Abstract
Objective
This prospective cohort study explored factors related to postoperative pain in gastric cancer patients.
Methods
A total of 236 patients who underwent gastrectomy were enrolled. All patients enrolled in the study completed the Hospital Anxiety and Depression Scale (HADS) questionnaire and Life Orientation Test-Revised (LOT-R) questionnaire on the day before surgery. Heat pain threshold (HPT), cold pain threshold (CPT) and pressure pain threshold (PPT) were measured for all patients one day prior to surgery and demographic details were collected. All patients were connected to a patient-controlled intravenous analgesia (PCIA) pump at the end of the surgery. The occurrence of postoperative pain was used as a dependent variable, and multivariate logistic regression analyses were conducted to screen for factors affecting postoperative pain.
Results
In total, 83 patients (35.2%) had postoperative pain. Body mass index (BMI) ≥28 kg/m2 [odds ratio (OR): 2.67; 95% confidence interval (95% CI): 1.07−6.67], total gastrectomy (OR: 2.64; 95% CI: 1.42−4.91), preoperative anxiety score ≥8 (OR: 2.37; 95% CI: 1.12−5.02), heat pain threshold ≤4.9 s (OR: 2.14; 95% CI: 1.06−4.32), pressure pain threshold ≤4 g (OR: 2.05; 95% CI: 1.05−4.03), and female gender (OR: 1.99; 95% CI: 1.04−3.83) were risk factors for postoperative pain.
Conclusions
Obesity, wide range of gastrectomy, high preoperative anxiety, low HPT and PPT, and female gender are associated with increased risk for postoperative pain.
Keywords: Anxiety level, gastrectomy, pain threshold, postoperative pain
Introduction
Pain is an unpleasant subjective experience that been defined as the 5th vital sign (1). Postoperative pain primarily occurs within the first 24−72 h after surgery. The intensity of postoperative pain varies between individuals, and poor pain management may increase postoperative complications and influence rehabilitation (2). More accurate prediction of patients’ postoperative pain would allow surgeons and anesthesiologists to preoperatively prepare improved analgesic plans for postoperative pain management. Age, sex, psychological variables, education level, type of surgery, and response to analgesics have all been reported to influence postoperative pain. Recent studies have revealed that dynamic interactions between these complex factors can further affect postoperative pain (2-4).
Studies have primarily focused on preoperative measure of pressure pain sensitivity (5,6) and heat or cold pain threshold (7-10) to predict postoperative pain. However, the association between preoperative pain thresholds and postoperative pain remain controversial. Levels of anxiety and depression have also been positively correlated with postoperative and chronic pain (11,12), and preoperative anxiety influences acute postoperative pain in children (13). Conversely, other studies have reported inconsistent results in terms of the relationship between anxiety level and postoperative pain (14,15).
The aim of this prospective cohort study was to explore factors related to postoperative pain in gastric cancer patients, with a focus on the relationships between heat, cold, and pressure pain thresholds, level of anxiety and postoperative pain.
Materials and methods
Patients
The study was approved by the Ethics Committee at Peking University Cancer Hospital & Institute (protocol number 2019YJZ22) and registered at Chinese Clinical Trial Registry (registration No. ChiCTR 1900023080). Written informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations. The data sets generated during the current study are available in the http://www.medresman.org/login.aspx (No. ChiCTR-1900023080).
Consecutive patients with gastric cancer, aged 18−80 years, with an American Society of Anesthesiologists (ASA) physical status of grade I−III, scheduled for gastrectomy at Peking University Cancer Hospital from May 2019 to September 2019, were enrolled in the study. Exclusion criteria included: 1) repeated gastrectomy and conjoined surgery; 2) multiple primary malignant tumors; 3) current and chronic use of analgesics, psychotropic medications, hormones, or non-steroidal anti-inflammatories; 4) history of chronic pain, schizophrenia, epilepsy, or severe dementia; 5) hepatorenal dysfunction; or 6) unable to complete preoperative assessment due to severe dementia or language barrier.
Preoperative data collection
Demographic details including sex, age, smoking and drinking history, body mass index (BMI), education level and the grade of ASA physical status were collected. Patients were asked to complete the Hospital Anxiety and Depression Scale (HADS) questionnaire (16,17) and the Life Orientation Test-Revised (LOT-R) questionnaire on the day before surgery (18). The HADS questionnaire consists of 14 items comprised of 2 subscales (anxiety and depression) with 7 items per subscale related to anxiety or depression. The total score for each subscale ranges from 0 to 21, with scores between 0 and 7 indicating a normal state and scores higher than 8 indicating a state of elevated anxiety or depression (16,17). The LOT-R questionnaire consists of 3 positive and 3 negative items to be rated on a 5-point scale ranging from 0 (strongly disagree) to 4 (strongly agree), with higher scores representing higher levels of optimism (18).
Measurement of pressure pain threshold (PPT)
The pain threshold of pressure pain stimuli was assessed as previously described (19). Subjects were seated with their eyes closed to avoid visual feedback. Von Frey hairs of different strengths (0.008, 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8, 10, 15, 26, 60, 100, 180, 300 g; Aesthesio®, UgoBasile, Comerio, Italy) were placed on the palmar skin of the forearm and bent slightly to apply pressure. Subjects had to give a clear verbal signal of “yes” or “no” to indicate whether the stimulus was painful. Each Von Frey hair was used three times with an interval of 10 s each time. PPT was defined as at least two of three stimuli causing pain, and the weight of Von Frey hair was recorded as the PPT.
Measurement of cold pain threshold (CPT)
CPT was assessed as previously described (10) using a temperature-controlled water bath with a maximum temperature variance of ±0.5 °C (TAWA Q-18, Beijing, China). Subjects placed their non-dominant hand in the cold water bath (5 °C) with their fingers spread apart and without touching the bath wall. They were asked to keep their hands still in the water as long as possible. CPT was defined as the time (s) when the subject began to feel pain, with 60 s set as the maximum to ensure safety.
Measurement of heat pain threshold (HPT)
HPT was assessed as previously described (8) using a computer-controlled thermostat (YOONING GH-100, Hangzhou, China). Study subjects placed the thenar eminence of their non-dominant hand on a 10×7.5 cm metal plate that was maintained at 47.5±0.1 °C. Subjects were asked to keep their hand on the metal plate as long as possible. HPT was defined as the time (s) when the subject could not keep their hand on the metal plate, with 60 s set as the maximum to ensure safety. Three consecutive tests with an interval of 10 s were conducted to determine the HPT. All pain threshold measurements were performed one day prior to surgery.
Anesthetic and analgesic techniques
All patients received general anesthesia induced by 2 mg/kg of intravenous propofol, 0.4 μg/kg of intravenous sufentanil and 0.2 mg/kg of intravenous cisatracurium. Anesthesia was maintained with inhalation of sevoflurane and intravenously administered remifentanil and propofol. Bispectralindex (BIS) was kept between 40 and 60 and 10 µg of intravenous sufentanil was administered 30 mins before the end of surgery.
At the end of surgery, all patients were connected to a patient-controlled intravenous analgesia (PCIA) containing sufentanil (3 μg/kg), tropisetron (10 mg), and dexmedetomidine (0.1 mg), in 100 mL of 0.9% sodium chloride solution. The PCIA pump was programmed to deliver a loading dose of 2 mL, a background dose of 1 mL/h, and a bolus dose of 2 mL with a lockout time of 10 min and a one-hour limit of 13 mL. The operation type, operative duration, range of gastrectomy, blood loss, and transfusion volume were recorded.
Postoperative data collection
Postoperative pain was assessed using the Numerical Rating Scale (NRS) ranging from 0 to 10 (0=no pain; 10=the worst imaginable pain). Sufentanil (0.1 μg/kg) was given by the anesthesiologist to achieve an NRS score ≤3 prior to leaving the post-anesthesia care unit. If the NRS score >3, sufentanil (0.1 μg/kg) was given repeatedly in at least 15 mins intervals until an NRS score ≤3 was achieved. The postoperative visit was performed by the same researcher. Additional analgesic drugs were used by surgery whenever the NRS score remained >3 after pressing the PCIA pump. After leaving the post-anesthesia care unit, patient NRS scores were recorded every 24 h and PCIA pump pressing times and the use of any additional analgesic drugs were recorded until the PCIA pump was removed. Postoperative pain score was defined as the highest NRS score recorded during this time.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA). Continuous variables with normal distribution are presented as
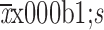 . Non-normal variables are presented as median (interquartile range), and categorical variable data are presented as percentage (%). The occurrence of postoperative pain was determined by the postoperative pain score and Youden’s index of the receiver operating characteristic (ROC) curve, with PCIA pump pressing times as a test variable and postoperative pain score >3 as a state variable.
. Non-normal variables are presented as median (interquartile range), and categorical variable data are presented as percentage (%). The occurrence of postoperative pain was determined by the postoperative pain score and Youden’s index of the receiver operating characteristic (ROC) curve, with PCIA pump pressing times as a test variable and postoperative pain score >3 as a state variable.
Multivariate logistic regression analysis was used to assess the relationship between postoperative pain and potential risk factors, with the occurrence of postoperative pain as a dependent variable. Independent variables were selected based on the literature, clinically validated models of the phenomenon being studied, and univariate logistic regression analysis. Variables with P<0.5 in univariate logistic regression analysis were subjected to multivariate logistic regression modeling. Gender, BMI, tobacco use, education level, operation type, range of gastrectomy, operative duration, preoperative anxiety score, preoperative depression score, LOT-R score, HPT, CPT and PPT were included as independent variables. HPT, CPT and PPT values were separated into two categories by interquartile range in the univariate or multivariate logistic regression modeling. Multivariate logistic regression modeling according to gender was used to further analyze relationships between postoperative pain and potential risk factors. A two-sided P value of <0.05 was the statistical significance level.
Results
General information
Among the total 251 patients enrolled, 15 dropped out, and 236 completed the study and were included in the statistical analysis. Demographic characteristics and perioperative factors of patients that completed the study are summarized in Table 1 .
Table 1. Demographic characteristics and perioperative factors of patients (N=236).
| Variables | n (%) |
| BMI, body mass index; ASA, American Society of Anesthesiologists; LOT-R, Life Orientation Test-Revised; PPT, pressure pain threshold; CPT, cold pain threshold; HPT, heat pain threshold; *, pulmonary diseases include COPD, chronic bronchitis, emphysema, bullae, and old pulmonary tuberculosis; *, cerebral diseases include old cerebral infarction, transient cerebral ischemia, and old cerebral hemorrhage. | |
Age (
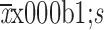 ) (year)
) (year)
|
60.31±9.93 |
| Gender: Female | 70 (29.7) |
BMI (
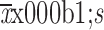 ) (kg/m2)
) (kg/m2)
|
23.52±3.20 |
| Smoking | 79 (33.5) |
| Drinking | 52 (22.0) |
| Education level [median (IQR)] (year) | 12 (9−15) |
| ASA grade | |
| I | 21 (8.9) |
| II | 211 (89.4) |
| III | 4 (1.7) |
| Preoperative complications | |
| Coronary heart disease | 14 (5.9) |
| Hypertension | 72 (30.5) |
| Arrhythmia | 30 (12.7) |
| Pulmonary disease* | 15 (6.4) |
| Diabetes | 30 (12.7) |
| Cerebral disease** | 11 (4.7) |
| Other (nephropathy, infectious diseases, rheumatoid) | 14 (5.9) |
| Operation type | |
| Laparoscopic gastrectomy | 89 (37.7) |
| Open gastrectomy | 147 (62.3) |
| Range of gastrectomy | |
| Total gastrectomy | 79 (33.5) |
| Distal or proximal gastrectomy | 157 (66.5) |
| Operative duration [median (IQR)] (min) | 188 (160−224) |
Blood loss (
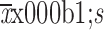 ) (mL)
) (mL)
|
78.39±62.73 |
Crystal liquid volume (
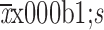 ) (mL)
) (mL)
|
1,670.13±384.61 |
Colloidal fluid volume (
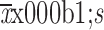 ) (mL)
) (mL)
|
646.61±256.05 |
Preoperative anxiety score (
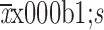 )
)
|
3.74±3.72 |
Preoperative depression score (
 )
)
|
3.40±3.21 |
LOT-R score (
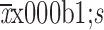 )
)
|
6.91±3.83 |
| Preoperative pain threshold | |
| PPT [median (IQR)] (g) | 6.00 (4.00−8.00) |
| CPT (s) | 10.85 (6.70−18.42) |
| HPT (s) | 7.85 (4.90−20.00) |
Postoperative pain
Based on the ROC curve (Youden’s index=21.5; area under curve=0.78), the occurrence of postoperative pain was defined as postoperative pain score >3 or PCIA pump pressing times ≥21 or the usage of additional analgesic drugs during a PCIA pump usage, as shown in Table 2 .
Table 2. Postoperative pain condition of all patients (N=236).
| Postoperative condition | n (%) |
| PCIA, patient-controlled intravenous analgesia; IQR, interquartile range. | |
| Postoperative pain score >3 | 62 (26.3) |
| PCIA press times [median (IQR)] | 6 (2−16) |
| Usage of additional analgesic drugs | 66 (28.0) |
| Occurrence of postoperative pain | 83 (35.2) |
Univariate and multivariate logistic regression analyses of postoperative pain
Univariate analysis indicated that HPT≤4.9 s [odd ratio (OR)=2.95], preoperative anxiety score ≥8 (OR=2.88), PPT≤4 g (OR=2.68), total gastrectomy (OR=2.13), female gender (OR=2.07) and CPT≤6.7 s (OR=2.00) were risk factors for postoperative pain, as shown in Table 3 . Multivariate analysis with logistic regression indicated that BMI≥28 kg/m2 (OR=2.67), total gastrectomy (OR=2.64), preoperative anxiety score ≥8 (OR=2.37), HPT≤4.9 s (OR=2.14), PPT≤4 g (OR=2.05), and female gender (OR=1.99) were risk factors for postoperative pain, as shown in Table 4 . ROC curve of multivariable regression analysis resulted in an area under curve of 0.728 (Figure 1 ).
Table 3. Univariate analysis of risk factors for postoperative pain.
| Variables | No. | Occurrence of postoperative pain [n (%)] | Univariate analysis | |
| OR (95% CI) | P | |||
| BMI, body mass index; LOT-R, Life Orientation Test-Revised; OR, odds ratio; 95% CI, 95% confidence interval; PPT, pressure pain threshold; CPT, cold pain threshold; HPT, heat pain threshold. | ||||
| Age (year) | 0.643 | |||
| ≥65 | 90 | 31 (34.4) | Reference | |
| <65 | 146 | 52 (35.6) | 1.14 (0.66−1.98) | |
| Gender | 0.013 | |||
| Male | 166 | 50 (30.1) | Reference | |
| Female | 70 | 33 (47.1) | 2.07 (1.17−3.68) | |
| BMI (kg/m2) | 0.113 | |||
| ≥28 | 24 | 12 (50.0) | 1.99 (0.85−4.64) | |
| <28 | 212 | 71 (33.5) | Reference | |
| Smoking | 0.096 | |||
| Yes | 79 | 22 (27.8) | 0.61 (0.34−1.09) | |
| No | 157 | 61 (38.9) | Reference | |
| Drinking | 0.924 | |||
| Yes | 52 | 18 (34.6) | 0.97 (0.51−1.85) | |
| No | 184 | 65 (35.3) | Reference | |
| Education level | − | − | 1.04 (0.96−1.11) | 0.340 |
| Operation type | 0.448 | |||
| Open gastrectomy | 147 | 49 (33.3) | 0.81 (0.47−1.40) | |
| Laparoscopic gastrectomy | 89 | 34 (38.2) | Reference | |
| Range of gastrectomy | 0.008 | |||
| Total gastrectomy | 79 | 37 (46.8) | 2.13 (1.21−3.72) | |
| Distal or proximal gastrectomy | 157 | 46 (29.3) | Reference | |
| Operative duration | − | − | 1.00 (0.99−1.01) | 0.310 |
| Preoperative anxiety score | 0.003 | |||
| ≥8 | 41 | 23 (56.1) | 2.88 (1.45−5.72) | |
| <8 | 195 | 60 (30.8) | Reference | |
| Preoperative depression score | 0.185 | |||
| ≥8 | 23 | 11 (47.8) | 1.80 (0.76−4.27) | |
| <8 | 213 | 72 (33.8) | Reference | |
| LOT-R score | − | − | 0.95 (0.89−1.02) | 0.152 |
| PPT (g) | 0.001 | |||
| ≤4 | 70 | 36 (51.4) | 2.68 (1.51−4.78) | |
| >4 | 166 | 47 (28.3) | Reference | |
| CPT (s) | 0.024 | |||
| ≤6.7 | 59 | 28 (47.5) | 2.00 (1.10−3.66) | |
| >6.7 | 177 | 55 (31.1) | Reference | |
| HPT (s) | <0.001 | |||
| ≤4.9 | 61 | 33 (54.1) | 2.95 (1.62−5.37) | |
| >4.9 | 175 | 50 (28.6) | Reference | |
Table 4. Multivariate analysis of risk factors for postoperative pain.
| Variables | OR (95% CI) | P |
| BMI, body mass index; OR, odds ratio; 95% CI, 95% confidence interval; PPT, pressure pain threshold; CPT, cold pain threshold; HPT, heat pain threshold. | ||
| Female | 1.99 (1.04−3.83) | 0.039 |
| BMI≥28 (kg/m2) | 2.67 (1.07−6.67) | 0.036 |
| Smoking | 0.72 (0.35−1.49) | 0.376 |
| Open gastrectomy | 0.90 (0.48−1.67) | 0.733 |
| Total gastrectomy | 2.64 (1.42−4.91) | 0.002 |
| Preoperative anxiety score ≥8 | 2.37 (1.12−5.02) | 0.024 |
| Preoperative depression score ≥8 | 0.69 (0.21−2.28) | 0.541 |
| PPT≤4 (g) | 2.05 (1.05−4.03) | 0.037 |
| CPT≤6.7 (s) | 1.10 (0.52−2.31) | 0.805 |
| HPT≤4.9 (s) | 2.14 (1.06−4.32) | 0.034 |
Figure 1.
ROC curve of multivariable regression analysis. ROC, receiver operating characteristic.
Multivariate logistic regression analyses of postoperative pain in males or females
Multivariate analysis with logistic regression indicated that total gastrectomy (OR=2.40), preoperative anxiety score ≥8 (OR=4.31), and HPT≤4.9 s (OR=3.01) were risk factors for postoperative pain in males (Table 5 ), while total gastrectomy (OR=5.92) and pressure pain threshold ≤4 g (OR=5.02) were risk factors for postoperative pain in females (Table 5 ).
Table 5. Multivariate analysis of risk factors for postoperative pain in males and females.
| Variables | OR (95% CI) | P |
| BMI, body mass index; HPT, heat pain threshold; PPT, pressure pain threshold; OR, odds ratio; 95% CI, 95% confidence interval. | ||
| Males | ||
| BMI≥28 (kg/m2) | 2.63 (0.94−7.37) | 0.066 |
| Total gastrectomy | 2.40 (1.16−5.01) | 0.019 |
| Preoperative anxiety score ≥8 | 4.31 (1.54−12.04) | 0.005 |
| HPT≤4.9 (s) | 3.01 (1.33−6.82) | 0.008 |
| Females | ||
| Total gastrectomy | 5.92 (1.67−20.99) | 0.003 |
| PPT≤4 (g) | 5.02 (1.57−16.08) | 0.004 |
Discussion
Our study results indicate a higher incidence of postoperative pain in females (OR=1.99). This is consistent with results from Vallerand et al. in which females demonstrated greater sensitivity to experimental noxious stimulation and showed lower pain threshold and tolerance (20). Hormonal variations during the menstrual cycle can also alter pain sensitivity, and females have lower pain thresholds during the peri-ovulatory, luteal, and premenstrual phases than during the follicular phase (21). Reduced adrenocortical activity is also associated with high pain sensitivity, and contributes to lower cortisol levels in females than in males after exposure to traumatic stimuli (22).
Our results also indicate that obese patients are more susceptible to postoperative pain than non-obese patients. This is consistent with studies from Campbell and Majchrzak suggesting that overweight individuals experienced more pain than those with normal BMI after total joint arthroplasty (23), thoracotomy, or thoracoscopic radical lung cancer surgery (24). Systemic inflammation due to increased release of inflammatory mediators by macrophages may contribute to a higher incidence of postoperative pain in obese patients (25).
Our study shows that gastric cancer patients with lower heat pain thresholds and lower pressure pain thresholds are more likely to experience postoperative pain. Notably, we report that low PPT is associated with more postoperative pain than low HPT in female patients, while low HPT is associated with more postoperative pain than low PPT in males. Several other studies support preoperative HPT measurement as a potential predictor of postoperative pain intensity in patients (8-10). The relationship between PPT and postoperative pain is less clear. PPT was significantly correlated with postoperative pain levels in patients after lower abdominal gynecologic surgery (6), but not after total joint arthroplasty (5). While CPT has been identified as an independent risk factor for postoperative pain after laparoscopic cholecystectomy (10), our study found no correlation between CPT and postoperative pain. Studies in mice have shown that heat, cold, and mechanical stimulation activate thermo- and mechano-specific neurons diversely. Therefore, pain caused by heat, cold, or pressure may transmit to different thalamus functional areas and cortical circuits (26). This may in part explain why TPT and HPT were predictive of postoperative pain in our study, while CPT was not.
Patients with a preoperative anxiety score of ≥8 were at increased higher risk for the occurrence of postoperative pain, and this was especially true for males. Several studies have reported a direct relationship between anxiety and postoperative pain (11,13,27), while others have found that a high probability of anxiety, depression, or other emotions may indirectly affect the occurrence of postoperative pain (28,29). In our study, the incidence of depression (9.7%) was comparable to the overall prevalence of depression in China (30). We did not observe a correlation between depression score or LOT-R score with postoperative pain in this study, consistent with previous reports (12).
We also observed that the incidence of postoperative pain occurrence was higher after total gastrectomy than after partial gastrectomy (OR=2.64). This may be due in part to the wider range of gastrectomy, which can potentially cause nerve and tissue damage resulting in hyperalgesia of the peripheral nervous system and increased postoperative pain (15,31).
Hermans et al. reported that the degree of education was negatively correlated with pain thresholds and degree of pain tolerance (32). Highly educated or high-income patients are also less likely to tolerate a given health condition and may thus show higher pain sensitivity (33). In our study, however, we did not observe any correlation between postoperative pain and education level.
Similarly, our study did not identify age as a predictor for postoperative pain, consistent with a report from Rudin et al. in tubal ligation patients (34). Studies from Europe and America have suggested that younger people maybe more likely to experience postoperative pain occurrence (35,36). However, Healey et al. suggested that the occurrence of postoperative pain was positively correlated with age in patients undergoing gynecological endoscopic surgery (37). Differences in the type of operation, the impact of patient ethnic or cultural origins on reported pain score, and opioid usage may account for these discrepancies.
One potential limitation of our study is uneven gender distribution due to the higher incidence of gastric cancer in males (38). Thus, while our multivariate logistic regression analyses identified differential factors related to postoperative pain in the male and female subgroups, this may be due to the limited number of female study participants (n=70). Further enrollment of female gastric cancer patients would provide more robust data for these analyses.
Conclusions
Female gender, obesity (BMI≥28 kg/m2), high levels of preoperative anxiety, low pain threshold including pressure pain threshold and heat pain threshold, and wide range of the gastrectomy are correlated with increased occurrence of postoperative pain in gastric cancer patients. Our preliminary findings suggest that several demographic characteristics and surgical factors influence acute postoperative pain. The anesthesiologists should improve analgesic plans on this kind of gastric cancer patients for postoperative pain management.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by Peking University Medicine Seed Fund for Interdisciplinary Research (No. BMU2020MX028) and Braun Anesthesia science research fund (No. BBFD-2011-006).
References
- 1.Berry PH, Dahl JL The new JCAHO pain standards: implications for pain management nurses. Pain Manag Nurs. 2000;1:3–12. doi: 10.1053/jpmn.2000.5833. [DOI] [PubMed] [Google Scholar]
- 2.Yang MMH, Hartley RL, Leung AA, et al Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ open. 2019;9:e025091. doi: 10.1136/bmjopen-2018-025091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wylde V, Dennis J, Beswick AD, et al Systematic review of management of chronic pain after surgery. Br J Surg. 2017;104:1293–306. doi: 10.1002/bjs.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix-Fralish ML, Mogil JS Progress in genetic studies of pain and analgesia. Ann Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghverdian BA, Wright DJ, Schwarzkopf R Pressure pain threshold as a predictor of acute postoperative pain following total joint arthroplasty. Surg Technol Int. 2016;29:320–7. [PubMed] [Google Scholar]
- 6.Hsu YW, Somma J, Hung YC, et al Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology. 2005;103:613–8. doi: 10.1097/00000542-200509000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Pan PH, Coghill R, Houle TT, et al Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–25. doi: 10.1097/00000542-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Werner MU, Duun P, Kehlet H Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100:115–9. doi: 10.1097/00000542-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Granot M, Lowenstein L, Yarnitsky D, et al Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–6. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Bisgaard T, Klarskov B, Rosenberg J, et al Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90:261–9. doi: 10.1016/s0304-3959(00)00406-1. [DOI] [PubMed] [Google Scholar]
- 11.Tasmuth T, Estlanderb AM, Kalso E Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain. 1996;68:343–7. doi: 10.1016/s0304-3959(96)03219-8. [DOI] [PubMed] [Google Scholar]
- 12.Kain ZN, Sevarino F, Alexander GM, et al Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J Psychosom Res. 2000;49:417–22. doi: 10.1016/s0022-3999(00)00189-6. [DOI] [PubMed] [Google Scholar]
- 13.Rabbitts JA, Groenewald CB, Tai GG, et al Presurgical psychosocial predictors of acute postsurgical pain and quality of life in children undergoing major surgery. J Pain. 2015;16:226–34. doi: 10.1016/j.jpain.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kain ZN, Sevarino FB, Rinder C, et al Preoperative anxiolysis and postoperative recovery in women undergoing abdominal hysterectomy. Anesthesiology. 2001;94:415–22. doi: 10.1097/00000542-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Kalkman JC, Visser K, Moen J, et al Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–23. doi: 10.1016/s0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 16.Bjelland I, Dahl AA, Haug TT, et al The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Ma F, Lan B, et al Validity of distress thermometer for screening of anxiety and depression in family caregivers of Chinese breast cancer patients receiving postoperative chemotherapy. Chin J Cancer Res. 2020;32:476–84. doi: 10.21147/j.issn.1000-9604.2020.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheier MF, Carver CS, Bridges MW Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 19.Drobek W, De Laat A, Schoenaers J Tactile threshold and pressure pain threshold during treatment of orofacial pain: an explorative study. Clin Oral Investig. 2001;5:185–93. doi: 10.1007/s007840100125. [DOI] [PubMed] [Google Scholar]
- 20.Vallerand AH Gender differences in pain. Image J Nurs Sch. 1995;27:235–7. doi: 10.1111/j.1547-5069.1995.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 21.Riley JL 3rd, Robinson ME, Wise EA, et al A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–35. doi: 10.1016/s0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 22.al’Absi M, Petersen KL, Wittmers LE Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 23.Campbell AL, Yu S, Karia R, et al The effects of body mass index on pain control with liposomal bupivacaine in hip and knee arthroplasty. J Arthroplasty. 2018;33:1033–9. doi: 10.1016/j.arth.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Majchrzak M, Brzecka A, Daroszewski C, et al Increased pain sensitivity in obese patients after lung cancer surgery. Front Pharmacol. 2019;10:626. doi: 10.3389/fphar.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisberg SP, McCann D, Desai M, et al Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/jci19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida TF, Roizenblatt S, Tufik S Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000:40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 27.Nelson FV, Zimmerman L, BarnasonS, et al The relationship and influence of anxiety on postoperative pain in the coronary artery bypass graft patient. J Pain Symptom Manage. 1998;15:102–9. doi: 10.1016/S0885-3924(97)00256-X. [DOI] [PubMed] [Google Scholar]
- 28.Corruble E, Guelfi JD Pain complaints in depressed inpatients. Psychopathology. 2000;33:307–9. doi: 10.1159/000029163. [DOI] [PubMed] [Google Scholar]
- 29.Dickens C, McGowan L, Dale S Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–75. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- 30.Sjöberg L, Karlsson B, Atti AR, et al Prevalence of depression: Comparisons of different depression definitions in population-based samples of older adults. J Affect Disord. 2017;221:123–31. doi: 10.1016/j.jad.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Chung F, Ritchie E, Su J Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85:808–16. doi: 10.1097/00000539-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Hermans L, Van Oosterwijck J, Goubert D, et al Inventory of personal factors influencing conditioned pain modulation in healthy people: a systematic literature review. Pain Prac. 2016;16:758–69. doi: 10.1111/papr.12305. [DOI] [PubMed] [Google Scholar]
- 33.d’Uva TB, O’Donnell O, van Doorslaer E Differential health reporting by education level and its impact on the measurement of health inequalities among older Europeans. Int J Epidemiol. 2008;37:1375–83. doi: 10.1093/ije/dyn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudin A, Wölner-Hanssen P, Hellbom M, et al Prediction of post-operative pain after a laparoscopic tubal ligation procedure. Acta Anaesthesiol Scand. 2008;52:938–45. doi: 10.1111/j.1399-6576.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 35.Gagliese L, Gauthier LR, Macpherson AK, et al Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Med. 2008;9:299–314. doi: 10.1111/j.1526-4637.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 36.Caumo W, Schmidt AP, Schneider CN, et al Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265–71. doi: 10.1034/j.1399-6576.2002.461015.x. [DOI] [PubMed] [Google Scholar]
- 37.Healey M, Maher P, Hill D, et al Factors associated with pain following operative laparoscopy: a prospective observational study. Aust N Z J Obstet Gynaecol. 1998;38:80–4. doi: 10.1111/j.1479-828x.1998.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 38.Torre LA, Siegel RL, Ward EM, et al Global cancer incidence and mortality rates and trends — An update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.Epi-15-0578. [DOI] [PubMed] [Google Scholar]



