Abstract
Aims
Treatment guidelines for patients with atrial fibrillation (AF) suggest that patients should be managed with an antiarrhythmic drug (AAD) before undergoing catheter ablation (CA). This study evaluated whether pulmonary vein isolation employing cryoballoon CA is superior to AAD therapy for the prevention of atrial arrhythmia (AA) recurrence in rhythm control naive patients with paroxysmal AF (PAF).
Methods and results
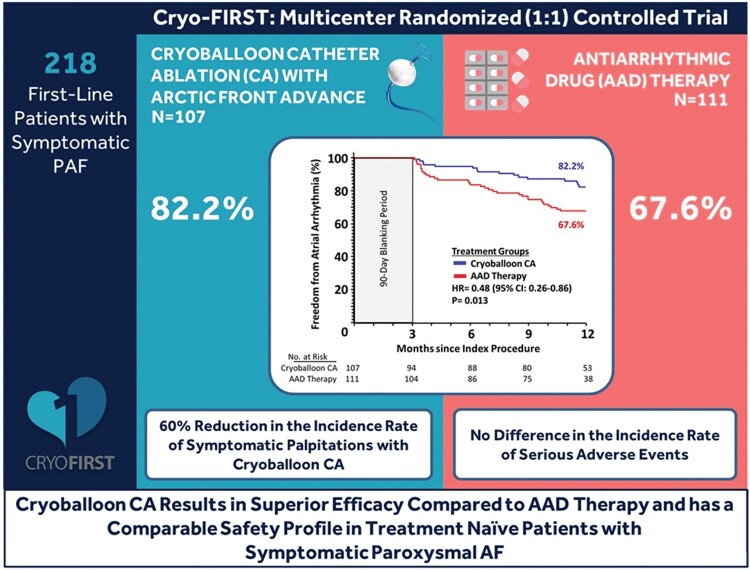
A total of 218 treatment naive patients with symptomatic PAF were randomized (1 : 1) to cryoballoon CA (Arctic Front Advance, Medtronic) or AAD (Class I or III) and followed for 12 months. The primary endpoint was ≥1 episode of recurrent AA (AF, atrial flutter, or atrial tachycardia) >30 s after a prespecified 90-day blanking period. Secondary endpoints included the rate of serious adverse events (SAEs) and recurrence of symptomatic palpitations (evaluated via patient diaries). Freedom from AA was achieved in 82.2% of subjects in the cryoballoon arm and 67.6% of subjects in the AAD arm (HR = 0.48, P = 0.01). There were no group differences in the time-to-first (HR = 0.76, P = 0.28) or overall incidence [incidence rate ratio (IRR)=0.79, P = 0.28] of SAEs. The incidence rate of symptomatic palpitations was lower in the cryoballoon (7.61 days/year) compared with the AAD arm (18.96 days/year; IRR = 0.40, P < 0.001).
Conclusions
Cryoballoon CA was superior to AAD therapy, significantly reducing AA recurrence in treatment naive patients with PAF. Additionally, cryoballoon CA was associated with lower symptom recurrence and a similar rate of SAEs compared with AAD therapy.
Keywords: Antiarrhythmic drug, Catheter ablation, Atrial fibrillation, Cryoballoon, First-line treatment, Randomized study
Graphical Abstract
What’s new?
This prospective, randomized global study evaluated cryoballoon catheter ablation vs. antiarrhythmic drug therapy as an initial, first-line rhythm control strategy in patients with symptomatic paroxysmal atrial fibrillation.
Cryoballoon ablation was superior to antiarrhythmic drug therapy for the prevention of atrial arrhythmia recurrence over 12 months and was associated with lower arrhythmia symptom recurrence.
There was no difference in the rate of serious adverse events between groups.
These findings suggest that cryoballoon ablation is an effective first-line treatment strategy in drug naive patients with symptomatic paroxysmal atrial fibrillation pursing rhythm control therapy.
Introduction
Treatment guidelines for patients with atrial fibrillation (AF) suggest that patients should be first managed with an antiarrhythmic drug (AAD).1P When a patient becomes AAD-refractory (intolerant or non-responsive), catheter ablation (CA) by pulmonary vein isolation (PVI) is recommended in those patients with recurrent symptomatic paroxysmal AF (PAF).1 Importantly, early rhythm control with AADs or ablation is associated with a reduction in adverse cardiovascular outcomes.2 Additionally, a shortened duration from AF diagnosis until CA has been shown to improve long-term rhythm control.3,4 To date, three randomized studies (RAAFT-1, RAAFT-2, and MANTRA-PAF) have compared radiofrequency current (RFC) CA to AAD therapy in treatment naive patients with PAF.5–7 In summary, RFC CA can be more effective in younger (otherwise healthy) patients compared with AAD therapy, but RFC CA might also yield a higher incidence of adverse events.5–7
The FIRE AND ICE trial demonstrated non-inferiority of cryoballoon compared with RFC CA with regards to the primary safety and efficacy endpoints in patients with drug-refractory symptomatic PAF8; however, cryoballoon CA-treated patients had significantly fewer repeat ablations, direct-current cardioversions, all-cause rehospitalizations, and cardiovascular rehospitalizations during the study period.9 More recently, two randomized studies conducted in North America evaluated initial rhythm-control therapy with cryoballoon CA vs. AAD treatment and found that first-line treatment with cryoballoon CA was superior to AAD for preventing atrial arrhythmia (AA) recurrence over 12 months. Moreover, serious procedure-related complications were uncommon.10,11 Here, we report the primary trial results of the randomized, global Cryo-FIRST study that evaluated cryoballoon CA vs. AAD therapy in patients undergoing first-line rhythm control treatment for symptomatic PAF.
Methods
Trial design
Detailed methods of the Cryo-FIRST trial (ClinicalTrials.gov Identifier: NCT01803438) have been previously published.12 In brief, this was a multicentre, prospective, open blind-endpoint controlled randomized (1:1) study to compare cryoballoon CA against AAD therapy in treatment naive patients with symptomatic, recurrent PAF. The steering and publication committees were composed of physicians who assumed a leadership role in the conduct of the study. This study complied with the Declaration of Helsinki and ISO 14155. Local ethics review committees approved the study at each participating centre. The study was sponsored by Medtronic. Data were inputted by each participating centre into an internet-based data collection system (Oracle Clinical), and an independent adverse event committee and Holter core laboratory were used to insure impartial classification of the study events. The final analysis was conducted by the sponsor; all authors take explicit responsibility for the accuracy and fidelity of the analyses performed by the corporate sponsor that are reported in this manuscript. The decision to publish the results and final decisions regarding the contents of the manuscript were made by the publication committee.
Study participants
Twenty centres in Europe, Australia, and Latin America participated in this trial (Supplementary material online, Table S1). This study enrolled patients 18 to 75 years old with a normal ECG (QRS width ≤120 ms; QTc <440 ms; PQ ≤210 ms), structurally normal heart [left ventricular ejection fraction ≥50%, thickness of the inter-ventricular septum ≤12 mm and left atrium diameter (short axis) <46 mm] and recurrent symptomatic PAF who were drug naive (had not previously received a Class I or III AAD for >48 h).12 All patients provided written informed consent prior to participation. After enrolment, patients were randomly assigned 1 : 1 to undergo cryoballoon CA or AAD therapy. A full list of inclusion and exclusion criteria has been previously published.12
Cryoballoon catheter ablation
The cryoballoon CA procedure has been previously described in detail.8,9,12–14 In brief, a 28- or 23-mm second-generation cryoballoon (Arctic Front Advance Cardiac Cryoablation Catheter, Medtronic) was delivered using a transseptal puncture and an over-the-wire delivery technique. The balloon was placed at each pulmonary vein (PV). Acute PVI was confirmed by entrance block (and where assessable, exit block) testing using a dedicated inner lumen, circular diagnostic mapping catheter (Achieve Mapping Catheter, Medtronic). Continuous phrenic nerve pacing with intervals of 1000–3000 was required during cryoballoon ablation of the right-sided PVs. Ablation was immediately stopped in the event of significantly altered movement of the diaphragm. In the case of incomplete electrical isolation of the PV or identification of focal triggers, additional freeze applications with the focal catheter (Freezor MAX Cardiac Cryoablation Catheter, Medtronic) or the alternative-sized cryoballoon were permitted. Patients were discharged and maintained on systemic anticoagulation therapy for a minimum of 3 months. Re-ablation and use of AADs were allowed during the first 90 days after the index procedure. After the 90-day blanking period, all AADs were discontinued except for β-blockers and calcium channel blockers, and repeat ablations were defined as primary endpoint failures.
Antiarrhythmic drug therapy
Subjects randomized to the AAD therapy arm received a Class I or III AAD in accordance with each hospital’s clinical practice and the 2012 ESC guidelines for the management of AF.15 However, amiodarone was discouraged due to its potential extracardiac toxicity. The use of other AADs was not allowed except for β-blockers. Drug, dose, and schedule changes were permitted during the first 90 days to optimize AAD therapy in case of inefficacy. Oral anticoagulation and use of calcium channel blockers were not governed by the study protocol.
Study follow-up
Scheduled follow-up visits took place at baseline, 1, 3, 6, 9, and 12 months, and included a 12-lead ECG and 7-day Holter. A patient diary was used to evaluate the occurrence of symptomatic palpitations. After the 90-day blanking period, prescription of AAD in the CA arm and CA in the AAD therapy arm was considered treatment crossover events.
End points
The primary study endpoint was freedom from any AA recurrence (at least one episode of AF, atrial flutter, or atrial tachycardia) lasting >30 s at 12 months documented by 7-day Holter ECG or any other ECG recording outside of the 90-day blanking period. Cardioversion and repeat CA for AA recurrence outside of the 90-day blanking period were also counted towards a primary endpoint failure event, as it was assumed that these procedures were conducted due to AA recurrence >30 s. Prespecified secondary endpoints reported here include the rate of serious adverse events (SAEs) and recurrence of patient-reported symptomatic palpitations. Serious adverse events include by definition events that led to death or serious deterioration in the health of the subject resulting in: a life-threatening illness or injury, a permanent impairment of a body structure or a body function, inpatient or prolonged hospitalization, or medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or a body function. Atrial arrhythmia recurrence was classified as a SAE if it resulted in inpatient or prolonged hospitalization per the above SAE definition.
Statistical analysis
It was assumed that 10% of subjects would withdraw from the study. Consequently, a sample size of 218 was chosen to achieve 80% power to detect a 20% reduction in the freedom from AA recurrence at 12-months with CA vs. AAD therapy at an alpha-level of 5% (two-sided Type I error).12 The primary analysis was based on the intention-to-treat (ITT) cohort. A sensitivity analysis was performed in a per-protocol cohort that included all subjects randomized until the point of crossover. Survival curves for the time-to-first primary endpoint and time-to-first SAEs were estimated by the Kaplan–Meier method and evaluated for statistical difference using the log-rank test. Differences in the rate of SAEs and recurrent symptomatic palpitations were estimated and compared between groups by means of the mixed Poisson model. No adjustments for multiple comparisons were performed, and missing data were not imputed.
Results
Patients
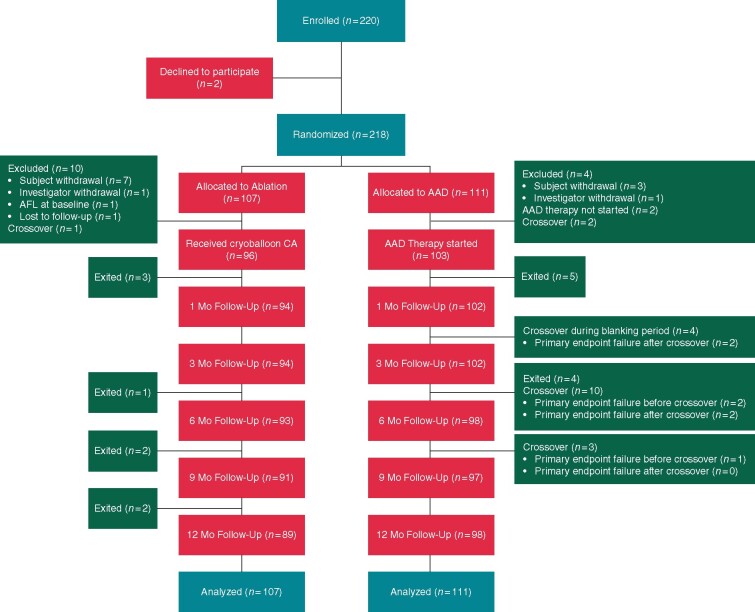
A total of 220 patients were enrolled between April 2014 and October 2018. The ITT cohort consisted of 107 patients randomized to cryoballoon CA and 111 patients randomized to AAD therapy. Subject baseline characteristics are presented in Table 1. Enrolment, randomization, and follow-up of study participants are summarized in Figure 1. Compliance with 7-day Holter monitoring was 87.1% in the cryoballoon group and 84.4% in the AAD group.
Table 1.
Baseline subject characteristics
| Cryoballoon CA (n = 107) | AAD (n = 111) | |
|---|---|---|
| Demographics and echocardiographic characteristics | ||
| Age (years) | 50.5 (13.1) | 54.1 (13.4) |
| Sex, male | 76 (71.0%) | 72 (64.9%) |
| Time from first ECG-documented AF to enrolment (years) | 0.7 (1.5) | 0.8 (2.1) |
| Left atrial diameter (short axis) (mm) | 37.0 (5.9) | 38.0 (4.9) |
| Left atrial diameter (long axis) (mm) | 46.8 (8.2) | 47.7 (6.3) |
| Left ventricular ejection fraction (%) | 62.8 (5.4) | 63.7 (5.4) |
| EHRA class | ||
| Class I | 0 (0.0%) | 0 (0.0%) |
| Class II | 75 (70.1%) | 83 (74.8%) |
| Class III | 30 (28.0%) | 25 (22.5%) |
| Class IV | 2 (1.9%) | 1 (0.9%) |
| Medical history | ||
| Hypertension | 33 (30.8%) | 40 (36.0%) |
| Diabetes | 1 (0.9%) | 4 (3.6%) |
| Hyperlipidaemia | 23 (21.5%) | 25 (22.5%) |
| Myocardial infarction | 2 (1.9%) | 0 (0.0%) |
| Coronary artery disease | 2 (1.9%) | 1 (0.9%) |
| Congestive heart failure | 0 (0.0%) | 0 (0.0%) |
| Stroke | 0 (0.0%) | 0 (0.0%) |
| Transient ischaemic attack | 0 (0.0%) | 1 (0.9%) |
| Valve dysfunction | 3 (2.8%) | 2 (1.8%) |
| CHA2DS2-VASc score | ||
| 0 | 49 (45.8%) | 38 (34.2%) |
| 1 | 33 (30.8%) | 40 (36.1%) |
| 2 | 13 (12.2%) | 15 (13.5%) |
| 3 | 4 (3.7%) | 10 (9.0%) |
| 4 | 3 (2.8%) | 2 (1.8%) |
| Baseline medications | ||
| Anticoagulant | 38 (35.5%) | 49 (44.1%) |
| Acetylsalicylic acid | 5 (4.7%) | 7 (6.3%) |
| β-Blocker | 54 (50.5%) | 56 (50.5%) |
| Calcium channel blocker | 9 (8.4%) | 15 (13.5%) |
Values are n (%) or mean (standard deviation).
AAD, antiarrhythmic drug; AF, atrial fibrillation; CA, catheter ablation; EHRA, European Heart Rhythm Association score.
Figure 1.
Patient flow diagram.
Treatment characteristics
For the cryoballoon arm, procedural details are available in Supplementary material online, Tables S2 and S3. During the index procedure complete isolation of all PVs was achieved in all 96 patients (100%). There were six re-ablations for AA recurrence(s) in six subjects assigned to cryoballoon, four of which occurred during the blanking period. Antiarrhythmic drug therapy was discontinued by the end of the blanking in all subjects that underwent cryoballoon CA and was not reinitiated during follow-up.
Two subjects never started AAD therapy, one was non-compliant and the other subject’s medical condition did not allow it. Drug and dosing information for the 103 subjects who initiated treatment in the AAD arm is available in Table 2. Antiarrhythmic drug therapy was discontinued in 20 patients due to patient non-compliance (n = 1), patient withdrawal from the study (n = 1), crossover to cryoballoon CA (n = 17 out of 19 AAD patients who crossed over) and physician discretion secondary to the development of side effects (n = 1). In subjects who did not cross over to cryoballoon CA, two had a change in dose after blanking; one of these subjects also had a change in AAD medication post-blanking.
Table 2.
Antiarrhythmic drug therapy and dosing
| Drug | Daily dose (mg) | Therapy start (n = 101)a | Month 3 (n = 97)b | Month 12 (n = 94)c |
|---|---|---|---|---|
| Flecainide | 50 | 3 (2.9%) | 2 (2.1%) | 2 (2.1%) |
| 80 | 1 (1.0%) | 0 (0%) | 0 (0%) | |
| 100 | 23 (22.3%) | 12 (12.4%) | 10 (10.6%) | |
| 120 | 0 (0%) | 1 (1.0%) | 0 (0%) | |
| 150 | 10 (9.7%) | 15 (15.5%) | 11 (11.7%) | |
| 200 | 24 (23.3%) | 13 (13.4%) | 12 (12.8%) | |
| Propafenone | 300 | 14 (13.6%) | 9 (9.3%) | 9 (9.6%) |
| 375 | 0 (0%) | 0 (0%) | 1 (1.1%) | |
| 450 | 13 (12.6%) | 13 (13.4%) | 10 (10.6%) | |
| 600 | 6 (5.8%) | 6 (6.2%) | 6 (6.4%) | |
| Sotalol | 40 | 0 (0%) | 1 (1.0%) | 0 (0%) |
| 80 | 3 (2.9%) | 7 (7.2%) | 4 (4.3%) | |
| 160 | 1 (1.0%) | 4 (4.1%) | 2 (2.1%) | |
| 240 | 1 (1.0%) | 0 (0%) | 0 (0%) | |
| Dronedarone | 800 | 2 (1.9%) | 5 (5.2%) | 4 (4.3%) |
| Amiodarone | 200 | 0 (0%) | 2 (2.1%) | 1 (1.1%) |
| 400 | 0 (0%) | 1 (1.0%) | 0 (0%) | |
| Stopped AAD therapyd | NA | 0 (0%) | 6 (6.2%) | 22 (23.4%) |
Values are n (%).
Dosing information was not available for one patient who was treated with flecainide and one patient treated with sotalol at therapy start. These patients are not included in the table.
Patients with available data. At 3 months, six patients had exited the study or missed the follow-up; these patients are not included in the table.
Patients with available data. At 12 months, nine patients had exited the study or missed the follow-up; these patients are not included in the table.
This category includes patients who crossed over to CA.
AAD, antiarrhythmic drug; CA, catheter ablation.
Crossovers
Crossovers occurred in 20 subjects (9%). One subject in the cryoballoon arm chose not to undergo an ablation procedure and instead used AAD therapy. This subject experienced a primary endpoint event (documented AA recurrence outside of the 90-day blanking period). Additionally, 19 subjects in the AAD arm had a crossover event, electing to have a cryoballoon CA during the study. Of these, seven had documented AA recurrence after the blanking period and were considered primary endpoint failures (AA recurrence before crossover: n = 3; and AA recurrence after crossover: n = 4). There were 12 subjects who crossed over from AAD to cryoballoon CA that did not have a primary endpoint event. Of these 12 subjects, four subjects had a crossover within the 90-day blanking period (among which, two subjects had not started AAD therapy).
Primary endpoint
In the ITT analysis, freedom from any AA was achieved in 82.2% of subjects in the cryoballoon arm and 67.6% of subjects in the AAD arm (HR = 0.48, 95% CI: 0.26–0.86; P = 0.013) as illustrated in Figure 2. Reasons for primary endpoint failure through 12 months in the ITT analysis are listed in Table 3. Study findings were similar in the per-protocol analysis, with freedom from any AA achieved in 83.1% of subjects in the cryoballoon arm and 67.2% of subjects in the AAD arm (HR = 0.44, 95% CI: 0.24–0.82; P = 0.008). Single procedure success of cryoballoon CA at 12 months was 80.2% (95% CI: 70.4–87.1%). Atrial fibrillation burden during Holter monitoring at each follow-up visit is presented in Supplementary material online, Table S4 and Figure S1.
Figure 2.
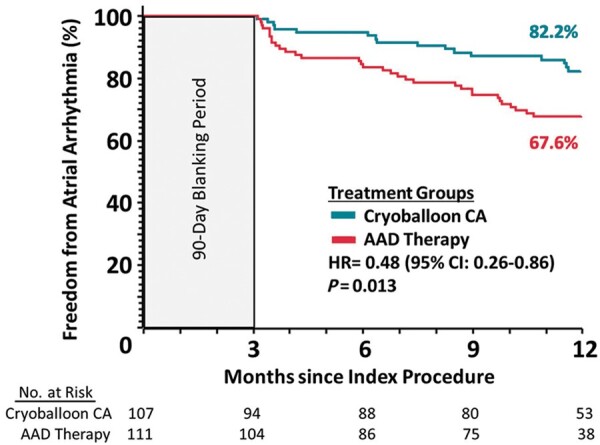
Time to first atrial arrhythmia recurrence in the intention-to-treat cohort.
Table 3.
Reason for primary endpoint failure through 12 months
| Primary endpoint failure event | Cryoballoon CA | AAD |
|---|---|---|
| Total | 16 | 33 |
| Atrial arrhythmia recurrence | 15 | 33 |
| Atrial fibrillation | 12 | 23 |
| Atrial flutter | 0 | 1 |
| Atrial tachycardia | 3 | 7 |
| Atrial fibrillation, atrial flutter | 0 | 1 |
| Atrial fibrillation, atrial flutter, and atrial tachycardia | 0 | 1 |
| Reablation | 1 | 0 |
| Cardioversion | 0 | 0 |
AAD, antiarrhythmic drug; CA, catheter ablation.
Secondary endpoints
During follow-up, there were 42 SAEs in 26 subjects in the cryoballoon arm and 56 SAEs in 37 subjects in the AAD arm [incidence rate ratio (IRR)=0.79; 95% CI: 0.51–1.22; P = 0.28, Table 4]. Atrial arrhythmia recurrence that met the definition of a SAE occurred in 11 subjects in the cryoballoon arm and 28 subjects in the AAD arm. There were 11 procedure related SAEs in the cryoballoon arm, including one case of transient ischaemic attack on the same day of cryoballoon CA; this patient was taking warfarin at the time of the procedure. Additionally, one subject randomized to AAD experienced transient phrenic nerve palsy after undergoing CA. There was no difference in time-to-first SAE in either the ITT (HR = 0.76, 95% CI: 0.46–1.26; P = 0.28, Figure 3) or per-protocol analyses (HR = 0.79, 95% CI: 0.48–1.32; P = 0.37). Additionally, there was no death, atrio-oesophageal fistula, pericardial tamponade, or stroke reported during the study, and there was no phrenic nerve injury present at the time of hospital discharge.
Table 4.
Serious adverse events
| Adverse event, events (subjects) | Cryoballoon CA (n = 107) |
AAD (n = 111) |
||||
|---|---|---|---|---|---|---|
| All | Procedure related | System related | All | Drug related | Procedure related (cross over) | |
| Total | 42 (26) | 11 (9) | 2 (1) | 56 (37) | 4 (4) | 1 (1) |
| Acute coronary syndrome | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Acute kidney injury | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Adverse drug reaction | 0 | 0 | 0 | 3 (3) | 2 (2) | 0 |
| Arteriospasm coronary | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 |
| Atrial arrhythmia recurrence | 15 (11) | 1 (1) | 0 | 34 (28) | 2 (2) | 0 |
| AVNRT | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Bronchitis | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Chest pain | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal pain | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Impaired gastric emptying | 1 (1) | 1 (1) | 0 | 1 (1) | 0 | 0 |
| Impaired healing | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Lung disorder/infection | 4 (1) | 1 (1) | 0 | 0 | 0 | 0 |
| Non-sustained ventricular tachycardia | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Oedema peripheral | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Orthostatic hypotension | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Palpitations | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Pericardial disordera | 3 (3) | 3 (3) | 1 (1) | 0 | 0 | 0 |
| Phrenic nerve paralysis | 0 | 0 | 0 0 | 1 (1)b | 0 | 1 (1) |
| Pneumonia | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Procedural failurec | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 |
| Pyrexia | 1 (1) | 1.(1) | 0 | 0 | 0 | 0 |
| Syncope | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Transient ischaemic attack | 1 (1) | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Vascular access site haemorrhage | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 |
| Other | 9 (9) | 0 | 0 | 9 (7) | 0 | 0 |
Atrial arrhythmia recurrence was only classified as a serious adverse event if it resulted in hospitalization.
None of these events resulted in cardiac tamponade.
Transient phrenic nerve palsy was experienced by one subject randomized to AAD who underwent CA.
Failure of the transseptal puncture was observed in one subject randomized to cryoballoon; a second procedure was successful.
AAD, antiarrhythmic drug; AVNRT, atrioventricular nodal reentry tachycardia; CA, catheter ablation.
Figure 3.
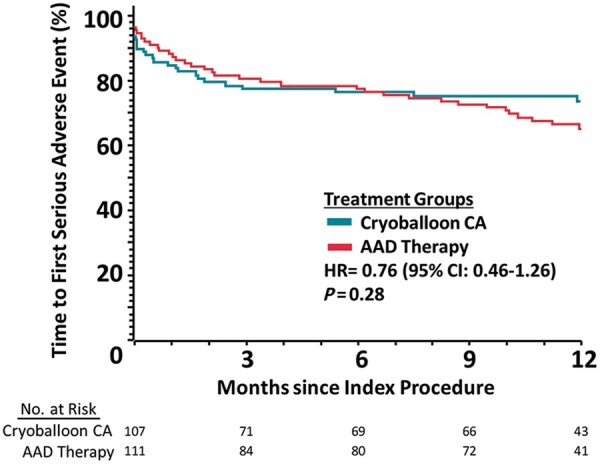
Time to first serious adverse event in the intention-to-treat cohort.
Throughout follow-up, the incidence rate of patient-reported days with symptomatic palpitations was lower in the cryoballoon arm (7.61 days/year) compared with the AAD arm (18.96 days/year, IRR = 0.40, 95% CI: 0.24–0.69; P < 0.001). At 12 months, 77 of 89 subjects (86.5%) in the cryoballoon arm and 69 of 98 subjects (70.4%) in the AAD arm were without AF-related symptoms (EHRA score 1; P = 0.017). The annualized rate of hospital or emergency service access due to symptoms caused by AA recurrence was 0.23 in the cryoballoon and 0.35 in the AAD group (IRR: 0.67, 95% CI: 0.41–1.10; P = 0.11).
Discussion
This was a global randomized study evaluating cryoballoon CA vs. AAD therapy as a first-line treatment in patients with recurrent symptomatic PAF. Cryoballoon CA was superior to AAD treatment for the prevention of AA recurrence over 12 months, and accordingly was associated with lower symptom recurrence. Moreover, a comparable safety profile was observed between both treatment strategies, with no significant group differences in the time-to-first SAE or the overall rate of SAEs throughout follow-up. Together these findings suggest that cryoballoon CA is an effective first-line treatment strategy in patients with symptomatic PAF.
Most comparisons between CA and AAD therapy have been completed in patients deemed as ‘AAD-refractory’ before study enrolment. These studies have consistently demonstrated that CA is superior to AADs for the reduction of AA recurrence and the avoidance of AF disease progression.16–19 Accordingly, the use of CA has received a Class I recommendation in patients with symptomatic, drug-refractory PAF in current guidelines.1 However, the safety and efficacy of CA vs. AADs as a first-line treatment has not been as extensively investigated in large randomized trials (especially when considering cryoballoon CA), and consequently, the usage of CA as a first-line treatment has received a Class IIa recommendation.1
Historically, three randomized studies (RAAFT-1, RAAFT-2, and MANTRA-PAF)5–7 have evaluated RFC CA vs. AAD therapy for preventing AF recurrence in treatment naive patients with PAF. A meta-analysis of the 491 patients enrolled in these trials observed a 37% reduction in AF recurrence with CA.20 However, these studies were limited by a small sample7 size or a relatively high rate of repeat ablation procedures.5,6
In our study, cryoballoon CA was associated with more than a 50% reduction in AA recurrence over 12 months. Importantly, in the present study, CA was found to be superior to AADs despite crossover events in 17% of patients randomized to AAD therapy. Overall, eight crossovers from the AAD to CA arm did not count as primary endpoint failure events due to the absence of documented AA recurrence outside of the blanking period. However, these events would likely be viewed as AAD treatment failure in clinical practice.
The procedural success observed with cryoballoon CA in this trial is generally aligned with that previously reported in three observational studies in treatment naive populations. In these studies, freedom from arrhythmia recurrence ranged from 71% to 89% over a follow-up period of 12–28 months.13,14,21 More recently, two randomized studies (EARLY-AF10 and STOP AF First11) have specifically evaluated cryoballoon CA vs. AAD therapy as a first-line treatment strategy in patients with symptomatic AF. Similar to the present investigation, both studies demonstrated that cryoballoon CA was superior to AAD therapy for preventing AA recurrence over 12 months. A strength of these trials was the use of more robust cardiac monitoring during follow-up; however, enrolment was limited to Canada10 and the USA.11 Cryo-First extends findings from these studies to new geographies including Europe, Australia, and South America.
A primary concern around the use of any CA as a first-line therapy is safety, considering the serious albeit uncommon complications associated with CA.1,17 In the present investigation, safety was comprehensively evaluated by collecting all SAEs. Importantly, the rate of SAEs was similar between groups. While more SAEs were adjudicated to be procedure-related compared with drug-related, there were more patients in the AAD arm who experienced AA recurrence leading to hospitalization. Moreover, AADs were discontinued in 20 patients randomized to AAD therapy. Importantly, there were no occurrences of death, atrio-oesophageal fistula, stroke, pericardial tamponade, or chronic phrenic nerve injury within the CA cohort in the present trial. Overall, these findings align with those recently reported in STOP AF First and EARLY-AF; both of these studies observed SAEs in a similar proportion of patients randomized to first-line cryoballoon CA as AAD therapy.10,11 Moreover, these results support a growing body of literature demonstrating that CA can be safely performed by experienced operators.16,17,22
A primary objective of restoring sinus rhythm in patients with recurrent PAF is to reduce symptoms.1 This is especially true in the present study population, which was relatively young and healthy with a low risk of embolic events. In our investigation, 87% of the patients in the cryoballoon CA arm were without symptoms at 12 months compared with 70% in the AAD arm. Moreover, the incidence of days with patient-reported symptomatic palpitations was lower in the cryoballoon arm, suggesting that CA is superior to AAD therapy for symptom reduction, even in patients who are relatively early in the AF disease process. In congruence, these findings are generally aligned with results from previous randomized first-line studies that have together demonstrated a trend towards a reduction in symptomatic AF recurrence with RFC CA compared with AAD therapy,20 and a lower rate of symptomatic AA recurrence with cryoballoon CA vs. AAD therapy.10
Limitations
Our trial has some limitations. There were 31 patients (14%) who exited the study early, of which 14 patients (6%) exited before initiating therapy. This could have introduced bias in the randomization. Also, crossovers occurred in 9% of patients, primarily from the AAD to cryoballoon arm. However, this rate is similar or lower than previously reported in ablation trials,6,17,23 and the per-protocol sensitivity analysis could not show any impact of crossovers on the study results. Moreover, the high rate of crossover further highlights the clinical challenge of long-term AAD therapy. Rather than specifying a specific AAD regimen, drug choice and dosing decisions were left to the discretion of individual investigators according to the 2012 ESC guidelines. While this adds variability, it also allows the AAD arm to reflect contemporary clinical practice. Some patients did receive doses of AAD drugs that were lower than recommended by the 2012 ESC guidelines, which may reflect intolerance to higher doses. Importantly, some of the lower AAD doses observed (including 100 mg of flecainide and 80 mg of sotalol) are in-line with the AHA/ACC/HRS guidelines for the management of patients with AF.24,25 Nevertheless, it is possible that undertreatment of the AAD group may have led to a higher rate of AA recurrence, and an overestimation of the benefit of ablation. Routine and symptom-based event recording was not required in this study. Consequently, we were not able to evaluate symptom-rhythm correlation. It is also possible that freedom from AA recurrence was overestimated. However, this was a randomized study and both groups completed the same robust Holter monitoring protocol with similar compliance. As such, it is unlikely that the absence of event recorders biased the group difference in AA recurrence observed. For centres to participate in this study, they were required to perform 100 ablation procedures per year, and to have performed a minimum of 50 cryoballoon ablation procedures before the start of the study. Although the outcomes of cryoballoon CA have been shown to be less dependent on operator experience than RFC ablation,26 the safety and efficacy results observed in the present study may not be generalizable to less experienced centres. Similarly, the study population was relatively young and healthy, and findings from this study may not be applicable to other populations. Lastly, patients were only followed for 12 months. While additional studies are needed to determine the longer term safety and efficacy of CA as a first-line treatment, frequent crossovers from AAD to CA pose a challenge for extended follow-up periods.
Conclusions
Cryoballoon CA was superior to AAD therapy, significantly reducing AA recurrence in relatively young, treatment naive patients with recurrent symptomatic PAF and a structurally normal heart. Moreover, CA was associated with lower symptom recurrence and a similar rate of SAEs compared with AAD treatment. Our findings suggest that cryoballoon CA using the Arctic Front Advance Cardiac Cryoablation Catheter is effective as a first-line therapy in relatively young otherwise healthy patients with symptomatic PAF.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors would like to thank the Cryo-FIRST sites and staff for their valuable contributions to this study. Also, the authors thank Prof. Thomas Neumann and Christine Scheld for their contributions to the Holter Core Lab, and Ralf Meyer, Rachelle Kaplon, Kelly van Bragt, Laura Manotta, Hae Lim, Federica Fonti, and Alessandra Gentili from Medtronic for support with the study.
Funding
This work was supported by Medtronic.
Conflict of interest: M.K. reports speaker fees from Abbott and Medtronic; proctoring, consultancy, and advisory board services for Medtronic; research grants from Medtronic and Biosense Webster. N.P. reports speaker’s honoraria from Medtronic and Biosense Webster; proctoring fees from Medtronic and Biosense Webster. V.V. reports speaker fees and proctoring honoraria for Medtronic, travel grants from Biotronik, Biosense-Webster, and Medtronic. J.S.H. reports honoraria for inclusion of patients and realization of the study. S.H., C.A., and G.-B.C. report speaker fees for Medtronic, Biotronik, Biosense Webster, and Abbott; teaching honoraria from Medtronic and Biotronik; proctoring honoraria from Medtronic. G.A. reports research support on Holter monitors. N.B. reports consultancy fees from Bisosense Webster, Boston Scientific SAS, and Medtronic. C.M. reports consultancy and speaker fees from Biosense Webster and Boston Scientific. J.C. reports a research grant from Medtronic. F.A. reports consultant for and lecture fees from Boston Scientific, Medtronic, and Microport CRM. D.L.P. in the past 12 months has provided consulting services for Abbott $0, Biosense Webster $0, Inc., Biotronik <$5000, Boston Scientific $0, CardioFocus $0, Johnson & Johnson $0, MediaSphere Medical, LLC<$5000, Medtronic $0, St. Jude Medical $0, and Siemens $0, SigNum Preemptive Healthcare, Inc. $0, Spectrum Dynamics $0, and Thermedical $0. He receives research funding from the Abbott, Biosense Webster, Boston Scientific/EPT, CardioInsight, CardioFocus, Endosense, German Heart Foundation, Hansen Medical, Medtronic, NIH, Robertson Foundation, St. Jude Medical, Siemens, and Thermedical. Mayo Clinic and D.L.P. and R. Robb have a financial interest in mapping technology. In accordance with the Bayh-Dole Act, this technology has been licensed to St. Jude Medical, and Mayo Clinic and D.L.P. and Robb have received annual royalties >$10 000, the federal threshold for significant financial interest. Mayo Clinic and Dr R. Robb have a financial interest in Analyze-AVW technology that may have been used to analyse some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic and Dr Robb have received royalties >$10 000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed. D.L.P. and Mayo Clinic jointly have equity in a privately held company, External Beam Ablation Medical Devices. Royalties from Wiley & Sons, Oxford, and St. Jude Medical. S.W. reports Speakers bureau/study funding from Abott and Boston Scientific; speakers bureau from Boehringer Ingelheim, Bristol Myers Squibb, Bayer Vital, and Daiichi -Sankyo. F.D.P. and D.B. are employed by and stockholders of Medtronic. S.I. and H.-F.P. reports no conflict of interest.
Data availability
The data underlying this article cannot be shared publicly due to privacy of the individuals that participated in the study.
References
- 1.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373-498. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A. et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 3.Bunch TJ, May HT, Bair TL, Johnson DL, Weiss JP, Crandall BG. et al. Increasing time between first diagnosis of atrial fibrillation and catheter ablation adversely affects long-term outcomes. Heart Rhythm 2013;10:1257–62. [DOI] [PubMed] [Google Scholar]
- 4.Lunati M, Arena G, Iacopino S, Verlato R, Tondo C, Curnis A. et al. Is the time between first diagnosis of paroxysmal atrial fibrillation and cryoballoon ablation a predictor of efficacy? J Cardiovasc Med 2018;19:446–52. [DOI] [PubMed] [Google Scholar]
- 5.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O. et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–95. [DOI] [PubMed] [Google Scholar]
- 6.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J. et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 7.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W. et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634–40. [DOI] [PubMed] [Google Scholar]
- 8.Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR. et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 9.Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M. et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J. et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2020; [DOI] [PubMed] [Google Scholar]
- 11.Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S. et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2020; [DOI] [PubMed] [Google Scholar]
- 12.Hermida JS, Chen J, Meyer C, Iacopino S, Arena G, Pavlovic N. et al. Cryoballoon catheter ablation versus antiarrhythmic drugs as a first-line therapy for patients with paroxysmal atrial fibrillation: rationale and design of the international Cryo-FIRST study. Am Heart J 2019;222:64–72. [DOI] [PubMed] [Google Scholar]
- 13.Akkaya E, Berkowitsch A, Zaltsberg S, Greiss H, Hamm CW, Sperzel J. et al. Second-generation cryoballoon ablation as a first-line treatment of symptomatic atrial fibrillation: two-year outcome and predictors of recurrence after a single procedure. Int J Cardiol 2018;259:76–81. [DOI] [PubMed] [Google Scholar]
- 14.Namdar M, Chierchia GB, Westra S, Sorgente A, Meir ML, Bayrak F. et al. Isolating the pulmonary veins as first-line therapy in patients with lone paroxysmal atrial fibrillation using the cryoballoon. Europace 2012;14:197–203. [DOI] [PubMed] [Google Scholar]
- 15.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S. et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- 16.Khan AR, Khan S, Sheikh MA, Khuder S, Grubb B, Moukarbel GV.. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:853–60. [DOI] [PubMed] [Google Scholar]
- 17.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, for the CABANA Investigators et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A. et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 19.Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Geller L, Kalejs O. et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ.. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 2015;17:370–8. [DOI] [PubMed] [Google Scholar]
- 21.Straube F, Dorwarth U, Ammar-Busch S, Peter T, Noelker G, Massa T. et al. First-line catheter ablation of paroxysmal atrial fibrillation: outcome of radiofrequency vs. cryoballoon pulmonary vein isolation. Europace 2016;18:368–75. [DOI] [PubMed] [Google Scholar]
- 22.Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur S. et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:854–60. [DOI] [PubMed] [Google Scholar]
- 23.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG. et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713–23. [DOI] [PubMed] [Google Scholar]
- 24.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr.. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Calkins H, Field ME, Chen LY, Furie KL. et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019;16:e66–e93. [DOI] [PubMed] [Google Scholar]
- 26.Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi F. et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy of the individuals that participated in the study.