Abstract
Objective
Hepatocellular carcinoma (HCC) development among hepatitis B surface antigen (HBsAg) carriers shows gender disparity, influenced by underlying liver diseases that display variations in laboratory tests. We aimed to construct a risk-stratified HCC prediction model for HBsAg-positive male adults.
Methods
HBsAg-positive males of 35−69 years old (N=6,153) were included from a multi-center population-based liver cancer screening study. Randomly, three centers were set as training, the other three centers as validation. Within 2 years since initiation, we administrated at least two rounds of HCC screening using B-ultrasonography and α-fetoprotein (AFP). We used logistic regression models to determine potential risk factors, built and examined the operating characteristics of a point-based algorithm for HCC risk prediction.
Results
With 2 years of follow-up, 302 HCC cases were diagnosed. A male-ABCD algorithm was constructed including participant’s age, blood levels of GGT (γ-glutamyl-transpeptidase), counts of platelets, white cells, concentration of DCP (des-γ-carboxy-prothrombin) and AFP, with scores ranging from 0 to 18.3. The area under receiver operating characteristic was 0.91 (0.90−0.93), larger than existing models. At 1.5 points of risk score, 26.10% of the participants in training cohort and 14.94% in validation cohort were recognized at low risk, with sensitivity of identifying HCC remained 100%. At 2.5 points, 46.51% of the participants in training cohort and 33.68% in validation cohort were recognized at low risk with 99.06% and 97.78% of sensitivity, respectively. At 4.5 points, only 20.86% of participants in training cohort and 23.73% in validation cohort were recognized at high risk, with positive prediction value of 22.85% and 12.35%, respectively.
Conclusions
Male-ABCD algorithm identified individual’s risk for HCC occurrence within short term for their HCC precision surveillance.
Keywords: Hepatocellular carcinoma, asymptotic HBsAg carriers, risk prediction model, screening, laboratory tests
Introduction
Cirrhotic patients are at particularly high risk of hepatocellular carcinomas (HCC), a leading cause of cancer-related death and accounts for a large proportion of health economic burden (1,2). HCC surveillance every six months using B-ultrasonography (US) with or without determining serum α-fetoprotein (AFP), US/AFP is strongly recommended by professional societies including Asian-Pacific Association for the Study of the Liver (APASL) (3), American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL). Hepatitis B virus (HBV) causes HCC in absence of cirrhosis (1,4), AASLD updates the recommendations of HCC surveillance to all HBV chronically infected adults. In China, chronic HBV infection remains the leading risk factor of HCC (1). Results of a HCC-screening demonstration program using US/AFP biannually to HBsAg-positive adults in some rural communities of China showed no reduction in liver cancer mortality within first 4 years of follow-up (5). Antiviral therapy rarely cures HBV infection and needs life-long medications, considerable carriers with seropositive for hepatitis B surface antigen (HBsAg) did not receive the therapy (1,6). There were 257−291 million HBV chronically infected adults in 2015 worldwide (6). Tailoring to individual HCC risk is required for precision surveillance, so that limited HCC-screening resources could be allocated to high-risk individuals who might benefit most from early intervention or intensive surveillance (7).
In HBV chronically infected Asians, HCC incidence is different with variated risk factors and underlying liver diseases (1), the incidence rate was 3.2 per 100 person-years in patients with cirrhosis, and 0.4 in patients without cirrhosis (8,9). To predict long-term HCC occurrence in Asians, several HCC-risk models were constructed including REACH-B (10), CU-HCC (11), GAG-HCC (12) and AGED (13), which weighted significantly on HBV replication status, i.e. HBV-DNA, HBV e antigen (HBeAg). With the usage of nucleos(t)ide analogs that inhibit HBV replication, PAGE-B for Caucasian (14) and mPAGE-B for Asians (15) were constructed employing patient’s age, gender, baseline platelet (PLT) counts, and serum albumin (ALB) levels, which reflect the underlying liver diseases. EASL recommends HCC surveillance to non-cirrhotic HBV patients according to PAGE-B classes. Nevertheless, these models were mostly tested in patients with chronic hepatitis B by 2019 (16), evidences of current modification in surveillance strategy based on risk stratification models are insufficient for high-grade recommendation. None of these models was tested in Chinese HBsAg-carriers from rural community where liver cancer incidence was 20.0/105, which was higher than the urban (16.1/105) (17).
Previous studies conducted in rural community showed that <40% of HBsAg-positive adults complied to the biannually repeat US/AFP tests after initial 2−3 years for HCC-screening ( 4,18). HCC development depends on presence of premalignant cells and is significantly influenced by the underlying severity and activity of liver diseases (1,19). Molecular and pathological analyses displayed that preneoplastic lesions harbored genetic alteration and abnormal expression of cellular proteins. For routine surveillance in community population, the risk prediction models of short-term HCC occurrence will avoid ineffective and wasteful distribution of demanding screening efforts to those relatively low-risk individuals (7). HBV-associated HCC developed more frequently in males than in females, with a female/male ratio of 1:4−7, due to the different effect of androgen and estrogen on HBV pathogenesis and carcinogenesis (3,20,21). In addition, healthy females have higher platelet and white blood cell counts than males (22). The serum level of alanine aminotransferase (ALT) was higher in healthy Chinese males than in females (23). The currently constructed HCC prediction models used the same variation and weigh in both gender of these laboratory tests, which reflect liver diseases at different severity and activity. In this study, based on a large population-level screening program in rural China, we aimed to build up a risk-stratified HCC prediction model for HBsAg-positive males using measurable tests which are currently performed in clinical laboratories.
Materials and methods
Participants and study design
The study participants were derived from the Community-based Cohort study on Population with high risk of Liver Cancer (the CCOP-LC cohort; Chinese Clinical Registry, ChiCTR-EOC-17012853), which was described previously (24). CCOP-LC conducted community-based liver cancer screening by US/AFP in six rural areas (Lingbi county, Mengcheng county, Sheyang county, Shenqiu county, Dancheng county and Qidong county) from three provinces (Anhui, Jiangsu and Henan) in China since October 2017. Cluster sampling was used to select candidate screening sites where counties had a relatively higher incidence and mortality of liver cancer. All females and males aged 35−69 years who had no history of cancer (self-reported) in the selected villages (the smallest unit) of the participating counties were approached by means of personal contact and phone invitation by trained local medical staff. After receiving immunochromatographic strip-test for HBsAg, the HBsAg-positive participants were included in the cohort. Face to face interviews were conducted to collect sociodemographic information, body weight and height, lifestyle (smoking, drinking, eating habits, etc.), and family history of liver cancer among first-degree relatives. HCC screening by US/AFP was provided annually. The study protocol (NCC201709011) was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (NCC/CH-CAMS) in Beijing. Each participant provided written informed consent before undertaking any study-related procedures in accordance with Good Clinical Practice and principles of the Declaration of Helsinki.
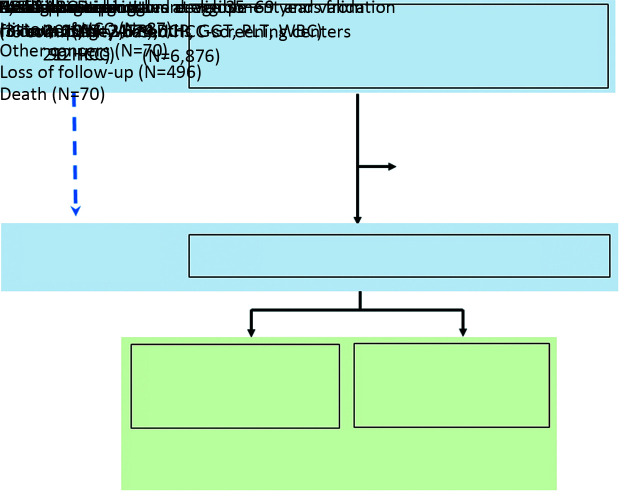
In the present study, we included HBsAg-positive males who met the inclusion criteria: 1) males at 35−69 years old; 2) HBsAg-positive; 3) no previously diagnosed HCC or other malignant diseases; and 4) no other diseases that restricted taking the examinations of dynamic computed tomography (CT) or magnetic resonance imaging (MRI). Three screening centers (Lingbi, Mengcheng, and Sheyang) were randomly assigned as training cohort, the other three centers (Shenqiu, Dancheng and Qidong) as validation cohort (Figure 1).
Figure 1.
Flowchart of study population from multicenter population-based liver cancer screening. HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; AFP, α-fetoprotein; DCP, des-γ-carboxy-prothrombin; GGT, γ-glutamyl-transpeptidase; PLT, platelet; WBC, white blood cell.
Laboratory tests
At baseline, for each participant, we measured PLT and white blood cell (WBC) counts; blood levels of ALT, aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), ALB; and HCC biomarkers of AFP and des-γ-carboxy-prothrombin (DCP). For participants in training cohort, we also detected their blood concentrations of HBV-DNA, HBsAg and HBeAg. PLT, WBC, ALT, AST, ALB and AFP were measured immediately after blood drawn in each screening center independently using unified reagents. Using commercialized Abbott reagents, blood levels of GGT, concentrations of HBV-DNA, HBsAg, HBeAg, DCP were determined, and AFP was double-checked in the central laboratory of NCC/CH-CAMS using the plasma that were snap frozen in −80 °C for 1−2 months. HBV-DNA concentration was determined by quantitative real-time PCR using the reagents from Kehua Biotechnology in Roche LightCycler 480 II.
Surveillance and HCC diagnosis
Every year, individuals were offered US/AFP examination and defined as US/AFP-positive, US/AFP-suspected, or US/AFP-negative. “US/AFP-positive” individuals had either of the following: 1) serum AFP levels of >400 ng/mL regardless of US-detected nodule; 2) US-detected nodule of ≥2 cm in size regardless of serum AFP concentration; and 3) US-detected nodule of ≥1 cm in size with serum AFP≥200 ng/mL. “US/AFP-suspected” individuals had either of the following: 1) serum AFP levels of ≥20 ng/mL regardless of US-detected liver nodule; and 2) US-detected nodule of ≥1 cm in size. “US/AFP-negative” individuals were defined as having serum AFP levels of <20 ng/mL without an US-detected liver nodule. Individuals with “US/AFP-positive” and “US/AFP-suspected” were referred to specialists for HCC confirmation diagnosis. All diagnosed HCC cases were ascertained by dynamic CT or MRI. CT/MRI images were independently evaluated by two radiologists from NCC/CH-CAMS. When diagnosis was unconfirmed, participants were offered and volunteered for US/AFP examination 3−6 months later. We also offered the examination to 20%−30% of participants with US/AFP-negatives 6 months later based on a randomized number after dividing them into groups of 35−44, 45−54, 55−64, and ≥65 years old. We further linked our data with local population-based cancer registries and bureaus of vital statistics of each center and confirmed liver cancer outcome (ICD-10 code C22.0 or C22.9) by Dec. 31, 2019.
Statistical analysis
All variables at baseline were first evaluated with unconditional univariate logistic regression analysis. The variables with P<0.1 in univariate analysis were further assessed by stepwise multivariable logistic regression. Variables with P<0.05 in multivariable logistic regression were subsequently included. The discrimination was evaluated by the area under the receiver operating characteristic curve (AUROC) and its 95% confidence interval (95% CI). Calibration was assessed by the Hosmer-Lemeshow test.
Then we created a point-based prediction rule based on coefficient-based model. The coefficient of each variable was divided by the smallest coefficient in the model and rounding to the nearest 0.1. In addition to overall performance, discrimination and calibration, the performance of the prediction rule was assessed according to its accuracy, including sensitivity, positive predictive value (PPV), negative predictive value (NPV), and the proportion of individuals of each risk group at different cutoff of risk score.
We validated the model internally using bootstrap procedure in the training cohort by sampling with the replacement for 1,000 iterations. The model was further externally validated in the independent validation cohort. With the same cutoff values defined in the training cohort, we measured the sensitivity, PPV, NPV, and the model predicted proportion of individuals in each risk group. The analyses were performed using R software (Version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant demographics
At baseline in 2017, from six HCC-screening centers of three provinces, a total of 116,542 males aged 35−69 years received HBsAg test; among them, 6,876 HBsAg-positive (confirmed with Abbott reagents) males were recruited. We excluded 723 male participants, of whom 87 were diagnosed with HCC before baseline, 70 were diagnosed with the other cancers, and 70 died from the causes other than HCC during the study period, and 496 lost follow-up. At the last follow-up in Oct. 2019, a total of 6,153 eligible HBsAg-positive males were included in our analyses. The training cohort recruited 3,629 HBsAg-positive males with 212 HCCs. Validation cohort recruited 2,524 HBsAg-positive males with 90 HCCs (Figure 1). The distribution in participants’ age and the other factors varied significantly at baseline among cohorts (Table 1).
Table 1. Baseline characteristics of training and validation cohorts.
| Factors | n (%) | P | |
| Training cohort (N=3,629) | Validation cohort (N=2,524) | ||
| HCC, hepatocellular carcinoma; AFP, α-fetoprotein; DCP, des-γ-carboxy-prothrombin; GGT, γ-glutamyl-transpeptidase; PLT, platelet; WBC, white blood cell; ALB, albumin; US, B-ultrasonography; BMI, body mass index. *, 9.80% of antiviral treated males in training cohort, 17.94% in validation cohort received the therapy less than one year, or stopped by himself without the instruction from his physician. | |||
| HCC cases | 212 (5.84) | 90 (3.57) | <0.001 |
| Age (year) [median (P25−P75)] | 51 (45−58) | 54 (48−62) | |
| 35−44 | 810 (22.32) | 379 (15.02) | <0.001 |
| 45−54 | 1,575 (43.40) | 890 (35.26) | |
| 55−64 | 906 (24.97) | 873 (34.59) | |
| 65−69 | 338 (9.31) | 382 (15.13) | |
| AFP (ng/mL) | |||
| <7.0 | 3,206 (88.34) | 2,309 (91.48) | <0.001 |
| 7.0−19.9 | 257 (7.08) | 145 (5.75) | |
| 20.0−199.9 | 113 (3.12) | 43 (1.70) | |
| ≥200.0 | 53 (1.46) | 27 (1.07) | |
| DCP (mAU/mL) | |||
| <40.0 | 3,510 (96.72) | 2,455 (97.26) | 0.189 |
| 40.0−139.9 | 48 (1.32) | 35 (1.39) | |
| ≥140.0 | 71 (1.96) | 34 (1.35) | |
| GGT (U/L) | |||
| <15 | 1,022 (28.16) | 474 (18.78) | <0.001 |
| 15−44 | 1,954 (53.85) | 1,572 (62.28) | |
| 45−79 | 400 (11.02) | 294 (11.65) | |
| ≥80 | 253 (6.97) | 184 (7.29) | |
| PLT (×109) | |||
| <100 | 409 (11.27) | 212 (8.40) | 0.001 |
| 100−299 | 3,094 (85.26) | 2,227 (88.23) | |
| ≥300 | 126 (3.47) | 85 (3.37) | |
| WBC (×106) | |||
| <4.0 | 395 (10.88) | 194 (7.69) | <0.001 |
| 4.0−9.9 | 3,145 (86.67) | 2,251 (89.18) | |
| ≥10.0 | 89 (2.45) | 79 (3.13) | |
| ALB (g/L) | |||
| <30.0 | 11 (0.30) | 5 (0.20) | <0.001 |
| 30.0−37.9 | 146 (4.02) | 65 (2.57) | |
| 38.0−44.9 | 1,498 (41.28) | 609 (24.13) | |
| ≥45.0 | 1,974 (54.40) | 1,845 (73.10) | |
| US-detected cirrhosis | |||
| Yes | 587 (16.18) | 266 (10.54) | <0.001 |
| BMI (kg/m2) | |||
| <18.5 | 21 (0.58) | 51 (2.02) | <0.001 |
| 18.5−23.9 | 1,361 (37.50) | 1,004 (39.78) | |
| 24.0−26.9 | 1,264 (34.83) | 844 (33.44) | |
| ≥27.0 | 983 (27.09) | 625 (24.76) | |
| Smoking (ever) | 1,954 (53.84) | 1,397 (55.35) | 0.244 |
| Alcohol drinking (ever) | 1,230 (33.89) | 1,124 (44.53) | <0.001 |
| Self-reported antiviral therapy received* | 982 (27.06) | 583 (23.10) | <0.001 |
| Self-reported diabetes | 228 (6.28) | 171 (6.77) | 0.441 |
| HCC family history | 363 (10.00) | 431 (17.08) | <0.001 |
Male-ABCD algorithm development and performance
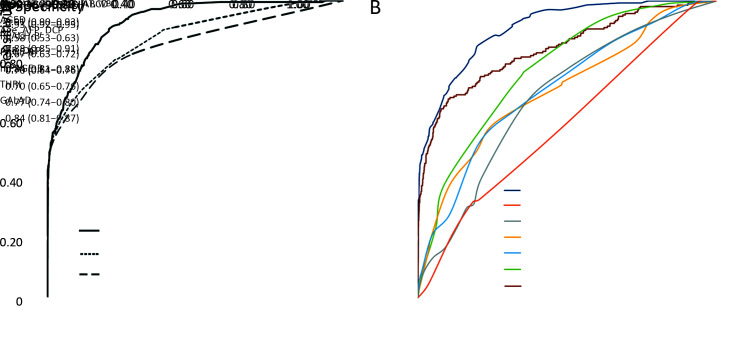
All parameters in the model development and validation were based on the baseline tests. In univariate analysis, the following variables showed significance of P<0.1 and they were used for further model selection (age, AFP, DCP, GGT, PLT, WBC, ALB, ALT, AST, and HBV-DNA) (Supplementary Table S1). In stepwise multivariate logistic regression, only age and five laboratory variables entered into the last model (Table 2). Based on these six variables, a coefficient-based model was employed to develop the score-based prediction rule, which was named male-ABCD algorithm, representing participant’s age, blood levels of GGT, counts of PLT, WBC, and concentration of DCP and AFP. Figure 2A shows the sensitivity and specificity of different combinations. AUROC of coefficient-based male-ABCD was 0.93 (0.92−0.95), which was higher than the AFP and DCP combination (AUROC=0.84, P<0.001, DeLong’s test), or the combination with age (AUROC=0.88, P<0.001, DeLong’s test). The χ2 of the Hosmer-Lemeshow test was 3.77 (P=0.88). Male-ABCD presented the lowest Akaike Information Criteria (AIC) of 887.18 compared with the AFP and DCP combination (AIC=1,007.08), or the combination with age (AIC=973.77), suggesting that it was the most suitable one. The algorithm was converted into a point-based prediction rule by dividing the coefficient of each variable by the smallest coefficient in the model (0.85) and rounding to the nearest 0.1. The total score ranges from 0 to 18.3. (Table 2).
Table S1. Univariate logistic regression analysis of training cohort (N=3,629).
| Risk factors | HCC cases/Total (n/N) | OR (95% CI) | P |
| AFP, α-fetoprotein; DCP, des-γ-carboxy-prothrombin; GGT, γ-glutamyl-transpeptidase; PLT, platelet; WBC, white blood cell; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; BMI, body mass index; HCC, hepatocellular carcinoma; OR, odds ratio; 95% CI, 95% confidential interval. | |||
| Age (year) | |||
| 35−44 | 14/810 | 1 (reference) | |
| 45−59 | 110/2,035 | 3.25 (1.85−5.70) | <0.001 |
| 60−69 | 88/784 | 7.19 (4.05−12.75) | <0.001 |
| AFP (ng/mL) | |||
| <7.0 | 100/3,206 | 1 (reference) | |
| 7.0−19.9 | 33/257 | 4.58 (3.02−6.94) | <0.001 |
| 20.0−399.9 | 49/135 | 17.70 (11.82−26.49) | <0.001 |
| ≥400.0 | 30/31 | 931.80 (125.82−6,900.90) | <0.001 |
| DCP (mAU/mL) | |||
| <20.0 | 61/2,735 | 1 (reference) | |
| 20.0−39.9 | 56/775 | 3.41 (2.35−4.95) | <0.001 |
| 40.0−49.9 | 5/17 | 18.27 (6.24−53.45) | <0.001 |
| 50.0−119.9 | 22/29 | 137.77 (56.71−334.68) | <0.001 |
| ≥120.0 | 68/73 | 596.17 (232.19−1,530.73) | <0.001 |
| GGT (U/L) | |||
| <20 | 38/1,697 | 1 (reference) | |
| 20−44 | 75/1,279 | 2.72 (1.83−4.05) | <0.001 |
| 45−79 | 49/400 | 6.10 (3.93−9.45) | <0.001 |
| ≥80 | 50/253 | 10.75 (6.88−16.80) | <0.001 |
| PLT (×109) | |||
| ≥150 | 68/2,452 | 1 (reference) | |
| 80−149 | 96/951 | 3.94 (2.86−5.42) | <0.001 |
| <80 | 48/226 | 9.45 (6.34−14.10) | <0.001 |
| WBC (×106) | |||
| ≥4 | 153/3,234 | 1 (reference) | |
| <4 | 59/395 | 3.54 (2.57−4.87) | <0.001 |
| ALB (g/L) | |||
| ≥38 | 190/3,472 | 1 (reference) | |
| <38 | 22/157 | 2.82 (1.75−4.52) | <0.001 |
| ALT (U/L) | |||
| <15 | 4/213 | 1 (reference) | |
| 15−44 | 118/2,515 | 2.57 (0.94−7.04) | 0.066 |
| ≥45 | 90/901 | 5.80 (2.11−15.97) | <0.001 |
| AST (U/L) | |||
| <15 | 3/138 | 1 (reference) | |
| 15−44 | 166/3,019 | 2.64 (0.83−8.36) | 0.100 |
| ≥45 | 43/472 | 4.54 (1.39−14.88) | 0.012 |
| HBsAg concentration (IU/mL) | |||
| <100 | 20/327 | 1 (reference) | |
| 100−999 | 97/1,225 | 1.32 (0.80−2.17) | 0.274 |
| ≥1,000 | 95/2,077 | 0.74 (0.45−1.21) | 0.225 |
| HBV DNA copies | |||
| <10 4 | 158/2,859 | 1 (reference) | |
| 104−99,999 | 33/394 | 1.56 (1.06−2.31) | 0.025 |
| ≥106 | 21/376 | 1.01 (0.63−1.62) | 0.963 |
| BMI (kg/m2) | |||
| <18.5 | 1/21 | 1 (reference) | |
| 18.5−23.9 | 93/1,361 | 1.47 (0.20−11.05) | 0.710 |
| 24.0−26.9 | 79/1,264 | 1.33 (0.18−10.06) | 0.780 |
| ≥27.0 | 39/983 | 0.83 (0.11−6.32) | 0.854 |
| Self-reported diabetes | 19/228 | 1.51 (0.92−2.47) | 0.100 |
| Drinking (ever) | 62/1,230 | 0.80 (0.59−1.08) | 0.141 |
| Smoking (ever) | 114/1,954 | 1.00 (0.76−1.32) | 0.980 |
| HCC family history | 26/363 | 1.28 (0.84−1.96) | 0.259 |
Table 2. OR and β coefficient of multivariate logistic regression analysis and point-based scoring system of male-ABCD.
| Factors | OR | β coefficient | P | Points assigned‡ |
| OR, odds ratio; AFP, α-fetoprotein; DCP, des-γ-carboxy-prothrombin; GGT, γ-glutamyl transpeptidase; PLT, platelets; WBC, white blood cells. ‡, The points were assigned by dividing the coefficient of each variable by the smallest coefficient in the model (0.85) and rounding to the nearest 0.1, for example, the coefficient for age 45−59 years was 1.11, and the smallest coefficient in the model was 0.85 (PLT, 80−149), so age 45−59 years were assigned 1.3 points (1.11/0.85). | ||||
| Age (year) | ||||
| 35−44 | Reference | 0 | ||
| 45−59 | 3.03 | 1.11 | 0.003 | 1.3 |
| 60−69 | 6.70 | 1.90 | <0.001 | 2.2 |
| AFP (ng/mL) | ||||
| <7.0 | Reference | 0 | ||
| 7.0−19.9 | 2.52 | 0.92 | 0.001 | 1.1 |
| 20.0−399.9 | 3.98 | 1.38 | <0.001 | 1.6 |
| ≥400.0 | 99.97 | 4.60 | <0.001 | 5.4 |
| DCP (mAU/mL) | ||||
| <20.0 | Reference | 0 | ||
| 20.0−39.9 | 3.98 | 1.38 | <0.001 | 1.6 |
| 40.0−49.9 | 20.15 | 3.00 | <0.001 | 3.5 |
| 50.0−119.9 | 91.07 | 4.51 | <0.001 | 5.3 |
| ≥120.0 | 224.66 | 5.41 | <0.001 | 6.3 |
| GGT (U/L) | ||||
| <20 | Reference | 0 | ||
| 20−44 | 2.52 | 0.92 | <0.001 | 1.1 |
| 45−79 | 3.18 | 1.16 | <0.001 | 1.4 |
| ≥80 | 2.57 | 0.94 | 0.009 | 1.1 |
| PLT (×109) | ||||
| ≥150 | Reference | 0 | ||
| 80−149 | 2.35 | 0.85 | <0.001 | 1.0 |
| <80 | 5.18 | 1.64 | <0.001 | 1.9 |
| WBC (×106) | ||||
| ≥4 | Reference | 0 | ||
| <4 | 2.56 | 0.94 | <0.001 | 1.1 |
| Intercept | − | −6.64 | <0.001 | − |
Figure 2.
Receiver operator characteristic curves of male-ABCD. (A) Comparison of male-ABCD with combination of age, AFP and DCP in training cohort; (B) Comparison of male-ABCD with some established models among 6,153 HBsAg-positive males. AUROC, area under the receiver operating characteristic; 95% CI, 95% confidence interval; AFP, α-fetoprotein; DCP, des-γ-carboxy-prothrombin; GGT, γ-glutamyl-transpeptidase; PLT, platelet; WBC, white blood cell.
Point-based HCC prediction and stratification by male-ABCD
For each given participant, the risk score was calculated based on the male-ABCD algorithm. At different risk-score cutoffs, male-ABCD grouped the HBsAg-positive males into the variated proportion of HCC at high risk or at low risk. Table 3 shows the sensitivity of identifying HCC within 2 years when the cutoff of risk score was set at different points, and PPV among the group at high risk, NPV at low risk. The performance of male-ABCD in training cohort was compared with previously developed risk-stratification models of AGED (13), REACH-B (10), PAGE-B (14), mPAGE-B (15) for general hepatitis B patients, and THRI (25) for cirrhotic patients and GALAD (26) for HCC diagnosis among the high-risk population. Male-ABCD generated the largest AUROC over AGED, REACH-B, PAGE-B, mPAGE-B, and THRI and GALAD (with all P-values less than 0.001, Delong’s Test) (Supplementary Table S2).
Table 3. Accuracy and HCC risk category at different cutoffs of risk score.
| Cutoff of
risk score |
Training cohort | Validation cohort | |||||||||
| Sensitivity (%) | at high risk | at low risk | Sensitivity (%) | at high risk | at low risk | ||||||
| Proportion (%) | PPV (%) | Proportion (%) | NPV (%) | Proportion (%) | PPV (%) | Proportion (%) | NPV (%) | ||||
| HCC, hepatocellular carcinoma; PPV, positive predictive value; NPV, negative predictive value. | |||||||||||
| 0.5 | 100 | 94.46 | 6.18 | 5.54 | 100 | 100 | 96.87 | 3.68 | 3.13 | 100 | |
| 1.0 | 100 | 94.41 | 6.19 | 5.59 | 100 | 100 | 96.87 | 3.68 | 3.13 | 100 | |
| 1.5 | 100 | 73.90 | 7.90 | 26.10 | 100 | 100 | 85.06 | 4.19 | 14.94 | 100 | |
| 2.0 | 99.53 | 71.07 | 8.18 | 28.93 | 99.90 | 98.89 | 83.40 | 4.23 | 16.60 | 99.76 | |
| 2.5 | 99.06 | 53.49 | 10.82 | 46.51 | 99.88 | 97.78 | 66.32 | 5.26 | 33.68 | 99.76 | |
| 3.0 | 97.17 | 44.53 | 12.75 | 55.47 | 99.70 | 95.56 | 55.74 | 6.11 | 44.26 | 99.64 | |
| 3.5 | 94.81 | 35.93 | 15.41 | 64.07 | 99.53 | 92.22 | 43.94 | 7.48 | 56.06 | 99.51 | |
| 4.0 | 88.21 | 29.15 | 17.67 | 70.85 | 99.03 | 87.78 | 32.37 | 9.67 | 67.63 | 99.36 | |
| 4.5 | 81.60 | 20.86 | 22.85 | 79.14 | 98.64 | 82.22 | 23.73 | 12.35 | 76.27 | 99.17 | |
| 5.0 | 74.06 | 15.40 | 28.09 | 84.60 | 98.21 | 73.33 | 16.32 | 16.02 | 83.68 | 98.86 | |
| 5.5 | 68.40 | 11.35 | 35.19 | 88.65 | 97.92 | 66.67 | 12.00 | 19.80 | 88.00 | 98.65 | |
| 6.0 | 58.96 | 7.85 | 43.86 | 92.15 | 97.40 | 58.89 | 7.49 | 28.04 | 92.51 | 98.42 | |
| 6.5 | 53.30 | 5.81 | 53.55 | 94.19 | 97.10 | 53.33 | 5.78 | 32.88 | 94.22 | 98.23 | |
| 7.0 | 48.58 | 4.02 | 70.55 | 95.98 | 96.87 | 48.89 | 4.00 | 43.56 | 96.00 | 98.10 | |
| 8.0 | 47.17 | 3.17 | 86.96 | 96.83 | 96.81 | 40.00 | 2.58 | 55.38 | 97.42 | 97.80 | |
| 9.0 | 42.45 | 2.70 | 91.84 | 97.30 | 96.54 | 35.56 | 1.94 | 65.31 | 98.06 | 97.66 | |
| 10.0 | 35.38 | 2.20 | 93.75 | 97.80 | 96.14 | 28.89 | 1.43 | 72.22 | 98.57 | 97.43 | |
| 11.0 | 27.83 | 1.71 | 95.16 | 98.29 | 95.71 | 25.56 | 1.11 | 82.14 | 98.89 | 97.32 | |
| 12.0 | 20.28 | 1.21 | 97.73 | 98.79 | 95.29 | 23.33 | 0.99 | 84.00 | 99.01 | 97.24 | |
| 13.0 | 15.57 | 0.94 | 97.06 | 99.06 | 95.02 | 17.78 | 0.71 | 88.89 | 99.29 | 97.05 | |
| 14.0 | 12.74 | 0.74 | 100 | 99.26 | 94.86 | 13.33 | 0.52 | 92.31 | 99.48 | 96.89 | |
| 15.0 | 7.55 | 0.44 | 100 | 99.56 | 94.58 | 10.00 | 0.36 | 100 | 99.64 | 96.78 | |
| 16.0 | 4.72 | 0.28 | 100 | 99.72 | 94.42 | 5.56 | 0.20 | 100 | 99.80 | 96.63 | |
| 17.0 | 1.42 | 0.08 | 100 | 99.92 | 94.24 | 1.11 | 0.04 | 100 | 99.96 | 96.47 | |
| 17.5 | 0 | 0 | Undefined | 100 | 94.16 | 0 | 0 | Undefined | 100 | 96.43 | |
Table S2. Comparison of AUROC of point-based male-ABCD and some established models for HCC in training cohort.
| Prediction model | AUROC (95% CI) |
| AUROC, area under the receiver operating characteristic; HCC, hepatocellular carcinoma; AGED: age, gender, HBeAg, HBV-DNA (13); REACH-B: age, gender, ALT, HBeAg, HBV-DNA (10); PAGE-B: age, gender, PLT (14); mPAGE-B: age, gender, PLT, ALB (15); THRI: age, gender, etiology, PLT (25); GALAD: age, gender, AFP, AFP-L3, DCP (26). | |
| Point-based male-ABCD | 0.91 (0.90−0.93) |
| AGED | 0.58 (0.53−0.63) |
| REACHB | 0.67 (0.63−0.72) |
| PAGE-B | 0.72 (0.68−0.77) |
| mPAGE-B | 0.73 (0.70−0.77) |
| THRI | 0.76 (0.72−0.79) |
| GALAD | 0.84 (0.81−0.87) |
Validation of male-ABCD algorithm
The male-ABCD was validated in an external cohort of 2,524 HBsAg-positive males (Table 3). At 1.5 points of risk score, prediction reached 100% of sensitivity both in training and validation cohort. At 2.5 points, 46.51% of the participants in training cohort, 33.68% in validation cohort were recognized at low risk with 99.06% and 97.78% of HCC prediction sensitivity, respectively. NPV among population at low risk was 99.88% and 99.76%, respectively. At 4.5 points, 20.86% of the participants in training cohort, 23.73% in validation cohort were recognized at high risk. The PPV among the population at high risk reached 22.85% in training cohort and 12.35% in validation cohort (Table 3).
Comparison of male-ABCD with AGED, REACH-B, PAGE-B, mPAGE-B, THRI and GALAD
For HCC prediction and stratification, male-ABCD was compared with previously constructed models by the other investigators among the total of 6,153 HBsAg-positive males (Table 4). Male-ABCD generated the largest AUROC (with all P values less than 0.001, Delong’s Test) in comparison with the models of AGED (13), REACH-B (10), PAGE-B (14) and mPAGE-B (15) that were constructed based on hepatitis B patients, with THRI (25) that was based on cirrhotic patients, and with GALAD (26) that was used for early diagnosis of HCC among high-risk population (Figure 2B). For risk-stratification, male-ABCD predicted 21.52% of total HBsAg-positive males into HCC at low risk with 100% of sensitivity at 1.5 points of risk score. With 100% of prediction sensitivity, REACH-B recognized 4.27% and PAGE-B predicted 3.17% of the total as HCC at low risk. At 2.5 points of risk score, male-ABCD predicted 41.25% of total HBsAg-positive males into HCC at low risk with 98.68% of sensitivity, and the NPV reached 99.84%. The missed four HCCs were detected 6.1, 9.9, 9.9, 19.9 months later since the baseline tests, respectively. While AGED predicted 6.37%, and mPAGE-B predicted 9.25% of total into HCC at low risk with 99.06% and 99.01% of sensitivity, and 99.13% and 99.47% of NPV, respectively. With a risk score of 2.5−4.5 points based on male-ABCD, 25.50% (13/51) of HCCs were detected between 12−24 months.
Table 4. Performance of male-ABCD and some established models for HCC prediction and stratification among 6,153 HBsAg-positive males.
| Models of HCC prediction | Cutoff* | Sensitivity (%) | Predicted proportion (%) at different HCC risks | PPV (%) | NPV (%) | |
| High | Low | |||||
| HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; AGED: age, gender, HBeAg, HBV-DNA (13); REACH-B: age, gender, ALT, HBeAg, HBV-DNA (10); PAGE-B: age, gender, PLT (14); mPAGE-B: age, gender, PLT, ALB (15); THRI: age, gender, etiology, PLT (25); GALAD: age, gender, AFP, AFP-L3, DCP (26); *, Cutoff values for AGED, REACH-B, PAGE-B, mPAGE-B, THRI and GALAD were selected as recommended from the original studies; †, HBV DNA was only detected in training cohort. The performance of AGED and REACH-B was calculated among training cohort of 3,629 HBsAg-positive males; ‡Z= −10.08+0.09×age +1.67×sex+2.3×log(AFP)+0.04×AFP-L3+1.33×log(DCP); PPV, positive predictive value; NPV, negative predictive value. | ||||||
| Male-ABCD (18.3 points in total) | ||||||
| 1.5 points | 100 | − | 21.52 | 6.25 | 100 | |
| 2.5 points | 98.68 | 58.75 | 41.25 | 8.24 | 99.84 | |
| 4.5 points | 81.79 | 22.04 | − | 18.22 | 98.85 | |
| AGED† (12 points in total) | ||||||
| 4 points | 99.06 | 81.45 (intermediate) | 6.37 | 6.18 | 99.13 | |
| 9 points | 12.18 (high) | |||||
| REACH-B† (17 points in total) | ||||||
| 5 points | 100 | 76.22 (intermediate) | 4.27 | 6.10 | 100 | |
| 11 points | 19.51 (high) | |||||
| PAGE-B (25 points in total) | ||||||
| 10 points | 100 | 52.41 (intermediate) | 3.17 | 5.07 | 100 | |
| 18 points | 44.42 (high) | |||||
| mPAGE-B (21 points in total) | ||||||
| 8 points | 99.01 | 54.61 (intermediate) | 9.25 | 5.35 | 99.47 | |
| 13 points | 36.14 (high) | |||||
| THRI (366 points in total) | ||||||
| 120 points | 100 | 32.91 (intermediate) | 0.00 | 4.91 | − | |
| 240 points | 67.09 (high) | |||||
| GALAD | ||||||
| Z‡=−1.36 | 90.73 | − | − | 8.39 | 99.03 | |
| Z=−0.63 | 80.79 | − | − | 13.27 | 98.66 | |
| Z= 0.88 | 52.98 | − | − | 42.44 | 97.54 | |
Discussion
Based on a large population-level screening program, this study included 6,153 HBsAg-positive male adults from six HCC-screening centers of three provinces in rural China. In this perspective, multicenter study, we developed and validated an HCC risk-stratified algorithm named male-ABCD, which represents participant’s age, blood levels of GGT, counts of PLT, WBC, and concentration of DCP and AFP. Based on this algorithm, for a given HBsAg-positive male, his HCC risk within 2 years was recognized at low risk when the risk score was ≤2.5 points, particularly ≤1.5 points. However, this HCC risk within 2 years was very high when the risk score reached >4.5 points. For the population of HBsAg-positive male adults with the point-based prediction, 1,324 of total 6,153 HBsAg-positives (21.52%) were grouped into HCC at low risk when risk score was ≤1.5 points, none HCC occurred within 2 years. At 2.5 points of cutoff, 2,538 of 6,153 (41.25%) HBsAg-positives were recognized HCC at low risk, only four HCCs occurred within 2 years. The sensitivities were 99.06% in training cohort, and 97.78% in validation cohort with 99.88% and 99.76% of NPV, respectively. At 4.5 points of cutoff, 22.04% (1,356/6,153) of the HBsAg-positives were recognized as HCC at high risk within 2 years with 18.22% of PPV. After head-to-head comparison with previously developed models of AGED ( 13), REACH-B (10), PAGE-B (14), mPAGE-B (15) for general hepatitis B patients, THRI (25) for cirrhotic patients, and GALAD (26) for HCC diagnosis among high-risk population, male-ABCD generated the largest AUROC. Our current study indicated that HBsAg-positive male adults could be identified at different HCC risk based on male-ABCD algorithm. The tests employed in are measurable and currently performed in clinical laboratories. As barriers in the community to screen all HBsAg carriers biannually, male-ABCD algorithm provided an applicable model for identifying the higher risk group who truly need intense screening intervals.
Several studies in clinic-based patients indicated that the combination of AFP/DCP, which are employed in the GALAD for early HCC diagnosis (26), discriminates early HCC from benign chronic liver diseases (1,27,28). AFP has been widely used for HCC surveillance (29), DCP elevation was observed several months before HCC occurred (24,27). When cutoff values were fixed at AFP=20 ng/mL, DCP=40 mAU/mL, the combination well discriminate HCC from benign liver diseases 12 months before the clinical HCC diagnosis based on patients of two genders (22). Among the HBsAg-positive males, we observed that HCC risk increased when DCP≥20 mAU/mL and AFP≥7 ng/mL. We didn’t include AFP-L3 because our previous results indicated that AFP-L3 addition decreased the sensitivity of HCC discrimination from cirrhosis (28). Increase of serum GGT is related to liver inflammation and is also recognized as an independent HCC risk factor (30,31). We previously reported that GGT with some clinical factors predicted 3-year HCC occurrence among HBsAg-positive individuals (30). Our current study showed that GGT at 20−44 U/L and ≥80 U/L showed similar impact, but higher impact at 45−79 U/L on HCC risk. PLT is a critical indicator of cirrhosis, which is at particularly high risk of HCC (25,29). Our study showed that adding these laboratory tests that reflect the underlying severity of liver diseases to age/AFP/DCP, the algorithm significantly improved the sensitivity and risk-stratification among the HBsAg-positive male adults.
The current male-ABCD was constructed based on the HBsAg-positive male adults from community population instead of patients from hospitals. HBV has long-term effects on HCC, that higher levels of serum HBV-DNA and HBsAg showed increased HCC risk in the models of REACH-B and GAG-HCC (10,12,30). We failed to observe their impact on HCC occurrence in short term. It has been documented that normal values of ALT and PLT in healthy males are different from healthy females (22,23). We failed to observe the significant effect of elevated blood levels of AST and ALT on REACH-B (10), and decreased blood albumin levels on CU-HCC (11), mPAGE (15) as the short-term HCC risk factor for the HBsAg-positive males.
HCC surveillance every six months using US/AFP is recommended by many professional societies. However less than 40% of HBsAg-positive adults complied to the biannually repeated US/AFP tests after initial 2−3 years for HCC-screening (5,18). Precision surveillance could be allocated to high-risk individuals who might benefit most from early intervention or intensive surveillance. As the limitation of B-ultrasonography in the detection of early HCC (32), individuals at the very early stage of HCC could be missed out. In the current study, we aimed to identify any of HCC cases who had developed clinical HCC and those who were at very high risk of HCC developing within short period. With the risk assessment using male-ABCD, the HBsAg-positive males were grouped into at high risk, or at low risk. This discrimination would be helpful to take different strategies to monitor their HCC. Based on male-ABCD, no HCC occurred among the 1,324 of total 6,153 (21.52%) HBsAg-positive males with risk score of ≤1.5 points. Instead of the biannually examination, the annually or longer interval follow-up would reduce the total US-examination number to avoid over-screening, and relieve the participant anxiety. However, 22% (1,356/6,153) of the HBsAg-positive males were recognized with the risk score of >4.5 points and the PPV reached to 18.22%. It is better for these population of HCC at high risk strictly follow the recommendation by professional societies, which is US/AFP examination every six months ( 3).
The male-ABCD is constructed based on HBsAg-positive males. The applicability for HBsAg-positive females may not be suitable, and needs to be evaluated in the future. Because the population of training and external validation cohorts were derived from different provinces, their characteristics were quite different. The discrimination of point-based male-ABCD in external validation cohort was acceptable, with an AUC of 0.90 (95% CI: 0.86−0.93) and sensitivity of 97.78% at the cutoff value of 2.5 points. However, the proportion of at low risk was 14.94% (at 1.5 points of cutoff) and 33.68% (at 2.5 points of cutoff) in validation cohort, lower than that of training cohort (26.10% and 46.51%, respectively). The generalizability of male-ABCD should be further evaluated by external validations based on randomized controlled trial of liver cancer screening. To avoid recall bias and self-reported bias, our model development was based on age and laboratory variables that are objective and measurable. Several potential factors, such as cirrhosis, were not included in the current analysis, which would impair the performance as well as generalizability of the model. Clinically, cirrhosis is typically classified as compensated and decompensated cirrhosis, and compensated cirrhosis is usually free of symptoms with a better quality of life than decompensated cirrhosis. The diagnosis of cirrhosis at clinical early state is depended on the liver stiffness measurement with sophisticated instrument or in combination with platelet count and spleen size (33). B-ultrasonography is not sensitive enough and is easily affected by the operator’s experience and subjective judgment. Therefore, in the current study, B-ultrasonography detected cirrhosis was not selected as a candidate predictor. We will perform further investigations when data are available to solve this issue. No significant impact of higher levels of HBV-DNA on HCC occurrence in short-term was observed, which potentially provides a window time for the standardized antiviral therapy to reduce HCC risk (10-13) as around 80% of participants in our study received no and/or not-standardized antiviral therapy. With the reduced cost and the use of more effective antiviral medicines, the applicability of male-ABCD needs to be further evaluated.
Different from REACH-B (10), AGED (13), PAGE-B (14), mPAGE-B (15) and THRI (25), male-ABCD was unable to class the HBsAg-positive males into HCC at highest and HCC at middle-high risk, respectively. However, when risk score was >4.5 points based on male-ABCD, our model recognized around 22% of HBsAg-positive males into HCC at high risk with 18.22% of PPV.
Conclusions
The male-ABCD algorithm could identify individual HCC risk within 2 years since initial laboratory tests among HBsAg-positive males. All tests are currently performed in clinics with an immediate clinical applicability for their HCC precision surveillance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by State Key Projects Specialized on Infectious Diseases (No. 2017ZX10201201-006), Key research projects for precision medicine (No. 2017YFC0908103), Innovation Fund for Medical Sciences of Chinese Academy of Medical Sciences (CIFMS, No. 2019-I2M-2-004, 2016-I2M-1-007, 2019-I2M-1-003), and National Natural Science Foundation Fund (No. 81972628, No. 81974492).
Contributor Information
Wanqing Chen, Email: chenwq@cicams.ac.cn.
Chunfeng Qu, Email: quchf@cicams.ac.cn.
References
- 1.Kulik L, El-Serag HB Epidemiology and management of hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei H, Lei L, Shi J, et al No expenditure difference among patients with liver cancer at stage I−IV: Findings from a multicenter cross-sectional study in China. Gastroenterology. 2020;32:516–29. doi: 10.21147/j.issn.1000-9604.2020.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omata M, Cheng AL, Kokudo N, et al Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao LH, Liu X, Yan HX, et al Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun. 2016;7:12992. doi: 10.1038/ncomms12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji M, Liu Z, Chang ET, et al Mass screening for liver cancer: results from a demonstration screening project in Zhongshan City, China. Sci Rep. 2018;8:12787. doi: 10.1038/s41598-018-31119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas DL Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041–50. doi: 10.1056/NEJMra1810477. [DOI] [PubMed] [Google Scholar]
- 7.Kansagara D, Papak J, Pasha AS, et al Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161:261–9. doi: 10.7326/M14-0558. [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G, Stroffolini T, Zagni I, et al Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(1 suppl):S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Chen CJ, Yang HI, Su J, et al Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Yang HI, Sherman M, Su J, et al Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol. 2010;28:2437–44. doi: 10.1200/JCO.2009.27.4456. [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Chan SL, Mo F, et al Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660–5. doi: 10.1200/JCO.2009.26.2675. [DOI] [PubMed] [Google Scholar]
- 12.Yuen MF, Tanaka Y, Fong D YT, et al Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–8. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Fan C, Li M, Gan Y, et al A simple AGED score for risk classification of primary liver cancer: development and validation with long-term prospective HBsAg-positive cohorts in Qidong, China. Gut. 2019;68:948–9. doi: 10.1136/gutjnl-2018-316525. [DOI] [PubMed] [Google Scholar]
- 14.Papatheodoridis G, Dalekos G, Sypsa V, et al PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–6. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Kim YD, Lee M, et al Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69:1066–73. doi: 10.1016/j.jhep.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Voulgaris T, Papatheodoridi M, Lampertico P, et al Clinical utility of hepatocellular carcinoma risk scores in chronic hepatitis B. Liver Int. 2020;40:484–95. doi: 10.1111/liv.14334. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JG, Parkin DM, Chen QG, et al Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–9. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Kondo F, Ebara M, et al Natural history of large regenerative nodules and dysplastic nodules in liver cirrhosis: 28-year follow-up study. Hepatol Int. 2015;9:330–6. doi: 10.1007/s12072-015-9620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SH, Yeh SH, Lin WH, et al Estrogen receptor α represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4α. Gastroenterology. 2012;142:989–98. doi: 10.1053/j.gastro.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Li CL, Li CY, Lin YY, et al Androgen receptor enhances hepatic telomerase reverse transcriptase gene transcription after hepatitis B virus integration or point mutation in promoter region. Hepatology. 2019;69:498–512. doi: 10.1002/hep.30201. [DOI] [PubMed] [Google Scholar]
- 22.Saxena S, Wong ET Heterogeneity of common hematologic parameters among racial, ethnic, and gender subgroups. Arch Pathol Lab Med. 1990;114:715–9. doi: 10.1097/00000478-199007000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Wu WC, Wu CY, Wang YJ, et al Updated thresholds for serum alanine aminotransferase level in a large-scale population study composed of 34 346 subjects. Aliment Pharmacol Ther. 2012;36:560–8. doi: 10.1111/j.1365-2036.2012.05224.x. [DOI] [PubMed] [Google Scholar]
- 24.Qu C, Wang Y, Wang P, et al Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A. 2019;116:6308–12. doi: 10.1073/pnas.1819799116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma SA, Kowgier M, Hansen BE, et al Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017;24:32248–1. doi: 10.1016/j.jhep.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Johnson PJ, Pirrie SJ, Cox TF, et al The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–53. doi: 10.1158/1055-9965.EPI-13-0870. [DOI] [PubMed] [Google Scholar]
- 27.Lok AS, Sterling RK, Everhart JE, et al Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Zhang Y, Li S, et al Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res. 2018;10:1947–58. doi: 10.2147/CMAR.S167036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tayob N, Christie I, Richardson P, et al Validation of the hepatocellular carcinoma early detection screening (HES) algorithm in a cohort of veterans with cirrhosis. Clin Gastroenterol Hepatol. 2019;17:1886–93. doi: 10.1016/j.cgh.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan C, Zhu J, Wang Y, et al A cohort study on analysis of high-risk population screening and risk factors for primary liver cancer in rural China based on Qidong. Zhongguo Xun Zheng Yi Xue. 2018;18:428–33. doi: 10.7507/1672-2531.201802031. [DOI] [Google Scholar]
- 31.Van Hemelrijck M, Jassem W, Walldius G, et al Gamma-glutamyltransferase and risk of cancer in a cohort of 545, 460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47:2033–41. doi: 10.1016/j.ejca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Tzartzeva K, Obi J, Rich NE, et al Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology. 2018;154:1706–18. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Amico G, Morabito A, D’Amico M, et al Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–76. doi: 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]