Abstract
Objectives
Symptom persistence weeks after laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) clearance is a relatively common long-term complication of Coronavirus disease 2019 (COVID-19). Little is known about this phenomenon in older adults. The present study aimed at determining the prevalence of persistent symptoms among older COVID-19 survivors and identifying symptom patterns.
Design
Cross-sectional study.
Setting and Participants
We analyzed data collected in people 65 years and older (n = 165) who were hospitalized for COVID-19 and then admitted to the Day Hospital Post-COVID 19 of the Fondazione Policlinico Universitario "Agostino Gemelli" IRCCS (Rome, Italy) between April and December 2020. All patients tested negative for SARS-CoV-2 and met the World Health Organization criteria for quarantine discontinuation.
Measures
Patients were offered multidisciplinary individualized assessments. The persistence of symptoms was evaluated on admission using a standardized questionnaire.
Results
The mean age was 73.1 ± 6.2 years (median 72, interquartile range 27), and 63 (38.4%) were women. The average time elapsed from hospital discharge was 76.8 ± 20.3 days (range 25−109 days). On admission, 137 (83%) patients reported at least 1 persistent symptom. Of these, more than one-third reported 1 or 2 symptoms and 46.3% had 3 or more symptoms. The rate of symptom persistence was not significantly different when patients were stratified according to median age. Compared with those with no persistent symptoms, patients with symptom persistence reported a greater number of symptoms during acute COVID-19 (5.3 ± 3.0 vs 3.3 ± 2.0; P < .001). The most common persistent symptoms were fatigue (53.1%), dyspnea (51.5%), joint pain (22.2%), and cough (16.7%). The likelihood of symptom persistence was higher in those who had experienced fatigue during acute COVID-19.
Conclusions and Implications
Persistent symptoms are frequently experienced by older adults who have been hospitalized for COVID-19. Follow-up programs should be implemented to monitor and care for long-term COVID-19–related health issues.
Keywords: SARS-CoV-2, COVID-19, long COVID, aging, fatigue, geriatrics
No age group is protected from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection; yet, the disease burden is disproportionally high among persons aged 65 years and older, with a mortality rate as high as 20% in octogenarians.1 The presence of multiple preexisting comorbidities (eg, cardiovascular disease, diabetes mellitus, renal failure, respiratory diseases, cancer) is typically associated with poorer prognosis in patients with Coronavirus disease 2019 (COVID-19),2 possibly as a consequence of diminished physiological reserves. Concerns are not only related to the acute phase of the disease, but also to its post-acute phase. The recovery path from COVID-19 is variable and depends on age and preexisting comorbidities, as well as the severity of acute disease. According to the World Health Organization (WHO), the recovery time from COVID-19 is approximately 2 weeks in mild cases and 3 to 6 weeks in more severe infections.3 , 4 Yet, a considerable share of patients complain of COVID-19–related symptoms weeks after disease onset, a condition known as post-acute COVID-19 syndrome.5 , 6 Common long-lasting signs and symptoms include cough, fever, dyspnea, fatigue, musculoskeletal (myalgia, joint pain) and gastrointestinal complaints, and anosmia/dysgeusia.7, 8, 9
Some information on the characteristics associated with COVID-19 symptom persistence in the general population is available, but little is known about the condition in older adults. To start filling this gap in knowledge, we followed up older patients after hospital discharge and recovery from acute COVID-19. The aims of the study were to determine the prevalence of persistent symptoms among older COVID-19 survivors and identify the most frequent symptom patterns.
Methods
The Gemelli Against COVID-19 Post-Acute Care (GAC19-PAC) project is an ongoing initiative developed by the Fondazione Policlinico Universitario "Agostino Gemelli" IRCCS and the Università Cattolica del Sacro Cuore (Rome, Italy) to offer COVID-19 survivors a multidisciplinary, individualized follow-up. To this aim, a post-acute outpatient service called “Day Hospital Post-COVID-19” was established on April 21, 2020. Details on the post-acute outpatient service and assessments have been published previously.10
Study Sample
All patients who met the WHO criteria for discontinuation of quarantine (ie, more than 10 days from symptom onset plus 3 additional days with no symptoms except for anosmia/dysgeusia) who tested negative for SARS-CoV-2 at real-time reverse transcriptase polymerase chain reaction of nasopharyngeal and oropharyngeal swab were eligible for inclusion in the GAC19-PAC project.11 Between April 21 and December 21, 2020, 691 persons who had recovered from COVID-19 were admitted to the Day Hospital Post-COVID-19. For the present study, analyses were conducted in patients 65 years and older (n = 165). All patients lived in the community and were able to walk independently.
Post-COVID-19 Assessment
Patients were offered a comprehensive assessment including collection of detailed medical history and a thorough physical examination. A multidisciplinary approach, encompassing internal medicine, geriatric, ophthalmological, otolaryngologic, pneumological, cardiological, neurological, immunological, and rheumatological evaluations, was adopted to explore all possible consequences of SARS-CoV-2 infection.10 , 12 The persistence of symptoms potentially related to COVID-19 was evaluated on admission using a standardized questionnaire, as previously described.5 For the present study, patients were categorized into persistent and nonpersistent COVID-19 symptom groups depending on the presence of at least 1 persistent symptom on admission or none, respectively.
Ethical Approval and Manuscript Preparation
The study protocol was approved by the Ethics Committee of the Fondazione Policlinico Universitario "Agostino Gemelli" IRCCS/Università Cattolica del Sacro Cuore, Rome, Italy (protocol number: 0013008/20). Written informed consent was obtained from all participants before enrollment. The manuscript was prepared in compliance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) reporting guidelines for observational studies.13
Statistical Analysis
Continuous variables are shown as mean ± standard deviation (SD), and categorical variables are reported as frequencies by absolute values and percentages. Descriptive statistics were used to describe demographic and key clinical characteristics of the study population according to sex and time elapsed from hospital discharge. Differences in proportions and means of covariates between participants with and without persistent COVID-19–related symptoms were assessed using Fisher's exact test and t-test statistics, respectively. Cox proportional hazard models with robust variance estimates were used to assess the association between clinical characteristics and persistent COVID-19–related symptoms. Candidate variables to be included in the Cox models were selected on the basis of biological and clinical plausibility as potential risk factors for persistent symptoms. To identify factors independently associated with COVID-19 symptom persistence, the crude prevalence rate ratio (PR) and the corresponding 95% confidence interval (CI), controlling for age and sex, were first estimated. A multivariable Cox model was computed including all variables associated with the outcome at an α level of 0.05, after adjustment for age and sex.
All analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL).
Results
The average time elapsed from hospital discharge was 76.8 ± 20.3 days (range 25−109 days). On admission to the Day Hospital Post-COVID-19, 137 (83%) patients reported at least 1 persistent symptom. The main characteristics of the study population according to the persistence of COVID-19–related symptoms are summarized in Table 1 . The mean age was 73.1 years (SD 6.2; median 72, interquartile range 27), and 63 (38.4%) were women. Demographic, anthropometric, functional, and clinical characteristics were similar between patients with and without persistent symptoms. Compared with patients with no persistent symptoms, those with persistent symptoms reported a greater number of symptoms during acute COVID-19 (5.3 ± 3.0 symptoms vs 3.3 ± 2.0 symptoms, respectively; P < .001). In particular, fatigue, cough, and dyspnea during acute COVID-19 were more frequently reported by patients with persistent symptoms (Table 1).
Table 1.
Main Characteristics of Patients According to Persistent COVID-19–Related Symptoms
| Characteristics | Total Sample (n = 165) | Nonpersistent Symptoms (n = 28) | Persistent Symptoms (n = 137) | P |
|---|---|---|---|---|
| General and clinical characteristics | ||||
| Age (y) | 73.1 ± 6.2 | 73.7 ± 5.6 | 73.0 ± 6.4 | .57 |
| Sex (women) | 63 (38.2) | 10 (35.7) | 53 (38.7) | .76 |
| Education (y) | 12.3 ± 4.9 | 13.3 ± 5.3 | 12.1 ± 4.9 | .31 |
| Active smoking | 71 (43.0) | 14 (50.0) | 57 (41.6) | .53 |
| Flu vaccination | 77 (46.7) | 16 (57.4) | 61 (44.5) | .29 |
| Hypertension | 106 (64.2) | 19 (67.9) | 87 (63.5) | .82 |
| Heart failure | 15 (9.1) | 1 (3.6) | 14 (10.2) | .47 |
| Diabetes mellitus | 26 (15.8) | 4 (14.3) | 22 (16.1) | 1.00 |
| Renal failure | 17 (10.3) | 5 (17.9) | 12 (8.8) | .17 |
| Chronic obstructive pulmonary disease | 36 (21.8) | 3 (10.7) | 33 (24.1) | .13 |
| Body mass index (kg/m2) | 26.3 ± 4.1 | 25.4 ± 4.5 | 26.4 ± 4.1 | .25 |
| Mini Mental State Examination score | 28.2 ± 1.5 | 27.9 ± 1.3 | 28.3 ± 1.5 | .37 |
| Probable sarcopenia∗ | 62 (37.6) | 7 (25) | 55 (40.1) | .19 |
| Symptoms experienced during acute COVID-19 | ||||
| Fatigue | 116 (70.3) | 12 (42.9) | 104 (75.9) | <.001 |
| Cough | 109 (66.1) | 12 (42.9) | 97 (70.8) | <.01 |
| Dyspnea | 96 (58.2) | 8 (26.6) | 88 (64.2) | <.001 |
| Loss of appetite | 76 (46.1) | 13 (46.4) | 63 (46.0) | 1.00 |
| Dysgeusia | 62 (37.6) | 9 (32.1) | 53 (38.7) | .66 |
| Myalgia | 54 (32.7) | 7 (25.0) | 47 (34.3) | .38 |
| Joint pain | 52 (31.5) | 6 (21.4) | 46 (33.6) | .26 |
| Smell disorders | 51 (30.9) | 5 (17.9) | 46 (33.6) | .11 |
| Chest pain | 45 (27.3) | 4 (14.3) | 41 (29.9) | .10 |
| Rhinitis | 34 (20.6) | 7 (25.0) | 27 (19.7) | .60 |
| Diarrhea | 38 (23.0) | 6 (21.4) | 32 (23.4) | 1.00 |
| Headache | 30 (18.2) | 4 (14.3) | 26 (19.0) | .78 |
| Sore throat | 27 (16.4) | 4 (14.3) | 23 (16.8) | 1.00 |
| Red eyes | 25 (15.2) | 1 (3.6) | 24 (17.5) | .08 |
| Number of symptoms | 5.0 ± 3.0 | 3.3 ± 2.0 | 5.3 ± 3.0 | <.001 |
Data are shown as mean ± SD for age, education, body mass index, total number of symptoms, and Mini Mental State Examination score; absolute numbers (percentages) are reported for all other variables.
Probable sarcopenia was defined as a handgrip strength lower than 27 kg in men and 16 kg in women.
The rate of patients free of symptoms increased according to the number of days elapsed from hospital discharge (2.4% at 0−60 days, 16.4% at 61−90 days, 27.4% at 91+ days). A similar pattern was observed for the number of persisting symptoms (3.4 ± 2.4 at 0−60 days, 2.7 ± 1.9 at 61−90 days, 2.3 ± 2.5 at 91+ days).
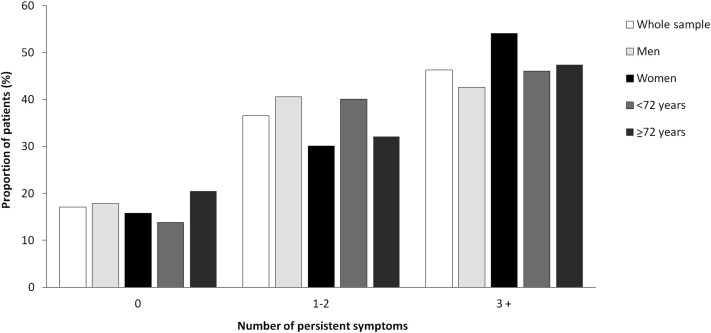
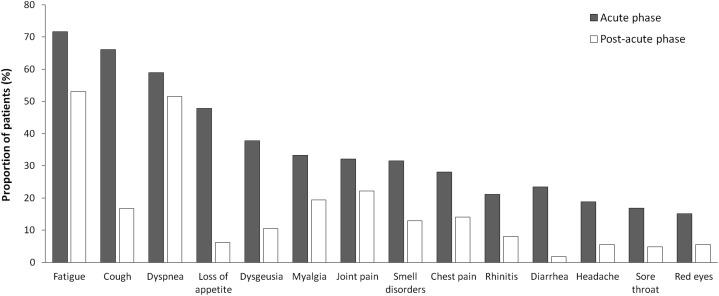
Figure 1 shows the proportion of patients with persistent symptoms on admission. More than one-third of patients reported 1 or 2 symptoms and 46.3% had 3 or more symptoms. The rate of symptom persistence was not significantly different when patients were stratified according to median age (P = .30). The persistence of specific symptoms in comparison with their prevalence during acute COVID-19 was also assessed. Figure 2 shows that a high proportion of patients reported persistent fatigue (53.1%), dyspnea (51.5%), joint pain (22.2%), and cough (16.7%).
Fig. 1.
Proportion of patients with persistent symptoms on admission in the whole sample and according to sex and median age (72 years).
Fig. 2.
COVID-19–related symptoms. The figure shows the proportion of patients with specific COVID-19–related symptoms during the acute phase of the disease and on admission.
Table 2 shows unadjusted and adjusted associations between potential risk factors and persistent COVID-19–related symptoms. In the unadjusted model, a direct association was determined between total number of persistent symptoms and some symptoms suffered during acute COVID-19 (ie, cough, fatigue, and dyspnea). Supplemental oxygen and prescription of enoxaparin during acute COVID-19 were associated with a higher likelihood of persistent symptoms. In contrast, the number of days from hospital discharge and the first follow-up visit was inversely correlated with symptom persistence (PR 0.98; 95% CI 0.97–0.99). These associations remained significant after adjusting for age and sex. In the fully adjusted model, the likelihood of symptom persistence was higher in those who had experienced fatigue during acute COVID-19 (PR 2.67; 95% CI 1.01–10.6). The number of days from hospital discharge was still significant in the fully adjusted model (PR 0.97; 95% CI 0.95–0.99).
Table 2.
Unadjusted and Adjusted Associations Between Potential Risk Factors and Persistence of COVID-19–Related Symptoms
| Characteristics | Unadjusted PR (95% CI) | Age/Sex Adjusted PR (95% CI) | Fully Adjusted PR (95% CI) |
|---|---|---|---|
| Age (y)∗ | 0.98 (0.92–1.04) | − | 1.00 (0.91–1.12) |
| Sex (female) | 1.14 (0.49–2.67) | − | 2.01 (0.48–8.61) |
| Cough | 3.23 (1.40–7.44) | 3.22 (1.38–7.49) | 1.49 (0.35–6.33) |
| Fatigue | 4.62 (1.97–10.8) | 4.62 (1.96–10.8) | 2.67 (1.01–10.6) |
| Dyspnea | 4.68 (1.91–11.4) | 4.61 (1.88–11.2) | 1.70 (0.87–21.1) |
| Chronic obstructive pulmonary disease | 2.64 (0.75–9.32) | 2.97 (0.82–10.7) | 5.05 (0.83–30.6) |
| Oxygen support | 2.52 (1.09–5.82) | 2.90 (1.19–7.06) | 1.21 (0.19–7.07) |
| Hydroxychloroquine | 1.87 (0.82–4.25) | 1.97 (0.85–4.56) | 1.85 (0.45–7.63) |
| Azithromycin | 1.89 (0.75–4.75) | 1.95 (0.76–5.00) | 5.09 (1.15–22.4) |
| Enoxaparin | 2.96 (1.27–6.89) | 3.03 (1.29–7.11) | 3.62 (0.68–19.2) |
| Number of symptoms | 1.30 (1.09–1.54) | 1.29 (1.08–1.55) | 1.18 (0.84–1.65) |
| Days from hospital discharge | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | 0.97 (0.95–0.99) |
Prevalence ratio per year increase.
Discussion
The present study showed that, among older patients who recovered from COVID-19, more than 80% reported persistence of at least 1 symptom, particularly fatigue, dyspnea, joint pain, and cough. The severity of acute COVID-19, expressed as the number of symptoms experienced and the treatments received (respiratory support and medications) during hospital stay, was found to be the main risk factor for persistent COVID-19–related symptoms. A longer time from acute COVID-19, expressed as the days elapsed from hospital discharge, was associated with a lower likelihood of suffering persistent symptoms.
During the last months, the post-COVID-19 syndrome has received a great deal of scientific and media attention.14, 15, 16 Indeed, COVID-19 has turned out to be a long-term illness for many patients. Hence, recovery from COVID-19 is far more complex than testing negative for SARS-CoV-2. As many as 50% to 80% of patients complain of persistent symptoms months after laboratory-confirmed virus clearance. Frequently reported persistent symptoms include fatigue, headache, shortness of breath, anosmia, and muscle weakness.5 , 17 However, most data refer to the general population and little evidence is available in older people. To the best of our knowledge, this is the first study specifically assessing the characteristics of persistent COVID-19–related symptoms in an older population.
Risk factors for negative outcomes during acute COVID-19 are well established.18 Yet, the identification of people at higher risk of developing persistent COVID-19–related symptoms is challenging. Sudre et al.19 found that advanced age, female sex, excessive body weight, and the presence of more than 5 symptoms during the first week of acute COVID-19 were strong predictors of symptom persistence. In a large cohort study, the severity of acute COVID-19 was found to be the main risk factor for persistent COVID-19–related symptoms.20 Our data suggest that, in older adults, the presence of more symptoms during the acute phase of COVID-19 is associated with a higher risk of persisting symptoms more than 2 months after hospital discharge. In particular, the presence of fatigue at the time of acute COVID-19 is a major risk factor for symptom persistence. As expected, the longer the time from acute COVID-19, the more likely it is to recover from all COVID-19–related symptoms.21 , 22
COVID-19 is an infectious disease with the manifestation of interstitial pneumonia and severe acute respiratory syndrome.23 The team of medical doctors who are mainly involved in patient care has preferably been composed of infectious disease specialists, pneumologists, and intensivists. However, considering the advanced age of the population at higher risk of negative outcomes, the involvement of geriatricians for the correct evaluation and management of these patients is important not only during acute COVID-19, but also in the post-acute phase.24 The geriatrician is the specialist who best can manage multiple health problems with great aptitude and skill to care for multimorbid and complex patients.25 The geriatrician is also well suited for the management of persistent signs and symptoms, such as fatigue, muscle weakness, malnutrition, mood disorders, and reduced quality of life associated with persistent COVID-19–related symptoms.26 , 27
Limitations of the present study include the lack of information on symptom history before acute COVID-19 and the lack of grading of symptom severity. Recall bias cannot be ruled out. Indeed, patients who suffered a more severe acute COVID-19 might be more alert to any sign or symptom during recovery than those who were asymptomatic or had a mild disease. On the other hand, patients with persistent symptoms might have a better recollection of signs and symptoms experienced during acute COVID-19 than those without persistent symptoms. Furthermore, ours was a single-center study with a relatively small number of patients and without a control group of patients who were followed after other acute infections. Older adults recovered from pneumonia or other viral diseases (eg, herpes and chickenpox) can also experience persistent symptoms. However, none of those conditions were present in our patient population at the time of evaluation.
Conclusions and Implications
This study provides a preliminary description of clinical sequelae of COVID-19 in older people. Our findings indicate that persistent symptoms are frequently experienced by older adults who have been hospitalized for COVID-19. As also recommended by WHO,28 follow-up programs should be implemented to monitor and care for long-term health issues.
Footnotes
This work was partly funded by Fondazione Memmo, Danone Nutricia Italia, ASSICA, Istituto Valorizzazione Salumi Italiani, the nonprofit research foundation “Achille e Linda Lorenzon”, and by an intramural grant from the Università Cattolica del Sacro Cuore (D1.2020).
The authors declare no conflicts of interest.
References
- 1.Prem K., Liu Y., Russell T.W. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: A modelling study. Lancet Public Health. 2020;5:e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyaolu A., Okorie C., Marinkovic A. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020 Jun 25. doi: 10.1007/s42399-020-00363-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): A review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Landi F., Carfì A., Benvenuto F., Gemelli Against COVID-19 Post-Acute Care Team Predictive factors for a new positive nasopharyngeal swab among patients recovered from COVID-19. Am J Prev Med. 2021;60:13–19. doi: 10.1016/j.amepre.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalbandian A., Sehgal K., Gupta A. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutten J.J.S., van Loon A.M., van Kooten J. Clinical suspicion of COVID-19 in nursing home residents: symptoms and mortality risk factors. J Am Med Dir Assoc. 2020;21:1791–1797.e1. doi: 10.1016/j.jamda.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landi F., Barillaro C., Bellieni A. The new challenge of geriatrics: Saving frail older people from the SARS-CoV-2 pandemic infection. J Nutr Health Aging. 2020;24:466–470. doi: 10.1007/s12603-020-1356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemelli Against COVID-19 Post-Acute Care Study Group Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32:1613–1620. doi: 10.1007/s40520-020-01616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liotti F.M., Menchinelli G., Marchetti S. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181:702–704. doi: 10.1001/jamainternmed.2020.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gemelli Against COVID-19 Geriatrics Team. Landi F., Barillaro C. The geriatrician: The frontline specialist in the treatment of COVID-19 patients. J Am Med Dir Assoc. 2020;21:937–938. doi: 10.1016/j.jamda.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenbroucke J.P., von Elm E., Altman D.G., STROBE initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 14.Calvani R., Picca A., Landi F., Marzetti E. Plasma therapies and parabiosis in the COVID-19 Era. J Am Med Dir Assoc. 2020;21:994–995. doi: 10.1016/j.jamda.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanièce Delaunay C., Saeed S., Nguyen Q.D. Evaluation of testing frequency and sampling for severe acute respiratory syndrome coronavirus 2 surveillance strategies in long-term care facilities. J Am Med Dir Assoc. 2020;21:1574–1576. doi: 10.1016/j.jamda.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S.M., Bakaev I., Chen H. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21:1378–1383. doi: 10.1016/j.jamda.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hägg S., Jylhävä J., Wang Y. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Smet R., Mellaerts B., Vandewinckele H. Frailty and mortality in hospitalized older adults with COVID-19: Retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudre C.H., Murray B., Varsavsky T. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Huang L., Wang Y. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landi F., Gremese E., Rota E., Gemelli Against COVID-19 Post-Acute Care Team Positive RT-PCR nasopharyngeal swab in patients recovered from COVID-19 disease: When does quarantine really end? J Infect. 2020;81:e1–e3. doi: 10.1016/j.jinf.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpato S., Landi F., Incalzi R.A. A frail health care system for an old population: Lesson from the COVID-19 outbreak in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:e126–e127. doi: 10.1093/gerona/glaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kistler C.E., Jump R.L.P., Sloane P.D., Zimmerman S. The winter respiratory viral season during the COVID-19 pandemic. J Am Med Dir Assoc. 2020;21:1741–1745. doi: 10.1016/j.jamda.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang O., Bigelow B.F., Sheikh F. Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: Results of universal testing of 1970 residents. J Am Med Dir Assoc. 2020;21:1767–1773. doi: 10.1016/j.jamda.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaur S., Pandya N., Dumyati G. A structured tool for communication and care planning in the era of the COVID-19 pandemic. J Am Med Dir Assoc. 2020;21:943–947. doi: 10.1016/j.jamda.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hefele J.G. Research needed: Better quantitative studies to identify causes of COVID-19 nursing home disparities. J Am Med Dir Assoc. 2021;22:263–264. doi: 10.1016/j.jamda.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogorzelska-Maziarz M., Chastain A.M., Mangal S. Home health staff perspectives on infection prevention and control: Implications for Coronavirus Disease 2019. J Am Med Dir Assoc. 2020;21:1782–1790. doi: 10.1016/j.jamda.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO WHO recommends follow-up care, low-dose anticoagulants for COVID-19 patients. https://www.who.int/news-room/feature-stories/detail/who-recommends-follow-up-care-low-dose-anticoagulants-for-covid-19-patients Available at: