
- |
30-day: 0 (0/1) |
|
| Conference proceedings | ||||||||
|
Iovleva et al. (2016) [44] Retrospective, single center, case series US |
2 (2) | Imipenem-resistant PsA HCAP (2) |
APACHE II mean = 13 CCI mean = 2 |
- |
100 (2/2) |
100 (2/2) |
Unclear timeframe: 0 (0/3) |
|
|
Nathan et al. (2016) [46] Retrospective, multicentre US |
28 (8) | Gram-negative infections, including pneumonia (5), bronchiectasis (2), chronic pansinusitis (1) | ICU n = 0 | Duration: med. (range) = 12 (4–40) days |
100 (8/8) |
- | - | |
| 2015 studies | ||||||||
| Peer-reviewed literature | ||||||||
|
Gelfand et al. (2015) [25] Retrospective, single center, case series US |
3 (3) | MDR PsA pneumonia (3) | IMC n = 2 |
Dose C/T: All patients received 3 g q8h Duration mean (range): 12.7 (10–14) days |
100 (3/3) |
100 (3/3) |
Unclear timeframe: 0 (0/3) |
|
|
Soliman et al. (2015) [38] Case report UK |
1 (1) | PDR PsA exacerbation of chronic pulmonary infection (bronchiectasis) (1) | - |
Dose C/T: 3 g q8h Empiric/confirmed C/T: Confirmed Duration: 14 days |
100 (1/1) |
100 (1/1) |
30-day: 0 (0/1) |
|
APACHE acute physiology and chronic health evaluation, CABP community-acquired bacterial pneumonia, CCI Charlson Comorbidity index, CI continuous infusion, CR carbapenem-resistant, CRRT continuous renal replacement therapy, C/T ceftolozane/tazobactam, ESBL extended-spectrum β-lactamase, HABP hospital-acquired bacterial pneumonia, HCAP healthcare-associated pneumonia, ICU intensive care unit, iHD intermittent hemodialysis, IMC immunocompromised, IQR interquartile range, MDR multidrug-resistant, NP nosocomial pneumonia, NR not reported, PDR pandrug-resistant, PsA Pseudomonas aeruginosa, RTI respiratory tract infection, UK United Kingdom, US United States, VABP ventilator-associated bacterial pneumonia, XDR extensively drug-resistant
aIt is unclear what proportion of patients with RTIs received 3 g q8h doses
bThe patient died of multi-organ failure; it is unclear from the publication whether this was due to the PsA infection or the Curvularia spp. brain abscess
cPatient was started on C/T 5 days after starting bacteriophage therapy. The publication notes that ‘at this time the patient had made “remarkable progress over the last week”’ (after starting bacteriophage therapy)
d2 patients were reported as cured that died of underlying diseases 25 and 33 days, respectively, after cure
e1 patient had favorable clinical and microbiological cure after 14 days of C/T, then died of refractory heart failure 3 weeks after discharge from the ICU
f1 death was attributable to infection
Patient Characteristics
Identified studies included a total of 658 patients with RTIs treated with C/T. Considering only studies with more than one patient (n = 18), the median number of patients included was 16 (range 2 [16, 44]–149 [31]). Of these studies, five included only patients with RTIs [16, 25, 36, 44, 45], and 14 included patients of multiple infections types and reported data for the respiratory subset [18–20, 22–24, 27, 28, 30, 31, 42, 43, 46, 47].
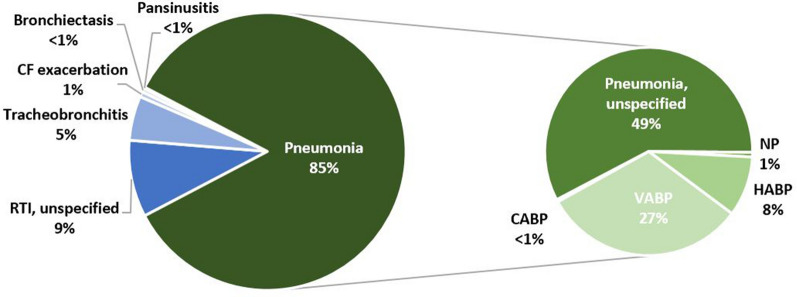
Pneumonia was the most common infection C/T was used to treat (85% of patients; n = 557; Fig. 2). Unspecified pneumonia—when the location (hospital/nosocomial or community) or ventilation status were not specified—comprised the majority of reported pneumonias and 49% (n = 322) of all RTIs reported. VABP (27% of all patients; n = 177) was more commonly reported than NP (1%; N = 4) or HABP (8%; n = 52) —though it is possible that a larger proportion of unspecified pneumonias were NP or HABP.
Fig. 2.
Types of RTIs treated with C/T in clinical practice. CABP community-acquired bacterial pneumonia, CF cystic fibrosis, HABP hospital-acquired bacterial pneumonia, NP nosocomial pneumonia, RTI respiratory tract infection, VABP ventilator-associated bacterial pneumonia
Of non-pneumonia infections, unspecified RTIs were most common (9%; n = 59). Tracheobronchitis was reported in 5% (n = 34) of patients. Pulmonary exacerbation of cystic fibrosis (CF), bronchiectasis, pansinusitis, and CABP were reported in < 1% of patients (Fig. 2).
The severity of patient illness was inconsistently reported, with the majority of multi-patient, multi-infection studies not reporting the severity of illness specific to patients with RTIs. However, of those studies that reported severity of illness, patients were often classified as seriously ill with multiple comorbidities. Eight studies reported that 57 patients with RTIs were admitted to the ICU [16, 21, 29, 32, 34, 35, 39, 45]. The majority of these studies were either single-patient case reports (6 studies) [21, 29, 32, 34, 35, 39], or a case series comprising two patients [16]. One larger study—conducted in patients with RTIs only—reported that 49 patients (of 62 recruited; 79.0%) were admitted to the ICU [45].
This literature review additionally captured two commonly used measures of severity of illness: Acute Physiology and Chronic Health Evaluation (APACHE) II and Charlson Comorbidity index (CCI). Two case series’ (n = 4 patients) reported mean APACHE II scores of 25.5 [16] and 13 [44]. In comparison, patients enrolled in the ASPECT-NP clinical trial, which assessed the efficacy and safety of C/T versus meropenem in 726 patients with Gram-negative NP, had a similar mean APACHE II score of 17 [13]. Two studies reported CCI scores [36, 44]—including the largest study identified that only reported solely on RTIs (n = 90 patients) [36]. The median CCI score in this study was 5, which is indicative of severe comorbidity [36]. The other study (n = 2 patients) reported a mean CCI score of 2, indicating less severe comorbidity [44].
Twelve studies reported 74 patients that were immunocompromised [17, 20, 25–29, 39, 40, 42, 43, 45]. Of these studies, two included only immunocompromised patients [28, 43]. As with ICU patients, this is likely an underestimation as most multi-infection studies did not provide a breakdown by infection type. This review considered immunocompromised patients as either those author-defined as immunocompromised, or as those with a history of organ transplant, disease suppressing immunity (e.g., HIV/AIDS, lymphoma, leukemia), receipt of chemotherapy, or immunosuppressive treatment (e.g., corticosteroids).
Thirty-two studies, comprising 650 patients (98.8% of patients), reported a causative pathogen [15–18, 20–45, 47]. The most prevalent causative pathogen reported was P. aeruginosa (92.8%; n = 603 patients; 31 studies), of which 11.9% (n = 72) were caused by non-resistant P. aeruginosa, or the resistance level was not specified, 0.5% (n = 3) were caused by carbapenem-resistant (CR) P. aeruginosa, 73.3% (n = 442) were caused by MDR P. aeruginosa, 13.8% (n = 83) were caused by extensively-drug-resistant (XDR) P. aeruginosa, and 0.5% (n = 3) were caused by pandrug-resistant (PDR) P. aeruginosa. In the other study that reported a causative pathogen, all patients (n = 47) had an extended-spectrum β-lactamase (ESBL)-positive Enterobacterales infection [19].
Treatment Characteristics
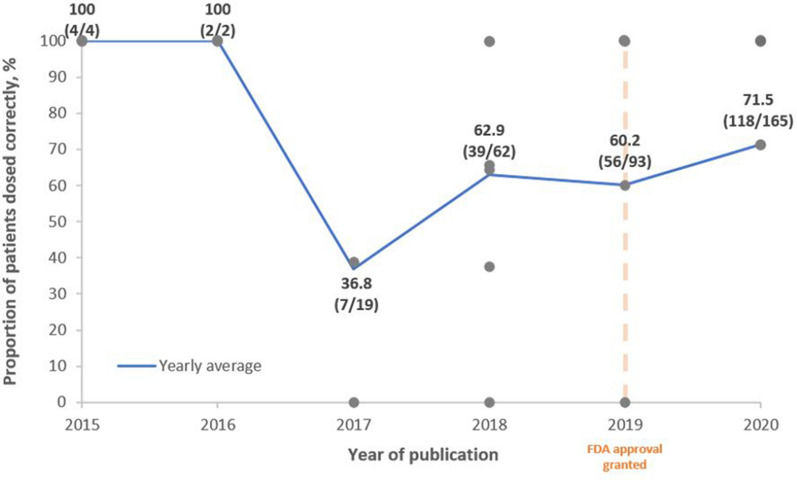
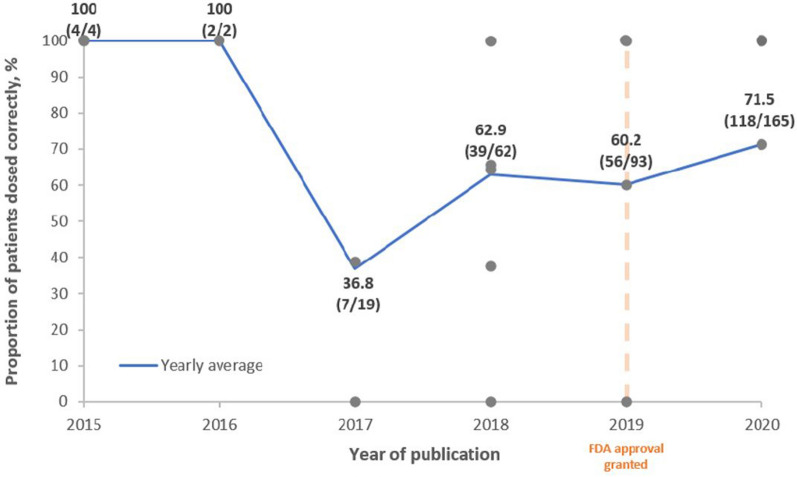
According to the C/T label, the Food and Drug Administration (FDA) recommends that the dosage of C/T to treat HABP/VABP is 3 g q8h for patients with creatinine clearance of > 50 mL/min. For patients with renal impairment, the recommended dosage is adjusted to account for decreased kidney function: estimated creatinine clearance of 30–50 mL/min = C/T 1.5 g q8h; 15–29 mL/min = 750 mg q8h; hemodialysis = 2.25 g loading dose followed by 450 mg q8h. To assess whether C/T was prescribed according to recommended doses, Fig. 3 shows the usage of the 3 g q8h (or adjusted for creatinine clearance) dose according to the year of publication. Nineteen studies reported dosing information (including information about renal impairment, where appropriate) [15, 17, 21–23, 25, 27–29, 31, 32, 34–38, 40–42]. Studies were not included in Fig. 3 if renal impairment status of patients was unclear. Case reports were recorded as 100% when dosed according to FDA recommendations, or 0% when not, hence the number of data points at either extreme. These data suggest that dosing of C/T used to treat RTIs has become more consistent with the current FDA recommendation overtime. Discounting 2015 and 2016, where few data were available, the proportion of patients dosed according to FDA recommendations has gradually increased from 36.8% in 2017 to 71.5% in 2020 in the published literature (Fig. 3).
Fig. 3.
FDA dosing* by year of publication. Each gray point represents a distinct study. The blue line represents the yearly average. The orange line represents the year in which the FDA approved the 3 g q8h dosing for HABP/VABP. *FDA dosing for RTIs: 3 g q8h (or creatinine clearance adjusted). FDA Food and Drug Administration, HABP hospital-acquired bacterial pneumonia; RTI respiratory tract infection, VABP ventilator-associated bacterial pneumonia
The duration of C/T was often significantly different to the label recommended duration of 8–14 days. Duration of therapy ranged from 7 to 42 days, irrespective of dose. In larger studies (> 30 patients), median duration ranged from 10 to 16.1 days, consistent with the indicated duration. Excluding single-patient case reports, 3 studies (67 patients) reported an average duration of C/T exceeding the label maximum dose of 14 days [16, 28, 45]; with one study (3 patients) reporting an average duration of > 28 days [28]. In this study, patients were immunocompromised, each of whom had hematologic malignancies or were hematopoietic-cell transplant recipients, and were infected by MDR P. aeruginosa [28]. Moreover, three single-patient case reports reported C/T durations exceeding the maximum label dose [17, 34, 39]; with two studies reporting a 42-day duration [34, 39]. In these two studies, C/T was used to treat a recurrent CR P. aeruginosa infection in a patient admitted to the ICU [39], and as desensitization therapy for a patient with VABP [34].
Outcomes
Overall Outcomes
Every included study reported outcomes for respiratory patients: 29 reported clinical outcomes [15–25, 27–30, 32–34, 36–46], 13 reported microbiological outcomes [15–17, 24, 25, 29, 32–35, 38, 42, 44], and 26 reported mortality outcomes [15–17, 20, 21, 24–29, 31–34, 36–45, 47].
Excluding single-patient case reports, 17 studies (including 494 patients) reported clinical success rates of 51.4 [22]–100.0% [16, 25, 30, 44, 46], with 10 studies (55.6%) reporting success rates of > 70% [16, 18, 19, 23, 25, 30, 42, 44–46]. In larger studies (> 30 patients; 7 studies) [18, 19, 22, 24, 36, 43, 45], clinical success rates ranged from 51.4 [22] to 78.3% [19]. Microbiological success rates were reported by five multi-patient studies (including 136 patients) [16, 24, 25, 42, 44], and ranged from 57.0 [24] to 100.0% [16, 25, 44], with three studies (60.0%) reporting success rates of > 70% [16, 25, 44]. In a larger study (> 30 patients; one study), microbiological success was 57.0% [24]. Thirty-day mortality rates were reported by 10 multi-patient studies (including 498 patients) [16, 20, 24, 27, 28, 31, 36, 42, 43, 45], and ranged from 0.0 [16, 28] to 33.0% [20]. In larger studies (> 30 patients; five studies) [24, 31, 36, 43, 45], 30-day mortality ranged from 20.5 [43] to 29.0% [45]. Two further multi-patient studies (including five patients) both reported 0.0% mortality rates, though did not specify a timeframe [25, 44].
Outcomes were consistent in studies including one patient (14 case reports and one case series with a single respiratory patient) [15, 17, 21, 26, 29, 32–35, 37–41, 47]. Clinical success was reported in 11 of 12 studies (91.7%) [15, 17, 21, 29, 32–34, 37–41], microbiological success was reported in 7 of 8 studies (87.5%) [15, 17, 29, 32–35, 38], 30-day mortality in 0 of 5 studies (0.0%) [37–41], and unspecified-timeframe mortality in 2 of 9 studies (22.2%) [15, 17, 21, 26, 29, 32–34, 47].
Outcomes by Infection Type
The nature of the studies captured—primarily reporting on patients of multiple infection types (with the respiratory subset not analyzed separately), or using a non-analytical, descriptive design—meant that analyses of factors associated with outcomes were uncommon. Only one study, Rodriguez-Nunez et al.—conducted solely in patients with either pneumonia or tracheobronchitis—performed an analysis of factors associated with 30-day mortality [36]. In a univariate analysis, they found a non-significant trend suggesting that pneumonia [64.6% of survivors vs. 84.0% of non-survivors, p = 0.072, OR = 2.9 (95% CI 0.9–9.4)] and use of a ventilator [30.8% of survivors vs. 52.0% of non-survivors, p = 0.061, OR = 2.4 (95% CI 0.9–6.3)] were associated with 30-day mortality [36].
One further study split outcomes by pneumonia type (VABP or non-VABP) [24]. In this study, patients with VABP had numerically lower clinical success (50.0% vs. 81.0%), microbiological success (53.4% vs. 60.3%), and higher 30-day or inpatient mortality (37.9% vs. 14.2%). However, no analysis was conducted to assess significance [24].
Excluding single-patient studies, studies including only patients with unspecified pneumonia (five studies; 110 patients) [25, 28, 30, 43, 45] reported clinical success rates ranging from 61.5 [43] to 100% [25, 30] (five studies) and 30-day mortality ranging from 0.0 [28] to 29.0% [45] (three studies). Studies including only patients with NP or HABP (two studies; 35 patients) reported clinical success rates ranging from 75 [18] to 100% [44] (two studies) and unspecified-timeframe mortality of 0% (one study) [18, 44]. One study reported solely on patients with VABP (two patients), reporting clinical success of 100% and 30-day mortality of 0% [16].
Outcomes by Treatment Characteristics
In a univariate analysis, Rodriguez-Nunez et al. found that there was no association between a 3 g q8h dose (or creatinine clearance adjusted equivalent) and mortality [63.1% of survivors received 3 g q8h dose vs. 52.0% of non-survivors, p = 0.349, OR = 0.6 (95% CI 0.3–1.6)] [36]. However, there was an association between pathogen susceptibility to C/T [as measured by minimum inhibitory concentration (MIC)], dosing, and 30-day mortality. Thirty-day mortality was significantly lower in patients who received a 3 g q8h dose and were infected by P. aeruginosa with an MIC ≤ 2 mg/L [vs. patients without these characteristics; 47.7% of survivors vs. 24.0% of non-survivors, p = 0.041, OR = 0.3 (95% CI 0.1–0.9)] [36].
Aside from Rodriguez-Nunez et al., outcomes were reported by dosing in three studies (including 32 patients) [20, 27, 42]. In each study, patients treated with a 3 g q8h dose (or creatinine clearance adjusted equivalent) had numerically similar or better outcomes than patients treated with a 1.5 g q8h dose. In Haidar et al., 18 patients (16 pneumonia, two tracheobronchitis) were treated with C/T [27]. Five patients received 3 g q8h (or creatinine clearance adjusted equivalent) and nine received 1.5 g q8h (or creatinine clearance adjusted equivalent) and were therefore underdosed according to FDA recommendations. Patients treated with 3 g q8h had numerically higher clinical success rates (80.0% vs. 66.7%), and lower 30-day mortality (0% vs. 11.1%) [27]. In Xipell et al., eight patients (four pneumonia, four tracheobronchitis), each with normal renal function, were treated with C/T [42]. Three of these patients received 3 g q8h; five received 1.5 g q8h. Patients treated with 3 g q8h had numerically higher clinical success rates (100.0% vs. 80.0%), higher microbiological success rates (100.0% vs. 50.0%), and lower 30-day mortality (0% vs. 20.0%) [42]. In Castón et al., six patients with unspecified RTIs, with unknown renal function, were treated with C/T [20]. Three patients received 3 g q8h; three received 1.5 g q8h. Patients treated with 3 g q8h had numerically similar clinical success rates (66.7% vs. 66.7%), higher microbiological success rates (100.0% vs. 0.0%), and similar 30-day mortality (33.3% vs. 33.3%) [20].
Outcomes by Pathogen and Resistance
In univariate analysis, Rodriguez-Nunez et al. found no association between infection with XDR P. aeruginosa and 30-day mortality [73.8% of survivors had an XDR P. aeruginosa infection vs. 84.0% of non-survivors, p = 0.308, OR = 1.9 (95% CI 0.6–6.2)] [36]. In five studies (including 72 patients) [18, 22, 30, 34, 47] that included patients with non-resistant P. aeruginosa, or the resistance was not specified, clinical success ranged from 51.4 [22] to 100.0% [30, 34] (four studies), and mortality (unspecified timeframe) was 0.0% in two case reports [34, 47]. In larger studies (> 30 patients; two studies), clinical success ranged from 51.4 [22] to 75.0% [18].
Excluding single-patient studies, in 9 studies [20, 24, 25, 27, 28, 31, 42, 43, 45] (including 409 patients) that included patients with MDR P. aeruginosa infections, clinical success ranged from 61.5 [43] to 100.0% [25], microbiological success ranged from 57.0 [24] to 100.0% [25], and 30-day mortality ranged from 0.0 [28] to 33.3% [20]. In larger studies (> 30 patients; four studies) [24, 31, 43, 45], clinical success ranged from 61.5 [43] to 72.6% [45], one study reported microbiological success of 57.0% [24], and 30-day mortality ranged from 20.5 [43] to 29.0% [45].
One study (14 patients) was identified that reported results for patients with XDR P. aeruginosa infections [23]. This study reported a clinical success rates of 79.0%. Furthermore, two studies (three patients) reported data on PDR P. aeruginosa [16, 38]. Both studies reported 100% clinical and microbiological success, and 0% 30-day mortality.
Aside from P. aeruginosa, one study was identified that solely included patients with ESBL-producing Enterobacterales infections [19]. This study found outcomes comparable with patients with P. aeruginosa infections, reporting a clinical success rate of 78.3% (36/46 patients) [19].
Comparative Study Outcomes
The literature search identified one study that compared a cohort of patients treated with C/T for RTIs (n = 62) with a cohort treated with mixed standard of care (SoC) antibacterials (n = 53) [45]. All patients had pneumonia with a MDR P. aeruginosa culture. This study found no difference in clinical cure rates (C/T: 72.6% vs. SoC: 67.9%, p = 0.683) or 30-day mortality rates (C/T: 29.0% vs. SoC: 26.4%, p = 0.840) between the study groups. However, patients treated with C/T had more comorbid conditions than patients treated with SoC antibacterials and were significantly more likely to be admitted to the ICU at diagnosis, both of which may indicate more severe disease [45].
Discussion and Conclusion
This SLR showed that a body of clinical data on the use of C/T to treat RTIs exists; however, reporting differences between studies often obscured the overall results. Despite the heterogeneity in the patient population, critical nature of infections, resistance profile of pathogens, and the large proportion of potentially underdosed patients, outcomes were generally high and comparable with the ASPECT-NP clinical trial. In larger studies (> 30 patients), clinical success rates ranged from 51.4 to 78.3% (eight studies), microbiological success was 57.0% (one study), and 30-day mortality ranged from 20.5 to 29.0% (five studies). These findings are comparable to those found in ASPECT-NP [13]: clinical cure = 54.4%, microbiological eradication = 73.1%, and 28-day mortality = 24.0%.
C/T was initially approved by the FDA in 2014 to treat complicated intra-abdominal infections and complicated urinary tract infections. In 2019, its label expansion to treat vHABP/VABP was approved by the FDA, based on the evidence from the clinical trial, ASPECT-NP [12, 13]. The recommended dose of C/T for HABP/VABP is 3 g q8h for patients with creatinine clearance of > 50 mL/min [12]. This dosing regimen, as used in ASPECT-NP [13], is based on optimized pharmacokinetic and pharmacodynamic properties, and ensures adequate penetration and target attainment in the lungs. However, the treatment characteristics of the studies identified in this SLR suggest that patients with RTIs were often underdosed. There was a trend that suggested more favorable outcomes were observed in a greater proportion of patients receiving the 3 g q8h dose (or creatinine clearance-adjusted equivalent) in recent years. The evaluation of appropriate dosing suggests improved outcomes among those receiving a 3 g q8h dosing regimen [20, 27, 36, 42].
According to the FDA label, the appropriate use of C/T to treat vHABP/VABP is pathogen-directed therapy rather than empiric therapy. C/T is indicated for the treatment of patients 18 years and older with vHABP/VABP, caused by the following susceptible Gram-negative microorganisms: Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Serratia marcescens [12]. It should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria to reduce the development of drug-resistant bacteria and maintain the effectiveness of antibacterial drugs [12]. Indeed, the majority of patients in this SLR had RTIs caused by P. aeruginosa, a difficult-to-treat pathogen for which there are limited treatment options [6]. In this study, most patients treated with C/T had at least a MDR pathogen with a high frequency of XDR (13.8% of patients) and CR (0.5%). This highlights a need for new and novel antibiotics in our Gram-negative armamentarium to combat these pathogens. Further, studies reviewed in this SLR report similar findings observed in the clinical trial, ASPECT-NP, which had extremely limited pathogen resistance, providing additional evidence for these pathogens that are seen in clinical practice. Further, one study in this SLR included ESBL-producing Enterobacterales infections and showed a 79% clinical success rate for C/T treated patients. The incidence of severe infections caused by ESBL-producing Enterobacterales is a rising concern worldwide owing to the successful dissemination of these species in both community and healthcare settings. Serious infections caused by these strains are usually treated with carbapenems; however this may potentially select for CR pathogens.
Specific measures of severity of illness, such as CCI or APACHE scores, were seldom reported across studies. In those that did report these measures, patients were typically seriously ill, had severe comorbidities, and had ICU stays. Furthermore, mechanically ventilated patients, such as those with VABP or vHABP, would likely have been receiving intensive care. This means that the number of ICU patients may be underestimated. This patient profile was consistent with expectations for patients with vHABP/VABP, and mirrors the population enrolled in ASPECT-NP [13]. Further, these clinical studies also included patients that were excluded from the clinical trials and yet the results still yielded comparable results to the trials.
Although this SLR is a comprehensive summary of the real-world use of C/T, the conclusions of this SLR are limited by the inconsistent reporting that is common within clinical data. The majority of studies included patients with multiple different infections, and only reported limited data on the subset of patients with RTIs. This meant that patient characteristics, treatment characteristics, and outcomes were often missing. As a result of inconsistencies in the reporting of data, further quantitative analyses, such as a random effect meta-analysis, were not applicable in this SLR as numerous factors could impact the results. These included, but were not limited to, the different components of effect modification observed within antibacterial evidence, the variability in pathogen susceptibility, infection types, causative pathogens, and changes of definitions over time. These challenges and limitations of quantitative analyses due to the heterogeneity of results have also been highlighted in the literature [48–51].
As described in detail in Puzniak et al. 2021, this SLR is subject to a number of limitations [14]. Briefly, variability in reported outcomes imposes challenges in attributing outcomes to the exposure studied. Moreover, the inclusion of non-peer-reviewed conference proceedings may have affected evidence included within this review. Some studies included portions of data that may have been reported in part by other studies. Since it was difficult to discern which patients were affected, this potential double counting was not adjusted. Many studies had small sample sizes and did not include comparison groups for statistical inference purposes. The vast majority of studies were of a retrospective design which are prone to selection bias. Finally, publication bias may have arisen due to potential non-publication of negative results. Although both IDWeek and ECCMID were searched, this review did not include a comprehensive search of all relevant microbiology conferences or search for studies that were not captured in biomedical databases. Although these are pragmatic limitations associated with all literature reviews, there remains a possibility that the studies included in this review overestimate the treatment effect [52].
In conclusion, this SLR identified and summarized the published clinical evidence on the use of C/T to treat RTIs. Despite the numerous inconsistencies in the reporting of data, these studies gathered from the relevant literature demonstrate and report the effectiveness of C/T in clinical practice. Further studies are required that evaluate C/T solely in patients with RTIs to allow for a better understanding of outcomes specific to RTIs and stratified by key parameters, such as dose and resistance patterns.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Employees of the study sponsor were involved in the study design, as well as collection, analysis, and interpretation of the data, and in critically revising the manuscript for important intellectual content. The sponsor also funded the Journal’s Rapid Service Fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Laura Puzniak and Ryan Dillon conceived and designed the research, contributed to the interpretation of results, and critically revised the manuscript for important intellectual content. Thomas Palmer, Hannah Collings, and Ashley Enstone conducted the literature review, analyzed the data, interpreted the results, and drafted the manuscript. All authors read and approved the final manuscript.
Disclosures
Laura Puzniak and Ryan Dillon are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA who may own stock and/or hold stock options in the Company Merck & Co., Inc., Kenilworth, NJ, USA. Thomas Palmer, Hannah Collings, and Ashley Enstone are employees of Adelphi Values PROVE, which received funding for this research.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
All data analyzed during this study are included in this published article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niederman MS. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis. 2010;51(Supplement_1):S12–S17. doi: 10.1086/653035. [DOI] [PubMed] [Google Scholar]
- 2.FNIH Biomarkers Consortium HABP/VABP Project Team. https://fnih.org/what-we-do/biomarkers-consortium/programs/ventilator-acquired-bacterial-pneumonia. Published 2017. Accessed 3 Dec 2020.
- 3.Frantzeskaki F, Orfanos SE. Treating nosocomial pneumonia: what's new. ERJ Open Res. 2018;4(2):00058–02018. doi: 10.1183/23120541.00058-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talbot GH, Das A, Cush S, et al. Evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. J Infect Dis. 2019;219(10):1536–1544. doi: 10.1093/infdis/jiy578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eccles S, Pincus C, Higgins B, Woodhead M. Diagnosis and management of community and hospital acquired pneumonia in adults: summary of NICE guidance. BMJ: Br Med J. 2014;349:6722. doi: 10.1136/bmj.g6722. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Antibiotic resistant threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc. Published 2020. Accessed 16 Dec 2020.
- 8.Tabak YP, Merchant S, Ye G, et al. Incremental clinical and economic burden of suspected respiratory infections due to multi-drug-resistant Pseudomonas aeruginosa in the United States. J Hosp Infect. 2019;103(2):134–141. doi: 10.1016/j.jhin.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Bonine NG, Berger A, Altincatal A, et al. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious gram-negative bacterial infections. Am J Med Sci. 2019;357(2):103–110. doi: 10.1016/j.amjms.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Lodise TP, Zhao Q, Fahrbach K, Gillard PJ, Martin A. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: how long is too long? BMC Infect Dis. 2018;18(1):625. doi: 10.1186/s12879-018-3524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Published 2017. Accessed 1 Oct 2020.
- 12.Ceftolozane/tazobactam (ZERBAXA®) [Prescribing information]. Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA. 2019.
- 13.Kollef M, Novacek M, Kivistik U, et al. ASPECT-NP: a randomised, controlled, double-blind, phase 3, non-inferiority trial of ceftolozane/tazobactam versus meropenem for treatment of nosocomial pneumonia. Lancet Infect Dis. 2019;19(12):1299–1311. doi: 10.1016/S1473-3099(19)30403-7. [DOI] [PubMed] [Google Scholar]
- 14.Puzniak L, Dillon R, Palmer T, Collings H, Enstone A. Real-world use of ceftolozane/tazobactam: a systematic literature review. Antimicrob Resist Infect Control. 2021. (in press). [DOI] [PMC free article] [PubMed]
- 15.Alessa MA, Almangour TA, Alhossan A, Alkholief MA, Alhokail M, Tabb DE. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa pneumonia in a patient receiving intermittent hemodialysis. Am J Health Syst Pharm. 2018;75(9):e184–e188. doi: 10.2146/ajhp170056. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez Lerma F, Munoz Bermudez R, Grau S, et al. Ceftolozane-tazobactam for the treatment of ventilator-associated infections by colistin-resistant Pseudomonas aeruginosa. Rev Esp Quimioterap. 2017;30(3):224–228. [PubMed] [Google Scholar]
- 17.Arena F, De Angelis LH, Maglioni E, et al. Ceftolozane-tazobactam pharmacokinetics during extracorporeal membrane oxygenation in a lung transplant recipient. AntimicrobAgents Chemother. 2019;63(3):e02131-18. doi: 10.1128/AAC.02131-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassetti M, Castaldo N, Cattelan A, et al. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents. 2019;53(4):408–415. doi: 10.1016/j.ijantimicag.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Bassetti M, Vena A, Giacobbe DR, et al. Ceftolozane/tazobactam for treatment of severe ESBL-producing enterobacterales infections: a multicenter nationwide clinical experience (CEFTABUSE II Study) Open Forum Infect Dis. 2020;7(5):ofaa139. doi: 10.1093/ofid/ofaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caston JJ, De La Torre A, Ruiz-Camps I, Sorli ML, Torres V, Torre-Cisneros J. Salvage therapy with ceftolozane-tazobactam for multidrug-resistant Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2017;61(3):e02136. doi: 10.1128/AAC.02136-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis SE, Ham J, Hucks J, et al. Use of continuous infusion ceftolozane-tazobactam with therapeutic drug monitoring in a patient with cystic fibrosis. Am J Health Syst Pharm. 2019;76(8):501–504. doi: 10.1093/ajhp/zxz011. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Canestro M, Perianez L, Mulet X, et al. Ceftolozane/tazobactam for the treatment of multidrug resistant Pseudomonas aeruginosa: experience from the Balearic Islands. Eur J Clin Microbiol Infect Dis. 2018;37(11):2191–2200. doi: 10.1007/s10096-018-3361-0. [DOI] [PubMed] [Google Scholar]
- 23.Escola-Verge L, Pigrau C, Los-Arcos I, et al. Ceftolozane/tazobactam for the treatment of XDR Pseudomonas aeruginosa infections. Infection. 2018;46(4):461–468. doi: 10.1007/s15010-018-1133-5. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher JC, Satlin MJ, Elabor A, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis. 2018;5(11):ofy280. doi: 10.1093/ofid/ofy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelfand MS, Cleveland KO. Ceftolozane/tazobactam therapy of respiratory infections due to multidrug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2015;61(5):853–855. doi: 10.1093/cid/civ411. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales Zamora JA, Varadarajalu Y. Fatal Curvularia brain abscess in a heart and kidney transplant recipient. IDCases. 2019;17(no pagination):e00576. doi: 10.1016/j.idcr.2019.e00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis. 2017;65(1):110–120. doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakki M, Lewis JS. Ceftolozane-tazobactam therapy for multidrug-resistant Pseudomonas aeruginosa infections in patients with hematologic malignancies and hematopoietic-cell transplant recipients. Infection. 2018;46(3):431–434. doi: 10.1007/s15010-018-1125-5. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Tejedor A, Merino-Vega CD, Martin-Vivas A, et al. Successful treatment of multidrug-resistant Pseudomonas aeruginosa breakthrough bacteremia with ceftolozane/tazobactam. Infection. 2017;45(1):115–117. doi: 10.1007/s15010-016-0944-5. [DOI] [PubMed] [Google Scholar]
- 30.Jones BM, Huelfer K, Bland CM. Clinical and safety evaluation of continuously infused ceftolozane/tazobactam in the outpatient setting. Open Forum Infect Dis. 2020;7(2):14. doi: 10.1093/ofid/ofaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Real-world experience with ceftolozane-tazobactam for multidrug-resistant gram-negative bacterial infections. Antimicrob Agents Chemother. 2020;64(4). [DOI] [PMC free article] [PubMed]
- 32.Kuti JL, Ghazi IM, Quintiliani R, Shore E, Nicolau DP. Treatment of multidrug-resistant Pseudomonas aeruginosa with ceftolozane/tazobactam in a critically ill patient receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents. 2016;48(3):342–348. doi: 10.1016/j.ijantimicag.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Lewis PO, Cluck DB, Tharp JL, Krolikowski MA, Patel PD. Failure of ceftolozane-tazobactam salvage therapy in complicated pneumonia with lung abscess. Clin Case Rep. 2018;6(7):1308–1312. doi: 10.1002/ccr3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddocks S, Fabijan AP, Ho J, et al. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2019;200(9):1179–1181. doi: 10.1164/rccm.201904-0839LE. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud A, Shah A, Nutley K, et al. Clinical pharmacokinetics of ceftolozane and tazobactam in an obese patient receiving continuous venovenous haemodiafiltration: a patient case and literature review. J Glob Antimicrob Resist. 2020;21:83–85. doi: 10.1016/j.jgar.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Nunez O, Perianez-Parraga L, Oliver A, et al. Higher MICs (>2 mg/L) predict 30-day mortality in patients with lower respiratory tract infections caused by multidrug- and extensively drug-resistant Pseudomonas aeruginosa treated with ceftolozane/tazobactam. Open Forum Infect Dis. 2019;6(10):ofz416. doi: 10.1093/ofid/ofz416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano MT, Premraj S, Bray JM, Murillo LC. Ceftolozane/tazobactam for pulmonary exacerbation in a 63-year-old cystic fibrosis patient with renal insufficiency and an elevated MIC to Pseudomonas aeruginosa. IDCases. 2020;21 (no pagination). [DOI] [PMC free article] [PubMed]
- 38.Soliman R, Lynch S, Meader E, et al. Successful ceftolozane/tazobactam treatment of chronic pulmonary infection with pan-resistant Pseudomonas aeruginosa. JMM Case Reports. 2015;2(2):e000025. doi: 10.1099/jmmcr.0.000025. [DOI] [Google Scholar]
- 39.Stewart A, Roberts JA, Wallis SC, Allworth AM, Legg A, McCarthy KL. Evidence of clinical response and stability of Ceftolozane/Tazobactam used to treat a carbapenem-resistant Pseudomonas aeruginosa lung abscess on an outpatient antimicrobial program. Int J Antimicrob Agents. 2018;51(6):941–942. doi: 10.1016/j.ijantimicag.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Stokem K, Zuckerman JB, Nicolau DP, Wungwattana M, Sears EH. Use of ceftolozane-tazobactam in a cystic fibrosis patient with multidrug-resistant pseudomonas infection and renal insufficiency. Respir Med Case Rep. 2018;23:8–9. doi: 10.1016/j.rmcr.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vickery SB, McClain D, Wargo KA. Successful use of ceftolozane-tazobactam to treat a pulmonary exacerbation of cystic fibrosis caused by multidrug-resistant Pseudomonas aeruginosa. Pharmacotherapy. 2016;36(10):e154–e159. doi: 10.1002/phar.1825. [DOI] [PubMed] [Google Scholar]
- 42.Xipell M, Paredes S, Fresco L, et al. Clinical experience with ceftolozane/tazobactam in patients with serious infections due to resistant Pseudomonas aeruginosa. J Glob Antimicrob Resist. 2018;13:165–170. doi: 10.1016/j.jgar.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Hart DE, Gallagher JO, et al. Ceftolozane–tazobactam (C/T) Treatment outcomes in immunocompromised (IC) patients with multidrug-resistant (MDR) Pseudomonas aeruginosa (PA) infections. Open Forum Infect Dis. 2019;6(2):781. doi: 10.1093/ofid/ofz360.1959. [DOI] [Google Scholar]
- 44.Iovleva A, Marshall SH, Perez F, Ray A, Jacobs MR, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam in treatment of pulmonary infections by Imipenem resistant Pseudomonas aeruginosa. Open Forum Infecti Dis. 2016;3(1).
- 45.Mills M, MacWhinnie A, Do T. Evaluating the impact of ceftolozane/tazobactam on clinical outcomes in patients with multi-drug-resistant Pseudomonas aeruginosa Pneumonia. Open Forum Infect Dis. 2019;6(2):783–784. doi: 10.1093/ofid/ofz360.1964. [DOI] [Google Scholar]
- 46.Nathan RV, Alvarado FS, Prokesch RC, et al. Ceftolozane/tazobactam: outpatient treatment of gram-negative infections at Physician Office Infusion Centers (POICs). In: Paper presented at: IDWeek2016.
- 47.Sheffield M, Nelson D, O'Neal M, et al. The use of continuous infusion Ceftolozane/tazobactam for resistant gram‐negative bacterial infections: a case series. In: Paper presented at: ACCP2019.
- 48.Thom H, Thompson JC, Scott DA, Halfpenny N, Sulham K, Corey GR. Comparative efficacy of antibiotics for the treatment of acute bacterial skin and skin structure infections (ABSSSI): a systematic review and network meta-analysis. Curr Med Res Opin. 2015;31(8):1539–1551. doi: 10.1185/03007995.2015.1058248. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell JN, Rhodes NJ, Lopez J, Jett R, Scheetz MH. Carbapenems vs/ alternative β-lactams for the treatment of nosocomial pneumonia: a systematic review and meta-analysis. Int J Antimicrob Agents. 2018;52(4):451–458. doi: 10.1016/j.ijantimicag.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Xu F, He LL, Che LQ, et al. Aerosolized antibiotics for ventilator-associated pneumonia: a pairwise and Bayesian network meta-analysis. Crit Care. 2018;22(1):301. doi: 10.1186/s13054-018-2106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mavros MN, Theochari NA, Kyriakidou M, Economopoulos KP, Sava JA, Falagas ME. Fluoroquinolone-based versus β-lactam-based regimens for complicated intra-abdominal infections: a meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2019;53(6):746–754. doi: 10.1016/j.ijantimicag.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Schmucker CM, Blümle A, Schell LK, et al. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS ONE. 2017;12(4):e0176210. doi: 10.1371/journal.pone.0176210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this published article.