Abstract
Aims
Adjuvant chemotherapy has been shown to improve survival in patients with completely resected early‐stage non‐small cell lung cancer (NSCLC). This study evaluated real‐world relapse rates and healthcare resource utilization in patients with stage II–IIIB NSCLC receiving adjuvant therapy in a community oncology setting after complete resection.
Patients and Methods
The study included patients with stage II–IIIB NSCLC and complete resection receiving any adjuvant therapy during 06/2008–04/2017 at US Oncology Network clinics, with follow‐up through 04/2019. Primary endpoints were rate of relapse, time to relapse (TTR), disease‐free survival (DFS), overall survival (OS), and monthly emergency department (ED) visits and hospitalizations before and after relapse.
Results
The study identified 456 patients; median age was 66 years, 50% were male. In patients with relapse (45.2%), median follow‐up was 31.7 months and median TTR was 13.7 months. Median DFS in the overall population was 42.9 months. Median OS was 82.4 months in the overall population and shorter in patients with relapse than without relapse (41.6 months vs. not reached, p < 0.0001). Patients with relapse had significantly more monthly ED visits (mean [SD] 0.10 [0.24] vs. 0.03 [0.08], p < 0.0001) and hospitalizations (mean [SD] 0.20 [0.43] vs. 0.05 [0.10], p < 0.0001) following relapse than before relapse.
Conclusions
Patients with stage II–IIIB NSCLC treated with adjuvant therapy after complete resection had high relapse rates, reduced survival, and significantly increased healthcare resource use when relapse occurred. New therapeutic options to reduce relapse rates in patients with early‐stage NSCLC could reduce healthcare utilization and costs.
Keywords: NSCLC, relapse, recurrence, survival, utilization
This retrospective study evaluated real‐world relapse rates and healthcare resource utilization in patients with stage II–IIIB non‐small cell lung cancer receiving adjuvant therapy after complete resection in community‐based oncology practices. Patients had high relapse rates, reduced survival, and significantly increased healthcare resource use when relapse occurred.
INTRODUCTION
In 2020, an estimated 228 820 new cases of lung cancer were diagnosed in the United States and approximately 135 720 patients died from the disease. 1 Approximately 87% of lung cancers are classified as non‐small cell lung cancer (NSCLC). 2 In the United States, the overall 5‐year survival rate for NSCLC was 26% during 2010–2017.
Whenever feasible, patients with early‐stage NSCLC are treated surgically with curative intent. Patients diagnosed with early‐stage NSCLC who are eligible for surgical resection can achieve 5‐year survival rates of over 60%. 2 The 5‐year survival rate in patients with resected NSCLC has been reported to be 63% for stage I disease but only 35% for stage IIIA disease. 2 Many patients with NSCLC are at risk of recurrence even after complete resection: approximately 30–55% will develop recurrence and die despite curative surgical resection. 3
Adjuvant chemotherapy has been shown to improve survival in patients with completely resected early‐stage NSCLC. 4 , 5 The Lung Adjuvant Cisplatin Evaluation (LACE) meta‐analysis suggested overall survival (OS) benefit for patients with stage II N1 and IIIA (mostly N2 cases) initiating adjuvant therapy after complete surgical resection, with an average survival benefit of 5% at 5 years compared to surgery alone without adjuvant therapy. 6 , 7 , 8 Adjuvant treatment with platinum‐based chemotherapy is considered the standard of care in patients with stages IIA, IIB, and IIIA disease after complete tumor resection. 4 , 5 , 9 , 10
While there are several published studies on relapse and survival outcomes in patients with NSCLC receiving adjuvant treatment after complete surgical resection, published studies are limited in assessing relapse rates, survival, and relapse‐associated healthcare resource utilization in real‐world clinical practice. Studies measuring relapse rates included patients with early‐stage NSCLC who may not have been at high risk for relapse post‐surgery, investigated limited treatment options, 11 or examined patients from a single tertiary academic institution and therefore did not represent community oncology patients at a national level. 7 Furthermore, no studies have evaluated utilization before and after relapse. The current study attempts to provide insight into the clinical and utilization outcomes of patients with NSCLC treated in a community oncology setting who are at high risk for relapse following surgery. Thus, the aim of this study was to use real‐world clinical practice data to describe demographic and clinical characteristics and assess relapse rates, clinical outcomes, and healthcare utilization in patients with stage II–IIIB NSCLC who received adjuvant therapy following complete surgical resection in a US community oncology setting.
MATERIALS AND METHODS
Study design and data sources
This was a retrospective observational study using clinical data from The US Oncology Network electronic health record (EHR) system, iKnowMed (iKM), to analyze patient profiles, relapse rates, and select clinical and healthcare resource utilization outcomes among patients with stage II–IIIB NSCLC (6th and 7th Edition of TNM classification for Lung Cancer Staging) with complete surgical resection and initiating adjuvant treatment in the community oncology setting. 12 , 13 iKM captures demographic, clinical, and treatment data for more than a million patients treated annually by more than 1000 community‐based oncologists within the US Oncology Network. A structured data extract of the iKM database was used to address most research questions of the study, and a targeted chart review provided supplemental information captured from unstructured fields of the EHR. The Social Security Administration Death Master File (DMF) was used to supplement the data available in iKM on vital status and dates of death.
The analysis included adult patients (at least 18 years of age) with stage II–IIIB NSCLC who underwent complete surgical resection and were identified as having initiated adjuvant treatment between 1 June 2008 and 30 April 2017. The index date was the date of complete surgical resection during the patient identification period. Baseline data were measured 30 days prior to and following the date of surgical resection, with preference given to data collected in the 30 days prior to the index date. Patients were required to have at least two visits within the US Oncology Network and were followed through 30 April 2019, date of death, or date of last visit where vital signs were taken, whichever occurred first. Patients without evidence of death had to have at least 24 months of follow‐up. Patients were excluded if they were enrolled in clinical trials at any time during the study period, were diagnosed with or treated for another documented primary cancer during the study period, received neoadjuvant treatment, or had evidence of conditions or medications that could impact or impair the immune system. Conditions and/or medications impacting the immune system included active/recurrent hepatic disorders, history of tuberculosis, suspected or proven immunocompromised state (history of select autoimmune disorders, known history of HIV, or diagnosis of immunodeficiency or receiving select immunosuppressive agents prior to or during the study period), prior treatment with canakinumab or other IL‐1β inhibitors, or current treatment with drugs targeting the immune system (tumor necrosis factor blockers, anakinra, rituximab, abatacept, or tocilizumab). The full list of conditions and medications impacting the immune system is presented in Supporting Information Table S1.
The final study population for analysis was defined after identification and review of the patient population meeting the inclusion criteria in the iKM database. All data were handled in compliance with the Health Insurance Portability and Accountability Act and the Health Information Technology for Economic and Clinical Health Act.
Statistical methods
Descriptive analyses were conducted to assess demographic, clinical, and treatment characteristics among the overall cohort of patients, stratified by relapse status following adjuvant treatment. Patient characteristics assessed included age, race, sex, geographic location, Eastern Cooperative Oncology Group (ECOG) performance status score, body mass index, smoking status, stage at diagnosis, baseline laboratory values, and baseline comorbidities. Treatment‐related variables abstracted from the iKM database included overall follow‐up time, surgery type, and radiation treatment start and stop dates. Hospitalizations and emergency department (ED) visits were evaluated for two time periods among patients with relapse: the interval between the date of surgical resection to the day before relapse, and from the date of relapse through the end of the follow‐up. To adjust for variable follow‐up times across patients, utilizations were calculated as per patient per month (PPPM) for each time period before and after relapse. Continuous variables were described by mean, standard deviation, median, and range. Categorical variables were defined by patient counts and percentages. For statistical comparisons between patients with and without relapse, continuous variables were analyzed with t‐test or analysis of variance, and categorical variables were analyzed with Pearson chi‐square or Fisher's exact test. An alpha level of 0.05 was considered the primary criterion for statistical significance. Due to the paired nature of the data, utilization measures before and after relapse were compared using the Wilcoxon signed‐rank test for continuous variables and McNemar's test for categorical variables.
Kaplan–Meier methods were used to examine time‐to‐event endpoints, including time to relapse or recurrence, physician‐assessed disease‐free survival (DFS), and OS with medians and 95% confidence intervals (CIs). Time to relapse or recurrence was defined as the interval between the date of surgery (index date) and the date of physician‐assessed relapse; patients who died before relapse were censored. Physician‐assessed DFS was the interval (in months) from the index date until the date of physician‐assessed relapse or recurrence or date of death (any cause) as documented in the DMF or the iKM EHR database. OS was calculated from the date of surgery (index date) until date of death from any cause. For all Kaplan–Meier time‐to‐event analyses, patients who did not experience an event for the respective time‐to‐event analysis were censored at the study end date or the last visit date available in the dataset, whichever occurred first.
RESULTS
Patient selection
Using the iKM database, we identified 51 178 patients diagnosed with stage II–IIIB NSCLC, 5498 of which received adjuvant therapy within the US Oncology Network during the study identification period. Among these patients, 2060 received care at a US Oncology Network site utilizing the full EHR capabilities of iKM at the time of adjuvant treatment and had data accessible for treatment purposes. We excluded 211 patients enrolled in clinical trials during the study observation period and 294 patients whose first recorded adjuvant treatment was recorded outside of the study identification period. A further 138 patients were excluded because they were diagnosed or treated for other primary cancers in the 3 years prior to their initiation of adjuvant therapy. Additional reasons for exclusion are shown in Figure 1. A total of 889 patients met all eligibility criteria. A stratified random sample of 650 patients were selected for screening and confirmation of eligibility criteria through chart review. Out of these patients, 91 patients without evidence of complete surgical resection were excluded. The final population included 456 patients whose selection criteria were confirmed during chart review. This population included 206 (45.2%) patients with relapse during the follow‐up period and 250 (54.8%) patients without relapse.
FIGURE 1.
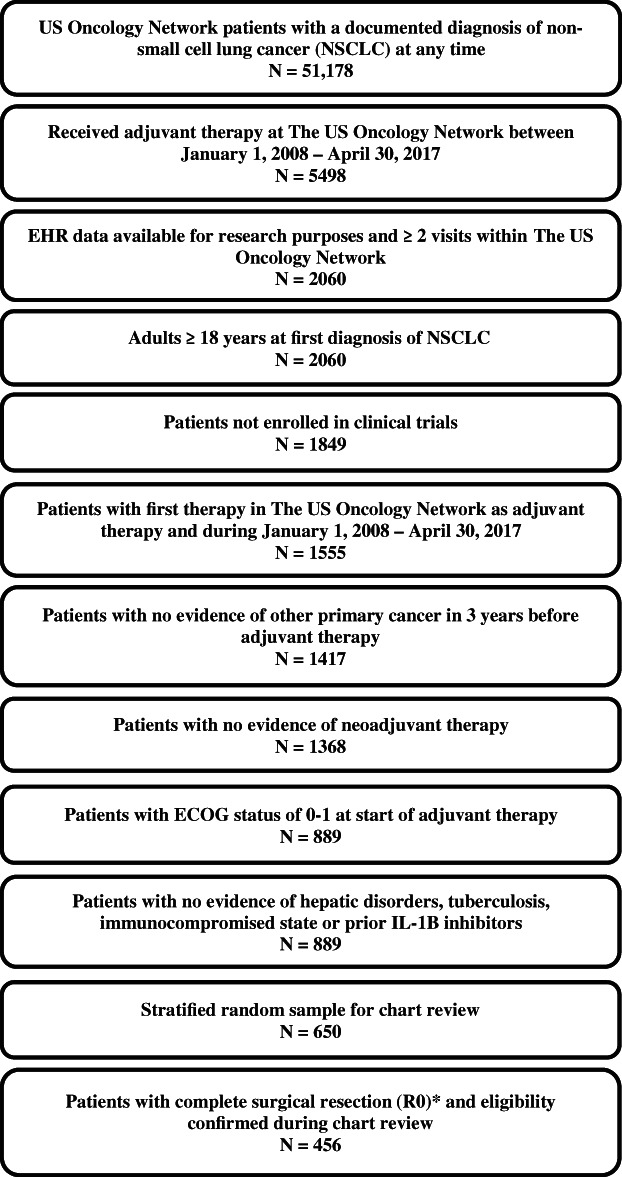
Study attrition. ECOG, Eastern Cooperative Oncology Network; EHR, electronic health record; NSCLC, non‐small cell lung cancer. *91 patients were excluded as not having complete surgical resection (R0)
Demographic and clinical characteristics of the study cohort, stratified by relapse status, are presented in Table 1. The median age of the overall population at the time of surgery was 66 years (range 29–85) and the proportions of male and female patients were equal. The majority of patients were White (68.0%), were former or current smokers (66.7%), had non‐squamous histology (67.3%), were either overweight or obese (56.1%), and at the time of surgery 63.8% had stage II and 35.1% stage III (1.5% stage IIIB) disease. The most common baseline comorbidities were cancer‐related signs and symptoms (36.4%), pain (28.7%), anemia (21.5%), and gastrointestinal disorders (25.0%).
TABLE 1.
Baseline demographic and clinical characteristics overall and by relapse status
| Characteristics | Overall n = 456 | Relapse n = 206 | No relapse n = 250 | p value |
|---|---|---|---|---|
| Number of patients | 456 | 206 | 250 | |
| Age at surgical resection, years | 0.4506 | |||
| Mean (SD) | 65.5 (9.2) | 65.2 (9.2) | 65.7 (9.2) | |
| Median (range) | 66 (29–85) | 65 (29–85) | 66 (35–84) | |
| Age group, n (%) | 0.9816 | |||
| ≤44 years | 10 (2.2) | 4 (1.9) | 6 (2.4) | |
| 45–64 years | 184 (40.4) | 84 (40.8) | 100 (40.0) | |
| ≥65 years | 262 (57.5) | 118 (57.3) | 144 (57.6) | |
| Sex, n (%) | 0.8507 | |||
| Female | 228 (50.0) | 102 (49.5) | 126 (50.4) | |
| Male | 228 (50.0) | 104 (50.5) | 124 (49.6) | |
| Race, n (%) | 0.7434 | |||
| White | 310 (68.0) | 136 (66.0) | 174 (69.6) | |
| Other | 103 (22.6) | 51 (24.8) | 52 (20.8) | |
| Black or African American | 31 (6.8) | 13 (6.3) | 18 (7.2) | |
| Asian | 12 (2.6) | 6 (2.9) | 6 (2.4) | |
| Body mass index at baseline, kg/m2 | 0.5956 | |||
| Mean (SD) | 25.2 (7.7) | 24.9 (7.3) | 25.5(8.0) | |
| Median (range) | 26.3 (8.5–52.0) | 26.2 (8.9,47.6) | 26.3(8.5–52.0) | |
| ECOG PS at start of adjuvant treatment, n (%) | 0.1768 | |||
| 0 | 121 (26.5) | 61(29.6) | 60(24.0) | |
| 1 | 335 (73.5) | 145(70.4) | 190(76.0) | |
| Smoking status, n (%) | 0.0180 | |||
| Current | 35 (7.7) | 15(7.3) | 20(8.0) | |
| Former | 269 (59.0) | 109(52.9) | 160(64.0) | |
| Never | 60 (13.2) | 28(13.6) | 32(12.8) | |
| Documented unknown | 64 (14.0) | 34(16.5) | 30(12.0) | |
| Not documented | 28 (6.1) | 20(9.7) | 8(3.2) | |
| Histology, n (%) | 0.0004 | |||
| Non‐squamous | 307 (67.3) | 155 (75.2) | 152 (60.8) | |
| Squamous | 140 (30.7) | 45 (21.8) | 95 (38.0) | |
| NSCLC (not otherwise specified) | 9 (2.0) | 6 (2.9) | 3 (1.2) | |
| Stage at initial NSCLC diagnosis, n (%) | 0.3404 | |||
| II | 1 (0.2) | 1 (0.5) | 0 (0.00) | |
| IIA | 204 (44.7) | 89 (43.2) | 115 (46.0) | |
| IIB | 106 (23.2) | 43 (20.9) | 63 (25.2) | |
| IIIA | 137 (30.0) | 68 (33.0) | 69 (27.6) | |
| IIIB | 8 (1.8) | 5 (2.4) | 3 (1.2) | |
| Stage at surgical resection, n (%) | 0.0600 | |||
| II (not specified) | 2 (0.4) | 2 (1.0) | 0 (0.00) | |
| IIA | 186 (40.8) | 77 (37.4) | 109 (43.6) | |
| IIB | 105 (23.0) | 44 (21.4) | 61 (24.4) | |
| III (not specified) | 1 (0.2) | 0 (0.00) | 1 (0.4) | |
| IIIA | 152 (33.3) | 75 (36.4) | 77 (30.8) | |
| IIIB | 7 (1.5) | 5 (2.4) | 2 (0.8) | |
| Not documented | 3 (0.7) | 3 (1.5) | 0 (0.00) | |
| ROS1 status | 0.0006 | |||
| Negative | 24 (5.3) | 19 (9.2) | 5 (2.0) | |
| Documented unknown | 21 (4.6) | 10 (4.9) | 11 (4.4) | |
| Not documented | 411 (90.1) | 177 (85.9) | 234 (93.6) | |
| ALK status | <0.0001 | |||
| Negative | 100 (21.9) | 66 (32.0) | 34 (13.6) | |
| Positive | 4 (0.9) | 3 (1.5) | 1 (0.4) | |
| Documented unknown | 125 (27.4) | 53 (25.7) | 72 (28.8) | |
| Not documented | 227 (49.8) | 84 (40.8) | 143 (57.2) | |
| EGFR status | <0.0001 | |||
| Negative | 100 (21.9) | 63 (30.6) | 37 (14.8) | |
| Positive | 25 (5.5) | 15 (7.3) | 10 (4.0) | |
| Documented unknown | 168 (36.8) | 74 (35.9) | 94 (37.6) | |
| Not documented | 163 (35.7) | 54 (26.2) | 109 (43.6) | |
| PD‐L1 expression | <0.0001 | |||
| Negative a | 20 (4.4) | 18 (8.7) | 2 (0.8) | |
| Documented unknown | 52 (11.4) | 29 (14.1) | 23 (9.2) | |
| Not documented | 384 (84.2) | 159 (77.2) | 225 (90.0) | |
| BRAF status | <0.0001 | |||
| Negative | 14 (3.1) | 13 (6.3) | 1 (0.4) | |
| Positive | 2 (0.4) | 2 (1.0) | 0 (0.00) | |
| Documented unknown | 20 (4.4) | 17 (8.3) | 3 (1.2) | |
| Not documented | 420 (92.1) | 174 (84.5) | 246 (98.4) | |
| Baseline comorbidities, n (%) | ||||
| Pain | 131 (28.7) | 67 (32.5) | 64 (25.6) | 0.1039 |
| Gastrointestinal | 114 (25.0) | 62 (30.1) | 52 (20.8) | 0.0225 |
| Anemia | 98 (21.5) | 46(22.3) | 52 (20.8) | 0.6922 |
| Arterial hypertension | 79 (17.3) | 39 (18.9) | 40 (16.0) | 0.4103 |
| Respiratory: asthma, COPD, emphysema | 77 (16.9) | 37 (18.0) | 40 (16.0) | 0.5780 |
| Hyperlipidemia | 45 (9.9) | 22 (10.7) | 23 (9.2) | 0.5980 |
| Neutropenia/leukopenia | 40 (8.8) | 20 (9.7) | 20 (8.0) | 0.5209 |
| Diabetes (type 1/2/NEC) | 35 (7.7) | 15 (7.3) | 20 (8.0) | 0.7743 |
| Infection | 23 (5.0) | 13 (6.3) | 10 (4.0) | 0.2618 |
| Follow‐up time from date of surgical resection, months | <0.0001 | |||
| Mean (SD) | 45.5 (26.6) | 38.3 (27.1) | 51.4 (24.8) | |
| Median (range) | 41.2 (1.7–124.7) | 31.7 (3.5–124.7) | 48.5 (1.7–122.4) |
Abbreviations: ALK, anaplastic lymphoma kinase; COPD, chronic obstructive pulmonary disease; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NEC, not elsewhere classified; NSCLC, non‐small cell lung cancer; PD‐L1, programmed death ligand‐1; SD, standard deviation.
Negative PD‐L1 expression defined as PD‐L1 percent staining of <1%.
Table 2 provides a summary of treatment patterns for the overall study population, stratified by relapse status. The median time from date of surgery to last follow‐up or death was 41.2 months (range 1.7–124.7) in the overall population and was shorter in patients with relapse (median 31.7 months) than patients without relapse (median 48.5 months, p < 0.0001). Overall, 80.7% of study patients had a surgery involving lobectomy or bilobectomy, and 80.9% received adjuvant chemotherapy without radiation treatment (chemotherapy only). Treatment with chemotherapy and sequential radiation was observed in 13.4% of the overall cohort, and treatment with chemotherapy and concurrent radiation in 5.7%. For the overall cohort of 456 patients, the most common chemotherapy‐only regimens were cisplatin/vinorelbine (32.0%), carboplatin/paclitaxel (25.7%), cisplatin/pemetrexed (16.3%), and carboplatin/paclitaxel (8.4%).
TABLE 2.
Treatment patterns overall and by relapse status
| Treatment characteristics | Overall n = 456 | Relapse n = 206 | No relapse n = 250 | p value |
|---|---|---|---|---|
| Surgery type, n (%) | 0.7024 | |||
| Lobectomy or bilobectomy | 368 (80.7) | 168 (81.6) | 200 (80.0) | |
| Pneumonectomy | 42 (9.2) | 18 (8.7) | 24 (9.6) | |
| Wedge or segmentectomy | 20 (4.4) | 9 (4.4) | 11 (4.4) | |
| Other a | 26 (5.7) | 11 (5.3) | 15 (6.0) | |
| Adjuvant treatment received | 0.4051 | |||
| Chemotherapy without radiation | 369 (80.9) | 163 (79.1) | 206 (82.4) | |
| Chemotherapy with sequential radiation | 61 (13.4) | 28 (13.6) | 33 (13.2) | |
| Chemotherapy with concurrent radiation | 26 (5.7) | 15 (7.3) | 11 (4.4) | |
| Treatments received | ||||
| Chemotherapy without radiation, n (%) | 369 (80.9) | 163 (79.1) | 206 (82.4) | |
| Chemotherapy duration, months, median (range) b | 2.1 (0.0,14.6) | 2.2 (0.0,14.6) | 2.1 (0.0,5.6) | 0.1790 |
| Treatments received, n (%) | ||||
| Cisplatin/vinorelbine | 118 (32.0) | 55 (33.7) | 63(30.6) | |
| Carboplatin/paclitaxel | 95 (25.7) | 43 (26.4) | 52(25.2) | |
| Cisplatin/pemetrexed | 60 (16.3) | 27 (16.6) | 33(16.0) | |
| Carboplatin/pemetrexed | 31 (8.4) | 12 (7.4) | 19(9.2) | |
| Other c | 152 (33.3) | 137 (33.5) | 167 (33.2) | |
| Chemotherapy with sequential radiation d | 61 (13.4) | 28 (13.6) | 33 (13.2) | |
| Chemotherapy duration, months, median (range) b | 2.1 (0.0,4.2) | 2.2 (1.0,4.2) | 2.1 (0.0,4.1) | |
| Treatments received, n (%) | ||||
| Carboplatin/paclitaxel | 24 (39.3) | 10 (35.7) | 14 (42.4) | |
| Cisplatin/vinorelbine | 17 (27.9) | 8 (28.6) | 9 (27.3) | |
| Cisplatin/pemetrexed | 10 (16.4) | 4 (14.3) | 6 (18.2) | |
| Cisplatin/etoposide | 4 (6.6) | 1 (3.6) | 3 (9.1) | |
| Other c | 6 (9.8) | 5 (17.9) | 1 (3.0) | |
| Chemotherapy with concurrent radiation e | 26 (5.7) | 15 (7.3) | 11 (4.4) | |
| Chemotherapy duration, months, median (range) | 2.1 (0.8,5.4) | 2.1 (1.0,5.4) | 2.1 (0.8,4.8) | 0.7950 |
| Treatments received, n (%) | ||||
| Carboplatin/paclitaxel | 14 (53.8) | 8 (53.3) | 6 (54.5) | |
| Cisplatin/etoposide | 4 (15.4) | 1 (6.7) | 3 (27.3) | |
| Cisplatin/pemetrexed | 2 (7.7) | 2 (13.3) | 0 (0.00) | |
| Cisplatin/vinorelbine | 2 (7.7) | 2 (13.3) | 0 (0.00) | |
| Other c | 4 (15.4) | 2 (13.3) | 2 (18.2) | |
Includes combination procedures and/or mediastinal resection or lymphadenectomy.
Treatment duration lasting only 1 day is presented as 0.0 months.
Adjuvant treatments used in <5.0% of the overall population.
Sequential radiation occurred when radiation dates were within 90 days of chemotherapy dates.
Concurrent radiation occurred when dates of chemotherapy and radiation overlapped.
Clinical outcomes
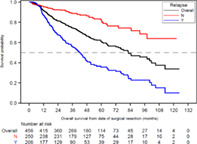
In the 206 patients who relapsed (45.2%) during a median 41.2 months of follow‐up time, median time to relapse was 13.7 months (95% CI 11.9–16.7 months). The median physician‐assessed DFS (death considered as events) was 42.9 months (95% CI 36.8–59.5; Figure 2(a)), with estimated DFS at 1, 2 and 5 years of 77.2%, 63.6%, and 44.9%, respectively. Among the overall population, 173 (37.9%) patients died during the follow‐up period. The median survival time, from date of surgery to death or censoring, was 82.4 months (95% CI 72.1–101.7; Figure 2(b)). Patients with relapse had a higher rate of death (62.1%) than patients without relapse (18.0%), and a shorter median survival time (41.6 months vs. median not reached, p < 0.0001). For all patients, the survival rates at 1, 2, and 5 years were 91.4%, 81.4%, and 61.2%, respectively. One‐, 2‐, and 5‐year survival rates were 86.8%, 67.5%, and 35.6%, respectively, for patients with relapse, and 95.2%, 92.4%, and 82.3%, respectively, for patients without relapse.
FIGURE 2.
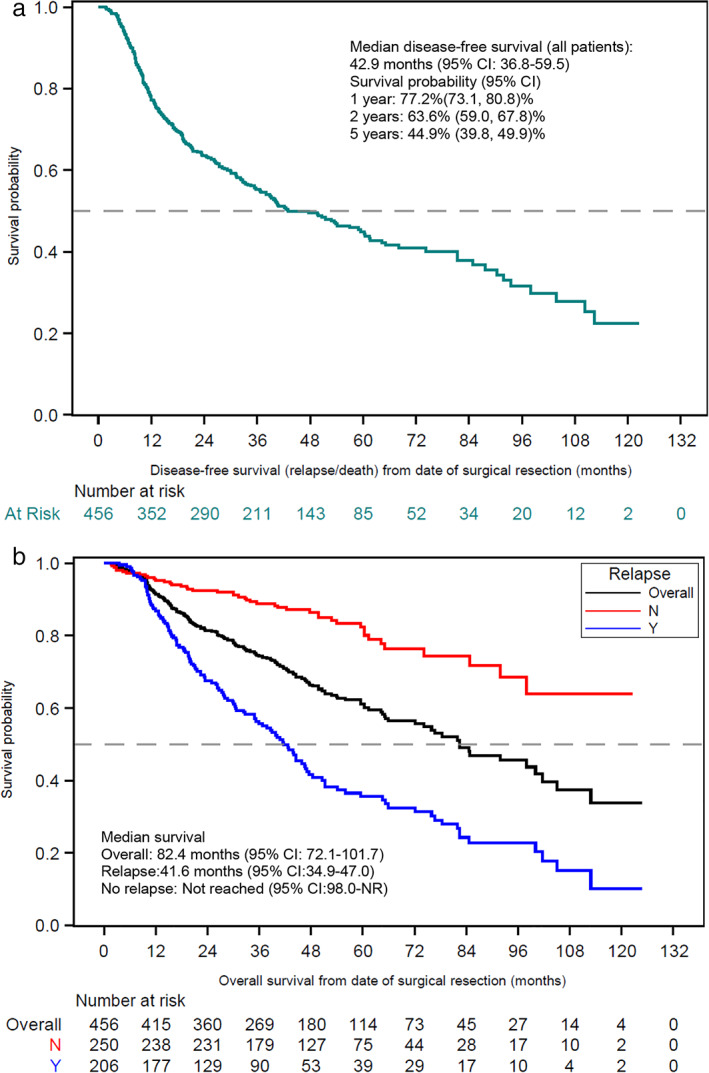
(a) Disease‐free survival from date of complete surgical resection. Disease‐free survival: defined as the interval between the date of surgery and date of physician‐assessed relapse or date of death from any cause. Patients who did not experience relapse or death were censored at the study end date or the last visit date available in the dataset, whichever occurred first. (b) Overall survival from date of complete surgical resection, overall and by relapse status. Survival defined as overall survival was defined as the time interval between the date of surgery until date of death from any cause. Patients who did not experience an event for the respective time‐to‐event analysis were censored at the study end date or the last visit date available in the dataset, whichever occurred first. CI, confidence interval
Healthcare resource utilization
Among study patients with relapse during the follow‐up period (death was not considered an event) and with at least 1 month of follow‐up time before and after relapse (n = 195), 26.2% had at least one ED visit before the date of relapse and 40.0% had at least one ED visit after relapse (p = 0.0016; Table 3). Similarly, the proportion of patients with at least one hospitalization was higher after relapse (59.5%) than before relapse (36.4%). Patients with relapse also had significantly more ED visits (PPPM visits, mean [SD] 0.10 [0.24] vs. 0.03 [0.08], p < 0.0001) and hospitalizations (PPPM hospitalizations, mean [SD] 0.20 [0.43] vs. 0.05 [0.10], p < 0.0001) following relapse than they had before relapse.
TABLE 3.
Healthcare resource utilization among relapsed patients, before and after relapse
| Time period | |||
|---|---|---|---|
| Utilization parameters | From surgery to relapse | From relapse to end of medical record c | p value d |
| Patients with available data a , b | 195 | 195 | |
| Time in period, months | 0.2291 | ||
| Mean (SD) | 20.1 (18.59) | 18.4 (20.16) | |
| Median (range) | 13.0 (3.02–110.23) | 11.3 (1.02–103.39) | |
| Emergency department visits | |||
| Patients with at least one visit, n (%) | 51 (26.2) | 78 (40.0) | 0.0016 |
| Emergency department visits, PPPM | |||
| Mean (SD) | 0.03 (0.08) | 0.10 (0.24) | <0.0001 |
| Median (range) | 0.00 (0.00–0.49) | 0.00 (0.00–1.74) | |
| Hospitalizations | |||
| Patients with at least one hospitalization, n (%) | 71(36.4) | 116(59.5) | <0.0001 |
| Hospitalizations, PPPM | |||
| Mean (SD) | 0.05 (0.10) | 0.201 (0.425) | <0.0001 |
| Median (range) | 0.00 (0.00–0.61) | 0.039 (0.00–2.706) | |
Abbreviations: NSCLC, non‐small cell lung cancer; PPPM, per patient per month; SD, standard deviation.
Patients were required to have at least 1 month of follow‐up time before relapse and at least 1 month of follow‐up time after relapse.
All patients experienced relapse event after surgical resection of NSCLC.
Inclusive of relapse date.
Due to paired nature of data, continuous variables were compared using Wilcoxon signed‐rank test and categorical variables were compared with McNemar's test.
DISCUSSION
This retrospective study presents data on healthcare resource utilization, rates of relapse, and survival outcomes in a large, longitudinal cohort of real‐world patients with completely resected stage II–IIIB NSCLC receiving adjuvant therapy in a US community oncology setting. Our findings showed high relapse rates among these patients, poor survival outcomes (especially among relapsed patients), and increased healthcare utilization after relapse.
A total of 456 patients with NSCLC receiving adjuvant treatment after complete surgical resection were included in the study. Consistent with prior studies, our population had a greater prevalence of non‐squamous histology (67.3%). 11 , 14 , 15 Our population was evenly split between males and females, unlike other studies, which included predominantly male patients 14 , 16 or predominantly female patients. 11 Our population was on average older (median 66 vs. 59 years) than what has been reported by other clinical trials, 16 but was similar in age to study populations in retrospective observational studies outside the clinical trial setting. 11 , 14 , 15
As expected, a majority of patients in the current study received platinum‐based adjuvant therapy. Cisplatin was the most common platinum agent in patients receiving chemotherapy alone or with sequential chemotherapy and radiation treatment. Carboplatin was the most commonly used platinum agent in patients receiving concurrent radiation. The median duration of adjuvant therapy was 2.1 months, regardless of receipt of radiation therapy.
Several distinctions with study sample and study design between existing literature and our study are worth noting. In our study, relapse was observed in 45.2% of patients, similar to Valdes et al., who reported relapse rates of 48% in a retrospective chart review study of patients with completely resected disease who received adjuvant chemotherapy at a single institution. 11 In patients who relapsed in our study, the median time to relapse was 13.7 months (95% CI 11.9–16.7 months) vs. 18.5 months in Valdes et al. A few aspects may help to explain the discrepancies: the Valdes study included patients diagnosed at an earlier stage (32% of patients had stage I disease), while our study only included those with stage II and IIIB (high risk of relapse); the Valdes study only included those receiving platinum doublet therapy (82% cisplatin‐vinorelbine) in a single institution, whereas our study included patients receiving any adjuvant treatments and is more representative of treatment in a community oncology setting. Another retrospective observational study, by Buck et al., assessed patients with completely resected stage IB to IIIA disease in a community oncology setting, where they found a recurrence rate of 24.3% and median time to recurrence of 12.6 months. However, only 57% of patients received adjuvant therapy after resection, and recurrence was not reported separately for these relapsed patients; those results limit the comparability of that study to our current study. 17
With a median follow‐up time of 41.2 months, median OS from date of surgery initiation was 82.4 months with 1‐, 2‐, and 5‐year survival rates of 91.4%, 81.4%, and 61.2%, respectively. OS in patients with relapse was shorter than in those without relapse, with 1‐, 2‐, and 5‐year survival rates of 86.8%, 67.5%, and 35.6%, respectively, for patients with relapse, and 95.2%, 92.4%, and 82.3%, respectively, for patients without relapse. Prior studies evaluating survival identified 5‐year survival rates ranging between 62% and 42%, respectively, for patients with pathological stage IB and IIIA disease in a clinical trial population of patients with completely resected tumor receiving adjuvant therapy, 18 , 19 and from 62.4% to 15.2%, respectively, in a retrospective examination of patients with pathologic stage IIA–IIIB disease receiving adjuvant care after surgical resection at a single institution. 20 However, while previous studies have been limited to clinical trial patients 7 , 19 , 21 or patients from a single institution, 11 our study provides real‐world results from a large sample of patients with NSCLC treated with adjuvant therapy after complete surgical resection in US community oncology clinics.
Our findings showed that among patients who relapsed after complete surgical resection and received treatment with adjuvant therapies, healthcare utilization of ED services and hospitalizations was significantly higher following relapse than before relapse. On average, patients had three times the number of ED visits after relapse than before relapse, and four times the hospitalizations. Furthermore, a higher proportion of patients had at least one recorded ED visit or hospitalization following relapse compared to before relapse. Buck et al. reported that 13.9% of patients with stage II–IIIA disease were hospitalized during adjuvant treatment. 17 However, this analysis did not examine the number of hospitalizations prior to relapse in a subset of patients with relapse and thus is not directly comparable to our study.
Strengths/limitations
The results of this study should be considered in the context of the strengths and limitations of the data source and study design. The iKM database used in our analysis includes a wealth of information about community‐based oncology practices in the United States, but the information in the iKM database is collected for clinical practice reasons and not research purposes, which has several implications for our findings. Reporting practices of individual physicians may differ and impede the standardization of data included in this study. The iKM EHR contains information on patients only when they are seen by US Oncology Network physicians. Services and procedures provided outside of the US Oncology Network (e.g. hospitalizations, surgeries, and radiation therapies) are not captured by the database, nor are drugs received by patients from pharmacies not affiliated with US Oncology Network practices. Our study was designed to assess only those patients with high risk of relapse after surgery and was limited to patients receiving adjuvant therapy after complete surgical resection, and thus we do not have line of sight on services and/or outcomes for patients who did not receive treatment. Therefore, the results of our analysis may not be generalized to the overall US patient population because community oncology practices in the US Oncology Network may be different from those not participating in the network and adhering to their evidence‐based practices and pathways. However, use of the iKM EHR data represents usual care in a large network of community oncology practices. Therefore, these data can be used to report real‐world findings that are more representative of typical patients with NSCLC.
To our knowledge, this is the first real‐world study conducted in the United States to assess clinical outcomes and healthcare utilization before and after relapse among patients with completely resected NSCLC and high risk of relapse receiving adjuvant therapy in the community oncology setting. The high risk of relapse among these patients, along with worse OS and the significant economic burden associated with relapse, jointly stresses the need for new therapeutic options for patients receiving adjuvant treatment for NSCLC.
CONCLUSIONS
In this real‐world analysis of patients with stage II–IIIB NSCLC receiving adjuvant therapy after complete resection, we have identified high relapse rates, reduced survival, and significantly increased healthcare resource use when relapse occurred. New treatment options to reduce relapse in patients with early‐stage NSCLC at high risk of relapse could reduce healthcare utilization and result in substantial cost savings. The high relapse and mortality rates, and the economic burden associated with relapse underscore the ongoing need for more effective treatments in this patient group.
DECLARATION OF FUNDING
This study was funded by Novartis Pharmaceuticals Corporation.
DECLARATION OF FINANCIAL AND OTHER INTERESTS
Dr Cai is an employee and stockholder of Novartis Pharmaceuticals Corporation. N. Fulcher and M. Boyd are employees of McKesson Life Sciences. Dr Spira has served in an advisory and/or consultant role for Amgen, Array BioPharma, AstraZeneca, AstraZeneca/MedImmune, Gritstone Oncology, Incyte, Jazz Pharmaceuticals, and Novartis Pharmaceuticals Corporation and also received research funding from AbbVie, ADCT, Amgen, Arch Therapeutics, Astellas Pharma, Astex Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, CytomX Therapeutics, Daiichi Sankyo, Gritstone, Ignyta, Incyte, Janssen Oncology, LAM Therapeutics, Loxo, Macrogenics, MedImmune, Mirati Therapeutics, Newlink Genetics, Novartis Pharmaceuticals Corporation, Plexxikon, Roche, Takeda, and Trovagene.
AUTHOR CONTRIBUTIONS
Dr. Cai, Ms. Fulcher, Mr. Boyd, and Dr. Spira contributed to the study design. Mr. Boyd contributed to the data acquisition. All authors contributed to the study concept, data analysis, and interpretation of the results, substantively contributed to and reviewed the manuscript, and gave final manuscript approval.
DATA AVAILABILITY
The health data used to support the findings of this study are restricted by the US Oncology Institutional Review Board to protect patient privacy. For this reason, data used to support the findings of this study have not been made available.
Supporting information
Supporting Information Table S1 Medications/conditions impacting the immune system
ACKNOWLEDGMENTS
No assistance in the preparation of this article is declared. Some of the data presented in this paper were presented at the International Association for the Study of Lung Cancer 2020 North America Conference on Lung Cancer, Virtual, October 16–17, 2020.
Cai B, Fulcher N, Boyd M, Spira A. Clinical outcomes and resource utilization after surgical resection with curative intent among patients with non‐small cell lung cancer treated with adjuvant therapies in a community oncology setting: A real‐world retrospective observational study. Thorac Cancer. 2021;12:2055–2064. 10.1111/1759-7714.14007
Funding information Novartis
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. SEER Cancer Statistics Review , 1975‐2017, National Cancer Institute, Bethesda, MD [Internet]. [updated April 15, 2020; cited September 17, 2020]. https://seer.cancer.gov/csr/1975_2017/
- 3. Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradbury P, Sivajohanathan D, Chan A, Kulkarni S, Ung Y, Ellis PM. Postoperative adjuvant systemic therapy in completely resected non‐small‐cell lung cancer: a systematic review. Clin Lung Cancer. 2017;18:259–73. e258. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Non‐Small Cell Lung Cancer, V1 (2020) [Internet]. [updated 2 October 2019; cited 20 March 2020]. https://merkelcell.org/wp-content/uploads/2017/10/MCC_v.2.2019-2.pdf
- 6. Scagliotti GV, Pastorino U, Vansteenkiste JF, Spaggiari L, Facciolo F, Orlowski TM, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non‐small‐cell lung cancer. J Clin Oncol. 2012;30:172–8. [DOI] [PubMed] [Google Scholar]
- 7. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–9. [DOI] [PubMed] [Google Scholar]
- 8. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. International adjuvant lung cancer trial collaborative group Cisplatin‐based adjuvant chemotherapy in patients with completely resected non‐small‐cell lung cancer. N Engl J Med. 2004;350:351–60. [DOI] [PubMed] [Google Scholar]
- 9. Pisters KM, Le Chevalier T. Adjuvant chemotherapy in completely resected non‐small‐cell lung cancer. J Clin Oncol. 2005;23:3270–8. [DOI] [PubMed] [Google Scholar]
- 10. Pisters KM, Vallières E, Crowley JJ, Franklin WA, Bunn PA Jr, Ginsberg RJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early‐stage non‐small‐cell lung cancer: southwest oncology group trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valdes M, Nicholas G, Goss GD, Wheatley‐Price P. Chemotherapy in recurrent advanced non‐small‐cell lung cancer after adjuvant chemotherapy. Curr Oncol. 2016;23:386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Joint Committee on Cancer . Lung. In: Greene FL, Page DL, Fleming ID, Amin MB, Edge S, Brookland RK, et al., editors. AJCC Cancer Staging Manual. New York: Springer; 2002. p. 167–77. [Google Scholar]
- 13. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- 14. Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams CD, Gajra A, Ganti AK, Kelley MJ. Use and impact of adjuvant chemotherapy in patients with resected non‐small cell lung cancer. Cancer. 2014;120:1939–47. [DOI] [PubMed] [Google Scholar]
- 16. Kreuter M, Vansteenkiste J, Fischer JR, Eberhardt WE, Zabeck H, Kollmeier J, et al. Three‐year follow‐up of a randomized phase II trial on refinement of early‐stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine (the TREAT study). J Thorac Oncol. 2016;11:85–93. [DOI] [PubMed] [Google Scholar]
- 17. Buck PO, Saverno KR, Miller PJ, Arondekar B, Walker MS. Treatment patterns and health resource utilization among patients diagnosed with early stage resected non‐small cell lung cancer at US community oncology practices. Clin Lung Cancer. 2015;16:486–95. [DOI] [PubMed] [Google Scholar]
- 18. Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I‐IIIA resectable non small‐cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. [DOI] [PubMed] [Google Scholar]
- 19. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles‐Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB‐IIIA non‐small‐cell lung cancer (adjuvant Navelbine international Trialist association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. [DOI] [PubMed] [Google Scholar]
- 20. Kameyama K, Takahashi M, Ohata K, Igai H, Yamashina A, Matsuoka T, et al. Evaluation of the new TNM staging system proposed by the International Association for the Study of Lung Cancer at a single institution. J Thorac Cardiovasc Surg. 2009;137:1180–4. [DOI] [PubMed] [Google Scholar]
- 21. Douillard JY, Tribodet H, Aubert D, Shepherd FA, Rosell R, Ding K, et al. Adjuvant cisplatin and vinorelbine for completely resected non‐small cell lung cancer: subgroup analysis of the lung adjuvant Cisplatin evaluation. J Thorac Oncol. 2010;5:220–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Medications/conditions impacting the immune system
Data Availability Statement
The health data used to support the findings of this study are restricted by the US Oncology Institutional Review Board to protect patient privacy. For this reason, data used to support the findings of this study have not been made available.


