Abstract
Aims
Radiofrequency ablation creates irreversible cardiac damage through resistive heating and this temperature change results in a generator impedance drop. Evaluation of a novel local impedance (LI) technology measured exclusively at the tip of the ablation catheter found that larger LI drops were indicative of more effective lesion formation. We aimed to evaluate whether LI drop is associated with conduction block in patients with paroxysmal atrial fibrillation (AF) undergoing pulmonary vein isolation (PVI).
Methods and results
Sixty patients underwent LI-blinded de novo PVI using a point-by-point ablation workflow. Pulmonary vein rings were divided into 16 anatomical segments. After a 20-min waiting period, gaps were identified on electroanatomic maps. Median LI drop within segments with inter-lesion distance ≤6 mm was calculated offline. The diagnostic accuracy of LI drop for predicting segment block was assessed using receiver operating characteristic analysis.
For segments with inter-lesion distance ≤6 mm, acutely blocked segments had a significantly larger LI drop [19.8 (14.1–27.1) Ω] compared with segments with gaps [10.6 (7.8–14.7) Ω, P < 0.001). In view of left atrial wall thickness differences, the association between LI drop and block was further evaluated for anterior/roof and posterior/inferior segments. The optimal LI cut-off value for anterior/roof segments was 16.1 Ω (positive predictive value for block: 96.3%) and for posterior/inferior segments was 12.3 Ω (positive predictive value for block: 98.1%) where inter-lesion distances were ≤6 mm.
Conclusion
The magnitude of LI drop was predictive of acute PVI segment conduction block in patients with paroxysmal AF. The thinner posterior wall required smaller LI drops for block compared with the thicker anterior wall.
Keywords: Radiofrequency ablation, Local impedance, Atrial fibrillation, Pulmonary vein isolation
What’s new?
Impedance drop during radiofrequency catheter ablation is a marker for tissue heating and thus lesion formation.
Generator impedance is a transthoracic measurement taken between the tip of the ablation catheter to a patch on the patient’s skin, whereas local impedance is measured exclusively with electrodes on the ablation catheter.
The magnitude of local impedance drop was associated with acute conduction block in segments with inter-lesion distance ≤6 mm in paroxysmal atrial fibrillation patients undergoing de novo pulmonary vein isolation.
Larger local impedance drops were required to create conduction block in anterior/roof regions compared with posterior/inferior regions, allowing more tailored left atrial ablation.
Introduction
The goal of radiofrequency (RF) catheter ablation is to permanently damage cardiac tissue required for arrhythmia maintenance via resistive heating. Pulmonary vein isolation (PVI) is the cornerstone treatment of atrial fibrillation (AF) and is achieved by creating contiguous transmural lesions in atrial tissue with varying tissue thickness.1 Under-ablating cardiac tissue will cause ineffective lesion formation potentially leading to recurrence of AF or other atrial arrhythmias, while over-ablating can lead to permanent injury of extra-cardiac structures and serious life-threatening complications.
Impedance is a direct measure of the resistive coupling at the catheter–tissue interface and is temperature dependent. Resistance to RF current is heavily influenced by tissue composition, current shunting to blood, and surface area of contact between the tissue and catheter. Greater resistive coupling between the ablation catheter and cardiac tissue prior to RF leads to more resistive heating and improved lesion formation. Creation of an RF lesion is accompanied by a resulting impedance drop and the magnitude of this drop depends on the temperature and amount of myocardium being heated.2
To date, generator impedance drop has been routinely monitored in clinical practice to assess RF therapy delivery and has been shown to be an important indicator for successful PVI.3,4 However, generator impedance is a transthoracic measurement taken between the tip of the ablation catheter to a patch on the patient’s skin. It is therefore affected by factors including body habitus, fluid status and changes in lung volume,5–7 and accordingly may not be consistent between patients or even within a procedure.
Local impedance (LI) measurement is a novel metric which involves injection of current between the tip electrode and proximal ring of the ablation catheter. This creates a local potential field which is distorted by proximity to or contact with myocardium. Mini-electrodes embedded in the tip electrode measure these potential field distortions, which are divided by the known injection current to convert to impedance. Pre-clinical and clinical evaluation of this metric found that LI outperformed generator impedance at characterizing tissue composition and predicting lesion formation.8–10 However, the relationship between LI drop and acute conduction block in patients with paroxysmal AF undergoing RF PVI has not previously been studied. We hypothesized that a higher LI drop value would predict acute conduction block of segments with close inter-lesion spacing (≤6 mm) after first-pass encirclement of the PVs. We also hypothesized that thicker anterior segments would require larger drops to obtain transmural lesions required for isolation.
Methods
Patient population
This study was a prospective, non-randomized, single arm, multi-centre trial entitled ‘Electrical Coupling Information from the RHYTHMIA HDx™ Mapping System and DIRECTSENSE™ Technology in the Treatment of Paroxysmal Atrial Fibrillation—A Non-Randomized, Prospective Study: LOCALIZE’ (clinicaltrials.gov NCT03232645). The trial was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by all national/local institutional review boards and all patients provided written informed consent.
The study inclusion criteria were as follows: patients with symptomatic paroxysmal AF (≥30 s that terminated within 7 days), refractory or intolerant to at least one antiarrhythmic drug (Class I or III, β-blocker, or calcium channel-blocker), and between the ages of 18 and 80. The key study exclusion criteria were as follows: previous left atrial (LA) ablation procedure, New York Heart Association Class III or IV, severe left ventricular systolic dysfunction (ejection fraction <35%), LA diameter >5.5 cm, unstable angina or myocardial ischaemia, myocardial infarction, prior prosthetic valve, and pregnancy.
Pulmonary vein isolation procedure
The catheter ablation procedure was performed under conscious sedation or general anaesthesia at the discretion of the operator and centre. A diagnostic catheter was placed in the coronary sinus for pacing and to serve as a timing reference for creation of high-density electroanatomic maps. Prior to or immediately following transseptal puncture, an intravenous heparin bolus was administered with repeat heparin administration as necessary to maintain an activated clotting time greater than 300 s.
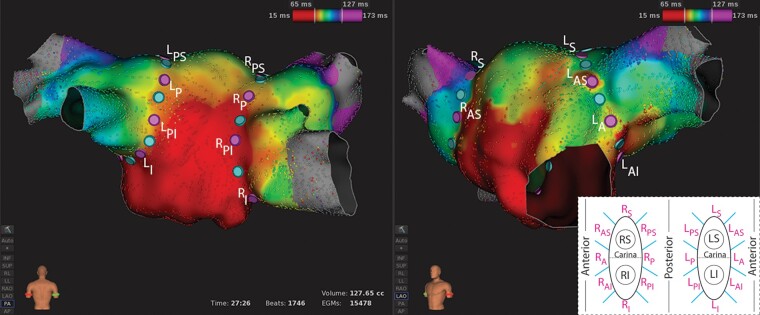
In all procedures, a 3D navigation system (RHYTHMIA HDx™ Mapping System, Boston Scientific, Marlborough, MA, USA) and 64-electrode basket mapping catheter (INTELLAMAP ORION™, Boston Scientific) were used to create an electroanatomic map of the LA. Ipsilateral PV rings were divided into 8 anatomical segments (16 segments per patient) using tags placed on the LA geometry (Figure 1). Segment borders were defined on the map prior to ablation in order to assign RF applications to segments.
Figure 1.
Representative example of a left atrial electroanatomic map with anatomical segments defined (n = 16). Segment borders are defined with light blue tags and segment centres with purple tags. A, anterior; I, inferior; L, left; P, posterior; R, right; S, superior.
First-pass encirclement was performed using point-by-point ablation (INTELLANAV MIFI™ Open-Irrigated, Boston Scientific) with blinding of operators to LI data. Catheter operators attempted to space lesions ≤6 mm apart and limit jumping between anatomical segments. Radiofrequency power and application duration were at the discretion of the catheter operator. Each RF application was denoted with a tag on the electroanatomic map. After a 20-min waiting period following initial encirclement of all PVs, a repeat electroanatomic map during coronary sinus pacing was created to identify conduction gaps within segments. If gaps were identified, touch-up ablations were performed while visualizing LI data. Pulmonary vein isolation was confirmed with entrance block in all cases, with exit block and/or adenosine challenge to unmask sites of dormant conduction also commonly performed depending on operator preference. No additional LA ablations were performed.
Data analysis
Local impedance data and RF tag location for first-pass ablations were processed offline using MATLAB R2019a (Mathworks, Natick, MA, USA). Raw LI data were filtered using a moving mean filter with a window length of 1.5 s. Starting LI and LI drop for each ablation lesion were calculated from filtered LI data, and starting and drop values for generator impedance were also collected. For each segment, the median LI drop, minimum LI drop, median generator impedance drop, and minimum generator impedance drop values were calculated. The maximum inter-lesion distance was calculated for each segment to characterize the largest distance between RF applications.
Statistics
Continuous variables were reported as median with inter-quartile range (Q1–Q3) or mean ± standard deviation. Categorical variables were summarized as count and percentage. Comparisons of LI in block vs. gap segments were performed using t-tests for equal or unequal variances, as appropriate. A Bonferroni adjustment was applied to account for multiple tests and a P-value of 0.0125 was considered significant in these analyses (SAS version 9.4, SAS Institute, Cary, NC, USA). A receiver operating characteristic (ROC) curve analysis was also performed for LI drop in segments with acute block and gaps. The area under the ROC curve (AUC) was calculated as a measure of diagnostic accuracy. A true positive was defined as when a physician adjudicated block and LI drop predicted block. From the ROC analysis, optimal cut-off values were calculated using the Youden Index.
Results
A total of 60 patients with documented paroxysmal AF underwent PVI performed by 8 operators at 6 centres. In one further patient, the procedure was commenced but was abandoned due to an adverse reaction to sedation/analgesia. Baseline demographics for the 60 patients with completed procedures [33 (55%) male, mean age 62 ± 11 years] are displayed in Table 1. Every patient left the PVI procedure with all veins isolated (n = 240 of 240). There were no reported device-related serious adverse events, including tamponade, pericardial effusion, or stroke.
Table 1.
Patient demographics
| Subjects (n = 60) | |
|---|---|
| Age (years) | 62 ± 11 |
| Male, n (%) | 33 (55) |
| LA diameter (mm)a | 39 ± 7 |
| Ejection fraction <55%, n (%) | 4 (8.2) |
| Hypertension, n (%) | 34 (56.7) |
| Diabetes, n (%) | 2 (3.3) |
| Anti-arrhythmic drugs, n (%) | 56 (93.3) |
| Class I | 22 (36.7) |
| Class III | 12 (20.0) |
| β-blockers | 39 (65.0) |
| Calcium channel blockers | 4 (6.7) |
| Oral anticoagulation, n (%) | 56 (93.3) |
| Non-vitamin K OAC | 48 (80.0) |
| Vitamin K OAC | 8 (13.3) |
LA, left atrium; OAC, oral anticoagulation.
Documented value in 41 subjects. All subjects (n = 60) left atrial diameter <55 mm.
Electroanatomic maps were reviewed for evidence of adequate conduction into the PVs beyond the level of the planned PVI ablation ring prior to ablation (Figure 1), as this was essential for determining which segments were blocked or reconnected at the re-map performed 20 min after initial encirclement. Five subjects and an additional two ipsilateral veins were removed from the analysis because there was minimal PV conduction beyond the planned PVI ablation ring prior to ablation, making segment block/gap adjudication unreliable (Supplementary material online, Figure S1). This resulted in 864 analysed segments in 55 subjects.
Dataset
Figure 1 displays a representative example of an activation map while pacing from the coronary sinus prior to starting ablation. Segment borders (light blue tags) and centres (purple tags) were defined prior to starting ablation and electrical conduction was evident beyond the segments. In the 55 subjects, a total of 3848 first-pass ablation lesions were analysed. The mean RF power and application duration were 33.7 ± 3.8 W and 28.3 ± 7.6 s, respectively. The distribution of power settings used was: <25 W: 0.6%, 25–35 W: 83.1%, and >35 W 16.3%. The distribution of application durations was: <25 s: 33.9%, 25–45 s: 64.2%, and >45 s 1.9%. Due to blinding of the operator to LI data, some artificial impedance measurements, caused by either ablating near the mapping catheter or ablating while the sheath was covering ring electrodes critical to the LI circuit, were identified during offline analysis. This affected 96 (2.5%) RF ablations in 72 of 864 (8.3%) segments and these segments were removed from the analysis.
Local impedance characterization
Table 2 displays the mean starting LI, mean LI drop, and reconnection rate for each of the 16 anatomical segments. When encircling the PVs while blinded to LI, the mean starting LI was 107.9 ± 16.0 Ω and mean LI drop was 19.8 ± 11.1 Ω. Generator data were available in 50 of 55 subjects, in whom the mean starting generator impedance was 126.6 ± 22.4 Ω and drop was 10.2 ± 5.6 Ω. At the LA remap 20 min following initial LI-blinded encirclement, 16.8% of segments showed acute reconnection. Higher gap rates were seen in the left anterior ridge (LA: 46.9% and LAS: 36.2%) and right posterior/roof (RS: 40%, RSP: 31.2%, and RP: 23.4%) segments. Lower acute gap rates were seen in the left posterior superior (3.9%) and inferior (3.9%) segments.
Table 2.
Mean segmental local impedances and acute reconnection rates
| Segment | Baseline (Ω) | Drop (Ω) | Gap rate (%) | |
|---|---|---|---|---|
| Left | Superior | 111.6 ± 18.1 | 21.6 ± 12.6 | 12.5 |
| Anterior superior | 106.9 ± 15.5 | 18.6 ± 9.9 | 36.2 | |
| Anterior | 105.2 ± 13.5 | 17.1 ± 8.7 | 46.9 | |
| Anterior inferior | 105.8 ± 16.2 | 18.9 ± 11.1 | 7.7 | |
| Inferior | 108.1 ± 15.4 | 21.3 ± 9.7 | 3.9 | |
| Posterior inferior | 109.2 ± 16.1 | 22.7 ± 9.9 | 5.8 | |
| Posterior | 110.6 ± 19.0 | 20.3 ± 12.1 | 12.0 | |
| Posterior superior | 110.7 ± 17.0 | 21.3 ± 12.1 | 3.9 | |
| Right | Superior | 105.1 ± 14.3 | 18.4 ± 11.7 | 40.0 |
| Anterior superior | 109.0 ± 15.5 | 22.7 ± 11.4 | 15.7 | |
| Anterior | 110.6 ± 16.7 | 24.3 ± 11.4 | 12.2 | |
| Anterior inferior | 108.2 ± 14.6 | 23.0 ± 10.3 | 8.2 | |
| Inferior | 110.0 ± 15.6 | 21.8 ± 9.6 | 7.7 | |
| Posterior inferior | 108.4 ± 15.7 | 16.0 ± 9.6 | 7.8 | |
| Posterior | 104.1 ± 14.2 | 14.5 ± 10.0 | 23.4 | |
| Posterior superior | 104.3 ± 14.8 | 15.9 ± 10.6 | 31.3 | |
| All | 107.9 ± 16.0 | 19.8 ± 11.1 | 16.8 | |
Comparison of segments with acute block or gap
Figure 2A shows a representative LI trace (raw data: grey and filtered data: yellow) with seven RF applications that are highlighted with an orange overlay. The trace has two separate time components, comprising a historical impedance stacking component (left) and a real-time impedance component (right). With each RF application, there is a pronounced drop in LI which is initially steep and then becomes shallower or reaches a plateau.
Figure 2.
Representative example of the local impedance trace and median local impedance drop calculation per anatomical segment. (A) Seven consecutive radiofrequency ablations (orange overlay and white arrows) with raw (white) and filtered (yellow) local impedance traces displayed. (B) Activation map identified a gap in the right posterior segment (grey tag, white asterisk) after the 20-min waiting period. Tag colours: black—RF ablation, light blue—segment border, and purple—segment centre. RF, radiofrequency.
For each segment, 4 parameters were calculated: median LI drop, minimum LI drop, median generator impedance drop and minimum generator impedance drop. Figure 2B shows a representative example of an activation map with median LI drops displayed at the centre of each segment (purple tags). A single gap (grey tag) was identified in the right posterior segment and had a median LI drop of 10 Ω. All other segments in the posterior view were acutely blocked and had median LI drops of 17–39 Ω.
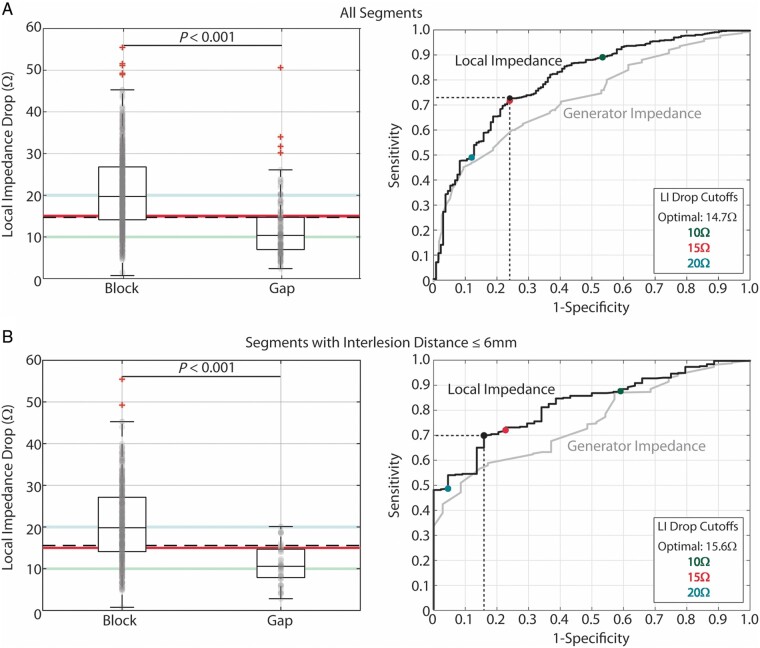
Data for these four parameters are presented for acutely blocked and gap segments in Table 3, along with the AUC value from ROC curve analysis. Impedance drop values for all 4 parameters were significantly larger for acutely blocked segments than for gap segments. However, median LI drop [19.7 (14.1–26.8) vs. 10.4 (7.0–14.7) Ω, P < 0.001] had the smallest P-value and the largest AUC, and these are shown graphically in Figure 3A. Receiver operating characteristic analysis (Youden Index) identified 14.7 Ω as the optimal LI drop cut-off value (sensitivity: 72.7%, specificity: 75.9%, positive predictive value for acute conduction block: 93.7%, Figure 3A, right). In comparison, the optimal generator impedance cut-off value (8.0 Ω) had a lower sensitivity (63.3%), specificity (71.8%), and positive predictive value (91.9%).
Table 3.
Median local (n = 55 subjects) and generator (n = 50 subjects) impedance drop values in block and gap segments
| Analysis | Block (Ω) | Gap (Ω) | P-value | AUC |
|---|---|---|---|---|
| Local impedance drop, median | 19.7 (14.1–26.8) | 10.4 (7.0–14.7) | <0.001 | 0.80 |
| Local impedance drop, minimum | 12.7 (8.6–18.5) | 6.3 (4.3–10.2) | <0.001 | 0.77 |
| Generator impedance drop, median | 10.0 (7.0–14.0) | 7.0 (4.5–8.9) | <0.001 | 0.74 |
| Generator impedance drop, minimum | 7.0 (4.5–10.0) | 4.0 (3.0–6.0) | <0.001 | 0.73 |
AUC, area under the receiver operating characteristic curve.
Figure 3.
Relationship between LI drop and acute block after first-pass encirclement. (A) Median LI drop in acutely blocked (n = 659) and gap (n = 133) segments (left) and corresponding ROC curve analysis (right). (B) Median LI drop in acutely blocked (n = 372) and gap (n = 44) segments with inter-lesion distance ≤6 mm and corresponding ROC curve analysis (right). Highlighted threshold values in the box-and-whisker plots (10 Ω: green, 15 Ω: pink, 20 Ω: blue, and calculated optimal: black dashed) are displayed as coloured points in the ROC curve plots. LI, local impedance; ROC, receiver operating characteristic.
The relationship between median LI drop and segmental block was further evaluated in segments with inter-lesion spacing ≤6 mm [416 of 792 (53%) segments]. Similar to the complete dataset, acutely blocked segments with inter-lesion spacing ≤6 mm had a significantly larger LI drop [19.8 (14.1–27.1) Ω] than segments with gaps [10.6 (7.8–14.7) Ω, P < 0.001, Figure 3B, left]. All gaps in segments with a median LI drop of >25 Ω (Figure 3A, left) were associated with inter-lesion spacing >6 mm, and when inter-lesion spacing was accounted for, the largest LI drop that was associated with a gap was 20.1 Ω.
For inter-lesion spacing ≤6 mm, the AUC was 0.82 for LI drop and 0.75 for generator impedance drop. Receiver operating characteristic analysis identified the optimal LI cut-off value as 15.6 Ω (sensitivity: 69.9%, specificity: 84.1%, and positive predictive value for acute conduction block: 97.4%, Figure 3B, right). The optimal generator impedance cut-off value (7.9 Ω) again had a lower sensitivity (62.2%), specificity (68.6%), and positive predictive value (95.0%).
Regional differences
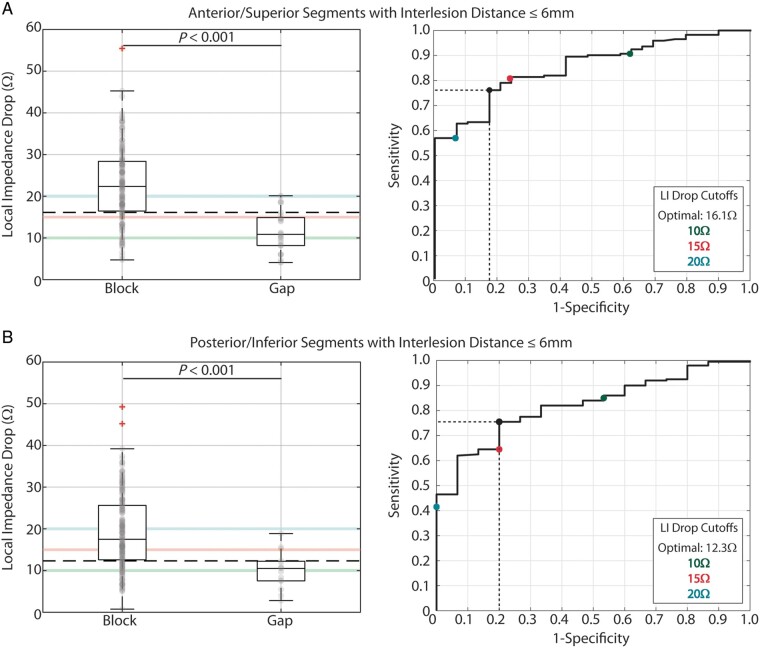
Data were further evaluated along LA wall thickness differences. Anterior and superior segments were found to have similar values and were combined, as were posterior and inferior segments. Acutely blocked anterior/roof segments with inter-lesion distance ≤6 mm had significantly larger median LI drops [22.4 (16.5–28.4) Ω] compared with segments with gaps [10.8 (8.2–14.9) Ω, P < 0.001, Figure 4A, left]. Similarly, acutely blocked posterior/inferior segments with inter-lesion distance ≤6 mm had significantly larger median LI drops [17.5 (12.6–25.6) Ω] compared with segments with gaps [10.5 (7.5–12.2) Ω, P < 0.001, Figure 4B, left]. Importantly, the median LI drop for blocked posterior/inferior segments was significantly lower than for blocked anterior/roof segments (P < 0.001).
Figure 4.
Relationship between LI drop and regional acute block after first-pass encirclement. (A) Median LI drop in anterior/superior acutely blocked (n = 172) and gap (n = 29) segments with inter-lesion distance ≤6 mm (Left) and corresponding ROC curve analysis (right). (B) Median LI drop in posterior/inferior acutely blocked (n = 200) and gap (n = 15) segments with inter-lesion distance ≤6 mm (left) and corresponding ROC curve analysis (right). Highlighted threshold values in the box-and-whisker plots (10 Ω: green, 15 Ω: pink, 20 Ω: blue, and calculated optimum: black dashed) are displayed as coloured points in the ROC curve plots. LI, local impedance; ROC, receiver operating characteristic.
The optimal LI cut-off value for anterior/roof segments was 16.1 Ω (sensitivity: 76.2%, specificity: 82.7%, and positive predictive value for acute conduction block: 96.3%), while the optimal LI cut-off value for posterior/inferior segments was lower at 12.3 Ω (sensitivity: 75.5%, specificity: 80.0%, and positive predictive value for acute conduction block: 98.1%).
The likelihood of achieving the clinically relevant LI drop in each region was examined. When blinded to LI, the majority of ablations in the anterior/roof region (59.8%) reached a drop that was larger than 16.1 Ω. For those ablations that achieved a 16.1 Ω drop, the mean time to reach this value was 10.4 ± 7.1 s. For the posterior/inferior region, 69.4% of ablations reached a drop that was larger than 12.3 Ω and the average time to reach this value was 8.2 ± 6.3 s.
Relationship between starting impedance and impedance drop
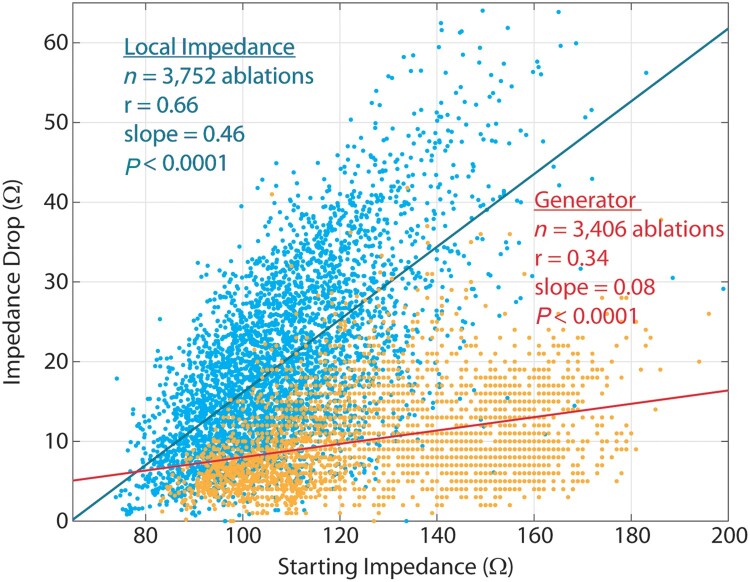
Figure 5 shows scatter plots and lines of best fit demonstrating the relationships between starting LI and LI drop and between starting generator impedance and generator impedance drop. Local impedance drop had a significant, strong and positive correlation with starting LI (Pearson R = 0.66, P < 0.0001). Generator impedance drop also significantly correlated to starting generator impedance, but the correlation was weaker (Pearson R = 0.34, P < 0.0001).
Figure 5.
Scatter plot with lines of best fit demonstrating the correlations between starting LI and LI drop (blue points/line) and between starting generator impedance and generator impedance drop (orange points/line). LI, local impedance.
Discussion
Main findings
There are three main findings from these initial results of the LOCALIZE clinical trial. Firstly, the magnitude of LI drop during ablation was predictive of acute PVI segment block, and this was particularly evident when inter-lesion distances were ≤6 mm. Secondly, LI drop showed improved diagnostic performance for acute conduction block than generator impedance drop, with greater sensitivity, specificity, and positive predictive value. Thirdly, a significantly smaller LI drop was required to achieve segment block in the thinner-walled posterior and inferior segments than in the thicker anterior and roof segments. These results suggest that RF ablation guided by LI may be beneficial in achieving acute first-pass PVI while tailoring ablation delivery to minimize risks of extra-cardiac damage.
Local and generator impedance
The impedance drop during ablation derived from the RF generator reflects tissue response to heating and has commonly been used as a marker of ablation efficacy. Previous studies have demonstrated that the generator impedance drop relates to the degree of contact force applied and is predictive of acute PV reconnection.3,4 However, as generator impedance is a transthoracic measurement from the catheter tip to the return skin patch, it is limited by a number of factors. These include the size and body habitus of the patient, their fluid status, and acute changes in lung volume, which are commonly seen due to erratic respiratory patterns in cases performed under conscious sedation.5–7 These factors may therefore lead to differences in values between patients and even within the same patient at different stages of an ablation procedure.
A LI metric derived entirely from the ablation catheter electrodes was therefore developed to provide a more direct measure. This may overcome some of the disadvantages of generator impedance, though LI measurement also has some limitations. The LI algorithm does not fully account for electrode-tissue surface area changes that can occur during respiration and can be affected by surface area changes during ablation, though the LI time graph can help operators distinguish between these changes. Additionally, if a long sheath is used to support the ablation catheter, care must be taken to avoid covering the catheter ring electrodes with the sheath as this can interact with the field that is being generated and cause a spurious rise in the measured LI, which is not the case for generator impedance until the tip electrode is covered.
In a pre-clinical study, LI drop predicted lesion size, with a better correlation than for generator impedance drop.10 In a prior clinical study, a moderate correlation was seen between local and generator impedance drops, with LI drops of greater magnitude (more than 2 times larger) than those from the RF generator.9 In a further study, the median LI drop for acutely successful LA lesions, as determined by lack of local tissue capture, was 14.6 (10.0–18.3) Ω, which was significantly larger than for unsuccessful lesions [6.8 (4.7–13.0) Ω, P = 0.049].8 In this study, the median LI drop in acutely blocked PVI segments was significantly higher than for gap segments. Although this was also true for generator impedance drop, LI drop showed improved diagnostic performance for predicting segment block in ROC curve analysis and again was larger in magnitude. Furthermore, starting LI showed a stronger correlation to LI drop than starting generator impedance did to generator impedance drop. This may allow operators to better predict the drop that is likely to be achieved prior to initiating energy delivery, thereby providing more effective pre-ablation planning.
Regional differences
It is well recognized that there are markedly different wall thicknesses in different regions of the LA, with thicker tissue present anteriorly, particularly at the left pulmonary vein/LA appendage ridge, and thinner tissue in the posterior region. This, coupled with the risk of inadvertent damage to extra-cardiac structures such as the posteriorly-located oesophagus, makes titration of ablation energy in different LA locations of vital importance. Historically, operators have empirically reduced RF power and application duration on the posterior wall, but other factors such as catheter contact also play a role and lesions may still vary significantly.
Analysis of Ablation Index, a metric incorporating contact force, power, and time, demonstrated that significantly lower values were required to achieve durable PVI segment block in the posterior/inferior region than in the anterior/roof region.11 These findings were replicated in this study, using a completely different technology, with a smaller LI drop required for conduction block in posterior/inferior segments and with this smaller drop achieved in a shorter time. These results indicate that LI-guided ablation allows tailoring of energy delivery within the LA, optimizing efficacy without increasing the risk of complications.
Other lesion metrics
Several other lesion metrics have previously been studied, including Force–Time Integral (FTI),12 Lesion Size Index,13 and Ablation Index,11 of which the latter is the most validated.14,15,16 These metrics share a common mechanism of incorporating factors known to be relevant to lesion creation including contact force, time and, with the exception of FTI, power. Local impedance drop differs from these metrics in that it is measuring a biological parameter directly from tissue, rather than combining ablation inputs. Further validation of LI-guided ablation is required to determine whether this theoretical advantage translates into improved clinical outcomes.
Clinical utility of local impedance drop
As LI drop has been shown in this study to be predictive of acute segment block, it would follow that LI-guided ablation may be of benefit in enhancing the likelihood of successful acute PVI, with different target LI drop values for anterior and posterior regions. Optimal values determined from ROC curve analysis, and therefore providing the best balance between efficacy and safety, were 16.1 Ω for anterior/roof segments and 12.3 Ω for the posterior/inferior region. However, concerns regarding efficacy and safety vary between LA regions, with less risk of extra-cardiac injury in the anterior wall. No acute reconnection was seen in anterior/roof segments when the median LI drop was >20.1 Ω and inter-lesion distance ≤6 mm, and therefore a strategy of aiming for LI drops of 16–20 Ω anteriorly and 12–13 Ω posteriorly may offer optimal results. However, a prospective, unblinded study is required in the future to validate these suggested target values in order to assess the safety and efficacy of this approach.
While LI drop in itself provides valuable information about tissue effect during ablation, it is possible that combining this in algorithms incorporating other parameters, such as time or rate of drop, may add additional value in guiding ablation lesion delivery and is likely to form the basis of future investigation.
Limitations
There are several limitations to this study. Firstly, the LOCALIZE trial included a relatively small number of subjects as the study design involved a protocol-mandated invasive repeat LA mapping procedure after 3 months. However, the study size was consistent with previous trials with a similar study design, and the segment-level analysis provided adequate power for analysis. Secondly, the optimal LI drop values identified in this study relate to acute conduction block and may not translate to durable lesion formation. Results from assessment for durable conduction block at the repeat LA mapping procedure after 3 months will be published following completion of the trial. Thirdly, although RF power and duration were at the discretion of the catheter operator, the evaluation did not cover the entire RF dosing range. The 8 operators at 6 centres primarily utilized standard powers (30–40 W) and durations (20–35 s) that were adjusted based on anatomical location. High power, short duration ablation was not utilized in this study and further assessment of LI to evaluate its performance with this form of ablation energy delivery is therefore required.
Conclusions
In the LOCALIZE study, the magnitude of LI drop was predictive of acute PVI segment conduction block in patients with paroxysmal AF. The thinner posterior/inferior wall required smaller LI drops for segment block compared to the thicker anterior/roof region, with LI drops of 16.1 and 12.3 Ω, respectively, having a high positive predictive value for acute segment block where inter-lesion spacing was ≤6 mm. These data support the potential use of LI drop as a guiding strategy for RF PVI.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contributions of the Boston Scientific Field Clinical teams involved in carrying out this study.
Funding
This study was funded by Boston Scientific.
Conflict of interest: M.D. has received research funding, Fellowship funding and speaker/proctorship fees from Boston Scientific. A.L. has received honoraria from Boston Scientific and Biosense Webster. E.S. has received speaker fees from Boston Scientific and ACUTUS Medical. M.S., T.O., and E.D. are salaried employees of Boston Scientific, and M.S. and E.D. hold stock in Boston Scientific. At the time of the study, J.L. was a salaried employee of and held stock in Boston Scientific. C.M. has received speaker fees from Boston Scientific and Abbott and is a Consultant for Boston Scientific, Biosense Webster, and Abbott. P.J. has received research funding and speaker fees from Boston Scientific. A.Y. has received speaker fees from Boston Scientific. W.U. has received research funding and speaker fees from Boston Scientific. I.G.-B. has received research funding and speaker/proctorship fees from Boston Scientific. All remaining authors have declared no conflicts of interest.
Data availability
The data underlying this article were provided by Boston Scientific Corp. by permission. Data will be shared on request to the corresponding author with permission of Boston Scientific Corp.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Viles-Gonzalez JF, Berjano E, d’Avila A.. Complications of radiofrequency catheter ablation: can we prevent steam pops? JACC Clin Electrophysiol 2018;4:501–3. [DOI] [PubMed] [Google Scholar]
- 3. Reichlin T, Knecht S, Lane C, Kühne M, Nof E, Chopra N. et al. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm 2014;11:194–201. [DOI] [PubMed] [Google Scholar]
- 4. Yazaki K, Ejima K, Kanai M, Kataoka S, Higuchi S, Yagishita D. et al. Impedance drop predicts acute electrical reconnection of the pulmonary vein-left atrium after pulmonary vein isolation using short-duration high-power exposure. J Interv Card Electrophysiol 2020;59:575–84. [DOI] [PubMed] [Google Scholar]
- 5. Gaspar T, Sih H, Hindricks G, Eitel C, Sommer P, Kircher S. et al. Use of electrical coupling information in AF catheter ablation: a prospective randomized pilot study. Heart Rhythm 2013;10:176–81. [DOI] [PubMed] [Google Scholar]
- 6. Piorkowski C, Sih H, Sommer P, Miller SP, Gaspar T, Teplitsky L. et al. First in human validation of impedance-based catheter tip-to-tissue contact assessment in the left atrium. J Cardiovasc Electrophysiol 2009;20:1366–73. [DOI] [PubMed] [Google Scholar]
- 7. van Es R, Hauck J, van Driel VJ, Neven K, van Wessel H, Doevendans PA. et al. Novel method for electrode-tissue contact measurement with multi-electrode catheters. Europace 2018;20:149–56. [DOI] [PubMed] [Google Scholar]
- 8. Martin CA, Martin R, Gajendragadkar PR, Maury P, Takigawa M, Cheniti G. et al. First clinical use of novel ablation catheter incorporating local impedance data. J Cardiovasc Electrophysiol 2018;29:1197–206. [DOI] [PubMed] [Google Scholar]
- 9. Gunawardene M, Munkler P, Eickholt C, Akbulak RO, Jularic M, Klatt N. et al. A novel assessment of local impedance during catheter ablation: initial experience in humans comparing local and generator measurements. Europace 2019;21:i34–42. [DOI] [PubMed] [Google Scholar]
- 10. Sulkin MS, Laughner JI, Hilbert S, Kapa S, Kosiuk J, Younan P. et al. Novel measure of local impedance predicts catheter-tissue contact and lesion formation. Circ Arrhythm Electrophysiol 2018;11:e005831. [DOI] [PubMed] [Google Scholar]
- 11. Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJ. et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. EP. Europace 2017;19:775–83. [DOI] [PubMed] [Google Scholar]
- 12. Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D. et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 13. Kanamori N, Kato T, Sakagami S, Saeki T, Kato C, Kawai K. et al. Optimal lesion size index to prevent conduction gap during pulmonary vein isolation. J Cardiovasc Electrophysiol 2018;29:1616–23. [DOI] [PubMed] [Google Scholar]
- 14. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A. et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE Study results. Circ Arrhythm Electrophysiol 2018;11:e006576. [DOI] [PubMed] [Google Scholar]
- 15. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Y. et al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 16. Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht Set al. . Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. EP Europace 2020;22:1645–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Boston Scientific Corp. by permission. Data will be shared on request to the corresponding author with permission of Boston Scientific Corp.