Clinical & Translational Immunology 2021; 10: e1317.
Correction to: Clin Trans Immunol 2021; 10: e1283.
https://doi.org/10.1002/cti2.1283. Published online 9 May 2021
Figure 5 originally published in this article was incorrect, as it contained a duplicated mouse image. The corrected Figure 5 and its caption appear below.
The authors apologise for this error.
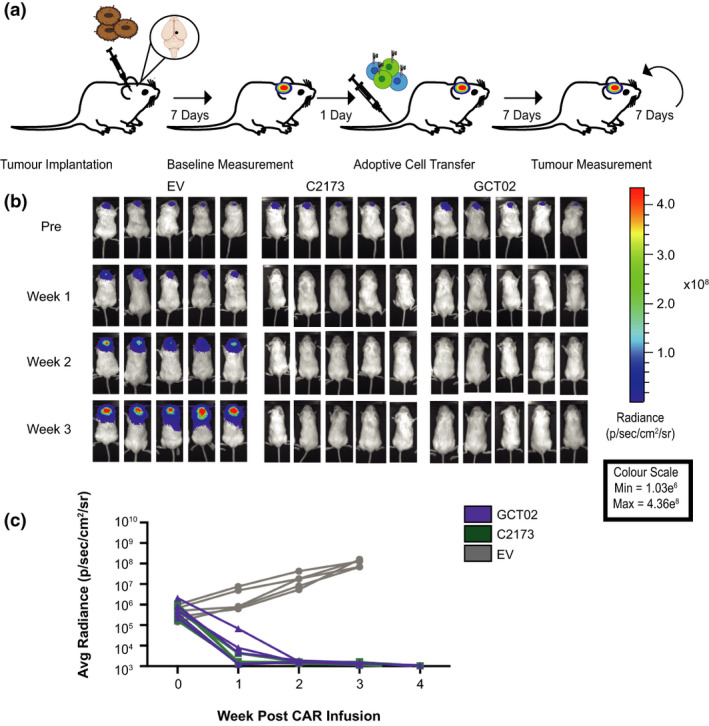
GCT02 CAR T cells effectively induce regression of intracranial tumors. (a) Schematic of the experimental protocol to evaluate the in vivo function of CAR T cells against EGFRvIII‐expressing intracranial tumors. Mice were intracranially injected with U87‐EGFRvIII GFP‐Luc tumor cells and 7 days later were imaged using bioluminescence. The mice were allocated to treatment groups before delivery of a single intravenous dose of 5 × 106 CD4+: 5 × 106 CD8+ T cells day 8 post‐activation GCT02 or C2173 CAR T cells. Empty vector T cells (EV) were injected as a negative control. Bioluminescence was examined weekly to monitor tumor size over time. The tumor injection site is indicated by black circle. (b) Bioluminescence imaging of U87‐EGFRvIII GFP‐Luc tumor‐bearing NSG mice, treated with either empty vector (EV), C2173 or GCT02 CAR T cells. Individual mice from each treatment group are shown for up to 3 weeks after CAR T cell infusion. Representative of two independent experiments. (c) Quantification of tumor growth in the mice in panel b. Tumor size was quantitated in radiance (photons/sec/area/sr). Each line represents a single mouse. n = 5 mice per group. Data are one representative of two independent biological replicates.


