Abstract
Background
Adenocarcinoma is the most common type of lung cancer and most adenocarcinomas have heterogeneous subtypes. Acinar‐predominant adenocarcinoma is the most common. This study aimed to identify the prognostic impact of other mixed histological subtypes in acinar‐predominant lung adenocarcinoma.
Methods
The medical records of patients with pathological stage IA acinar‐predominant lung adenocarcinoma between January 2010 and April 2016 were reviewed. The patients were divided into two groups according to the proportion of the lepidic subtype, with a cutoff value of 20%, and prognostic factors were analyzed.
Results
A total of 215 patients with stage IA acinar‐predominant adenocarcinoma were reviewed. The 20% or more lepidic subtype group had a low value of SUVmax (p = 0.001), good differentiation (p < 0.001) and a low incidence of the solid histological subtype (p = 0.016). Recurrence was significantly lower in the 20% or more lepidic subtype group (p = 0.008). The disease‐free survival (p = 0.007) and overall survival (p = 0.046) were significantly different between the two groups. Multivariate analysis showed that lymphovascular invasion (p = 0.006) and no or less than 20% lepidic subtype (p = 0.036) were significant prognostic factors for disease‐free survival.
Conclusions
The lepidic proportion may be useful to predict recurrence in acinar‐predominant stage IA lung adenocarcinoma.
Keywords: cancer risk factors, lung cancer, surgery, surgical oncology
Acinar‐predominant lung adenocarcinoma is the most common histological subtype and is usually combined with other histological subtypes. It has the widest range in terms of prognosis. In this study, we investigated the prognostic role of lepidic proportion in acinar predominant lung adenocarcinoma.
INTRODUCTION
Adenocarcinoma is the most common histological type in lung cancer and most adenocarcinomas show heterogeneous patterns and variety in terms of histological subtypes and their proportion. 1 Although surgical resection is the most effective treatment for early lung cancer, 2 approximately 30% of patients experience recurrence. For the management of adenocarcinoma, a new subtype for the histological classification of invasive lung adenocarcinoma was proposed by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS) in 2011. 3 This classification was based on the predominant histological subtype and divided into three prognostic groups: low grade (lepidic predominant), intermediate grade (acinar or papillary predominant) and high grade (micropapillary or solid predominant). 4 Numerous studies have tried to demonstrate differences in prognosis according to the predominant histological subtype. It is well known that the micropapillary (MPP) and solid‐predominant histological subtypes have worse prognoses than the other subtypes, although curative resection and mediastinal lymph node dissection are carried out in stage I lung adenocarcinoma. 5 , 6 In contrast, lepidic‐predominant subtype indicates good prognosis. 7 However, the most common of the predominant subtypes are acinar lung adenocarcinoma and the incidence rate of the MPP or solid‐predominant subtype is lower in early lung adenocarcinoma. 8 Furthermore, multiple other subtypes are usually mixed in most acinar‐predominant adenocarcinomas. We wondered about prognosis according to the presence or proportion of other histological subtypes in stage IA acinar‐predominant lung adenocarcinoma. We hypothesized that the prognosis may be different according to the proportion of other mixed subtypes and we investigated the prognostic factors in stage IA acinar‐predominant lung adenocarcinoma.
METHODS
We reviewed the electronic medical records (EMRs) of patients who underwent curative resection for lung cancer from January 2010 to April 2016. Written informed consent from the patients was waived because the study was a retrospective analysis of data. We classified the pathological stage of the patients according to the eighth edition of the tumor‐node‐metastasis (TNM) classification. Patients with pathological stage IA were included. Among these patients, those with adenocarcinoma in situ, minimally invasive adenocarcinoma, multifocal ground glass‐opacity (GGO), neoadjuvant chemotherapy and missing medical records were excluded, and patients with incomplete resection and wedge resection were also excluded. We classified the patients with acinar‐predominant stage IA lung adenocarcinoma on the basis of the pathological report, and 215 patients with pathological stage IA acinar‐predominant invasive adenocarcinoma were analyzed.
Data collection
The patient characteristics including age, sex, underlying disease, other previous malignancies and surgical procedures, were retrieved from the EMRs. Preoperative assessments were chest computed tomography (CT), positron emission tomography‐CT (PET‐CT), brain magnetic resonance imaging (MRI), bone scanning and bronchoscopy. Demographic and clinical data and pathological data after curative resection were collected for analysis.
The patients were classified into two groups according to the proportion of the lepidic subtype, with a cutoff value of 20%, and variables were compared between the two groups, including the disease‐free survival (DFS) and overall survival (OS). A new grading system for lung adenocarcinoma has recently been proposed. 9 A cutoff value of 20% of high‐grade patterns showed worse prognosis. We applied this cutoff value in our study for the proportion of the lepidic subtype.
All patients were followed until recurrence and death or loss of follow‐up (F/U). OS was defined as the interval from the date of surgery to the date of death. Recurrence was defined as local or extrathoracic metastasis based on clinical and pathological evidence, and the DFS was obtained.
Statistical analysis
All statistical analyses were carried out using SPSS version 18 (SPSS Inc.). Continuous variables were compared using the Mann–Whitney U test, and categorical variables were compared using the chi‐square test and Fisher's exact test.
DFS and OS were estimated by the Kaplan–Meier method and log‐rank test. Prognostic factors associated with recurrence and survival were determined using the Cox proportional hazards model after checking the proportionality assumption. Variables with p‐values less than 0.05 was considered statistically significant.
RESULTS
The baseline characteristics of the patients are shown in Table 1. The median age was 62 years (range 25–82). The patients included 85 males and 130 females. GGO features were identified in 118 patients on preoperative CT. The median values of carcinoembryonic antigen (CEA) and maximum standardized uptake value (SUVmax) were 1.4 (range 0.22–14.19) and 2.4 (range 0–17.6), respectively. For the surgical procedure, lobectomy was performed in 198 patients (92.1%).
TABLE 1.
Baseline patient characteristics
| Characteristic | Total (n = 215) |
|---|---|
| Median (range) or n (%) | |
| Age | 62 (25–82) |
| Sex | Male: 85 (41) |
| Smoking | 58 (28.2) |
| CEA | 1.4 (0.22–14.19) |
| SUVmax | 2.4 (0–17.6) |
| GGO | 118 (54.9) |
| Procedure | |
| Lobectomy | 198 (92.1) |
| Segmentectomy | 17 (7.9) |
| VATS | 180 (83.7) |
Note: Data are presented as the median (minimum‐maximum) or frequencies and percentages as appropriate.
Abbreviations: CEA, carcinoembryonic antigen; GGO, ground‐glass opacity; SUVmax, maximum standardized uptake value; VATS, video‐assisted thoracoscopic surgery.
According to the pathological data (Table 2), the median tumor size was 1.8 cm (range 0.3–3). Moderate differentiation was the most common (59.1%). A total of 37 patients had the pure acinar histological subtype (17%). Others showed multiple combinations of subtypes and proportions. For the analysis of other mixed subtypes, lepidic components were the most common (71.6%). A total of 50 patients had the mixed papillary subtype (23.3%). Less than 15% of patients had the MPP and solid subtypes. Lymphovascular invasion (LVI) was identified in 60 patients (27.9%). The median number of dissected lymph nodes (LNs) was 11 (range 0–37). If the tumor was a pure GGO or a GGO‐dominant lesion located in the central portion, segmentectomy or lobectomy without mediastinal LN evaluation was performed according to the surgeon's decision based on the preoperative imaging study. Mediastinal LN dissection (MLND) was performed in 179 patients (83.3%) according to the guidelines. 2 For the pathological stage, IA2 was the most common (53%). After the operation, adjuvant chemotherapy was conducted in three patients.
TABLE 2.
Pathological characteristics of stage IA acinar‐predominant subtype adenocarcinoma
| Characteristic | Total (n = 215) |
|---|---|
| Median (range) or n (%) | |
| Size | 1.8 (0.3–3) |
| Differentiation | |
| Well | 76 (35.3) |
| Moderate | 127 (59.1) |
| Poor | 12 (5.6) |
| Other histological subtype | |
| Papillary | 50 (23.3) |
| Lepidic | 154 (71.6). |
| MPP | 30 (14) |
| Solid | 24 (11.2) |
| Margin | 3.5 (0.1–10) |
| LVI | 60 (27.9) |
| Number of dissected LNs | 11 (0–37) |
| MLND | 179 (83.3) |
| pStage IA1 | 26 (12.1) |
| pStage IA2 | 114 (53) |
| pStage IA3 | 75 (34.9) |
| Adjuvant treatment | 3 (1.4) |
| Recurrence | 28 (13) |
| Death | 21 (9.8) |
Note: Data are presented as the medians (minimum‐maximum) or frequencies and percentages as appropriate.
Abbreviations: LN, lymph node; LVI, lymphovascular invasion; MLND, mediastinal lymph node dissection; MPP, micropapillary.
The patients were categorized as lepidic, with a cutoff value of 20% (Table 3). Age, sex and the value of CEA were not different between the two groups. However, the value of SUVmax was significantly higher in the less than 20% lepidic subtype group (p = 0.001). GGO lesions were also lower in the less than 20% lepidic subtype group (p = 0.013). Tumor size was not different between the two groups (p = 0.553). The presence of a well‐differentiated adenocarcinoma was significantly higher in the 20% or more lepidic subtype group (p < 0.001). For the mixed histological subtype, the papillary and solid subtypes were more common in the less than 20% lepidic subtype group (p = 0.023, p = 0.016). However, no significant difference (p = 0.172) in the presence of the MPP subtype or LVI was observed between the two groups (p = 1.000).
TABLE 3.
Clinical and pathological variables according to the proportion of lepidic subtype in stage IA acinar‐predominant lung adenocarcinoma
| Variables | Lepidic ≥20% | Lepidic <20% | p‐value |
|---|---|---|---|
| N = 105 | N = 110 | ||
| Age | 63 (39–82) | 64 (25–82) | 0.567 |
| Sex (male) | 36 (34.3) | 49 (44.5) | 0.128 |
| Smoking | 22 (21) | 36 (32.7) | 0.065 |
| CEA | 1.4 (0.22–9) | 1.4 (0.5–14.19) | 0.076 |
| SUVmax | 2 (0–11.6) | 2.8 (0–17.60) | 0.001 |
| GGO | 67 (63.8) | 51 (46.4) | 0.013 |
| Lobectomy | 97 (92.4) | 101 (91.8) | 1.000 |
| VATS | 87 (82.9) | 93 (84.5) | 0.854 |
| Size | 1.8 (0.6–3) | 1.9 (0.3–3) | 0.553 |
| Well differentiated | 52 (49.5) | 24 (21.8) | <0.001 |
| Other mixed subtype | |||
| Papillary | 17 (16.2) | 33 (30) | 0.023 |
| MPP | 11 (10.5) | 19 (17.3) | 0.172 |
| Solid | 6 (5.7) | 18 (16.4) | 0.016 |
| Margin | 3.5 (0.1–7) | 3.5 (0.5–10) | 0.948 |
| Number of dissected LNs | 11 (0–32) | 11 (0–37) | 0.681 |
| LVI | 29 (27.6) | 31 (28.2) | 1.000 |
| Recurrence | 7 (6.7) | 21 (19.1) | 0.008 |
Note: Data are presented as the medians (minimum‐maximum) or frequencies and percentages as appropriate.
Abbreviations: CEA, carcinoembryonic antigen; GGO, ground‐glass opacity; LVI, lymphovascular invasion; SUVmax, maximum standardized uptake value; VATS, video‐assisted thoracic surgery.
A total of 28 patients had recurrence after operation (13%) and 23 patients received adjuvant treatment.
The sites for recurrence were lung (39.3%), mediastinum (17.9%), pleura (21.4%) and extrathoracic area (21.4%). Chemoradiation treatment was conducted in seven patients. Systemic chemotherapy was conducted in 10 patients and tyrosine kinase inhibitor was initiated in six patients.
A total of 21 patients died during the F/U period after the operation. The cause of death was cancer progression in 10 patients.
Significant differences in DFS (Figure 1(a)) and OS (Figure 1(b)) were identified between the two groups (p = 0.007, p = 0.047). A lower proportion of the lepidic subtype indicated a worse prognosis for DFS and OS in acinar‐predominant stage IA adenocarcinomas (median DFS: 58 months, range 2–125, median OS 62 months, range 2–125).
FIGURE 1.
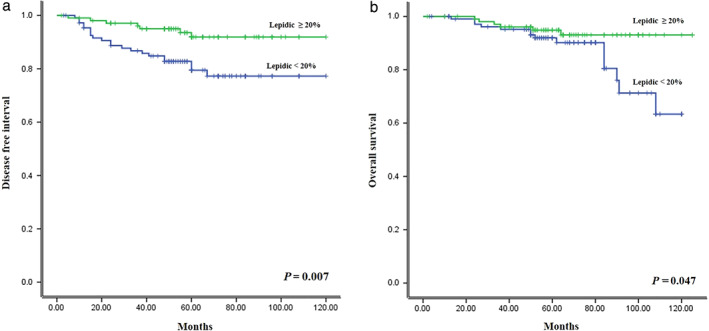
Survival curves for (a) disease free survival and (b) overall survival for stage IA acinar‐predominant lung adenocarcinoma according to the proportion of lepidic subtype
According to the Cox regression analysis of prognostic factors for DFS, SUVmax (p = 0.018), CEA (p = 0.044), moderate differentiation (p = 0.002), solid subtype (p = 0.030), less than 20% lepidic subtype (p = 0.010) and LVI (p = 0.001) were identified as prognostic factors for recurrence. In the multivariate analysis, moderate differentiation (p = 0.01), less than 20% lepidic subtype (p = 0.036) and LVI (p = 0.006) were independent poor prognostic factors for DFS (Table 4).
TABLE 4.
Cox proportional analysis for disease‐free survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | |
| SUVmax | 1.118 | 1.020–1.226 | 0.018 | 0.974 | 0.857–1.106 | 0.682 |
| CEA | 1.151 | 1.004–1.321 | 0.044 | 1.086 | 0.928–1.270 | 0.304 |
| Moderate differentiation | 10.037 | 2.382–42.299 | 0.002 | 6.801 | 1.597–28.966 | 0.010 |
| Solid subtype | 2.737 | 1.105–6.776 | 0.030 | 1.533 | 0.567–4.147 | 0.400 |
| Lepidic <20% | 3.060 | 1.300–7.200 | 0.010 | 2.621 | 1.065–6.452 | 0.036 |
| LVI | 3.568 | 1.691–7.528 | 0.001 | 3.244 | 1.409–7.470 | 0.006 |
Abbreviations: CEA, carcinoembryonic antigen; LVI, lymphovascular invasion; SUVmax, maximum standardized uptake value.
For overall survival, LVI was the only prognostic factor in multivariate analysis (p = 0.048) (Table 5).
TABLE 5.
Cox proportional analysis for overall survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | |
| SUVmax | 1.150 | 1.036–1.277 | 0.009 | 1.064 | 0.943–1.202 | 0.314 |
| Lepidic <20% | 2.521 | 0.976–6.513 | 0.056 | 2.430 | 0.903–6.536 | 0.079 |
| LVI | 2.920 | 1.233–6.915 | 0.015 | 2.624 | 1.010–6.820 | 0.048 |
Abbreviations: CEA, carcinoembryonic antigen; LVI, lymphovascular invasion; SUVmax, maximum standardized uptake value.
DISCUSSION
Adenocarcinoma is the most common histological type in lung cancer, and invasive adenocarcinoma was classified based on the predominant histological pattern in 2011 by a multidisciplinary group. 3 The 2015 WHO guidelines adopted this classification, and lung adenocarcinoma was separated into three prognostic groups. A lepidic‐predominant adenocarcinoma has a low‐grade prognosis. The acinar or papillary predominant subtype has an intermediate prognosis, and it is well known that the solid or MPP subtype has the worst prognosis (high grade). 10
In stage IA lung adenocarcinoma, the disease is curable and the best candidate for surgery; in terms of a favorable prognosis, the lepidic subtype shows a good clinical result, and this tendency is more pronounced in the early stage of lung adenocarcinoma. The incidence of the lepidic‐predominant subtype in the early stage is higher than that in the advanced stage due to less invasiveness. However, the acinar‐predominant subtype is the most common histological subtype throughout the entire course of lung adenocarcinoma, accounting for more than 50% of lung adenocarcinomas. 8 The most interesting thing is that the acinar‐predominant subtype is more heterogeneous than any other subtypes. Generally, invasive lung adenocarcinoma is histologically heterogeneous, but the lepidic‐predominant subtype with the solid or MPP mixed subtype is rare, and the solid‐predominant subtype is not usually mixed with the lepidic subtype. 11 However, the acinar‐predominant subtype is histologically heterogeneous, containing all other subtypes, and has a wide range of distributions and proportions. As a result, acinar‐predominant adenocarcinoma has the widest range in terms of prognosis. 12 , 13 However, there have been few studies regarding prognosis in acinar‐predominant lung adenocarcinoma. Ito et al. investigated the intermediate group of adenocarcinomas. 8 They classified the intermediate group of adenocarcinomas according to the second‐most predominant histological subtype. They found that the intermediate group of adenocarcinomas in which the second most predominant subtype was the lepidic subtype had a favorable prognosis. The results of this study are similar to our study. However, the different point is that the proportion is more important than second predominant in our study. We also investigated the prognosis according to the second‐predominant subtype and the lepidic subtype was identified as the second most predominant subtype in 120 patients, but there was no significance difference in DFS in multivariate analysis. Tsubokawa et al. demonstrated that vascular invasion is an independent prognostic factor in the intermediate group of adenocarcinomas. 14 Our study indicated that LVI is the valuable prognostic factor. LVI is a significant prognostic factor in lung cancer, and the solid‐predominant subtype is more associated with LVI, reflecting a worse prognosis. 15 However, the relationship between LVI and acinar‐predominant adenocarcinomas is not well known.
Grading classification with a cutoff value, such as TNM stage, is a classic guideline for disease in the clinical field of medicine. However, there is no grading system for invasive lung adenocarcinoma that uses only the predominant pattern. Furthermore, the current grading system using the predominant pattern is sometimes conflicting because of the wide spectrum of heterogeneous histological patterns and proportions. A new grading system for lung adenocarcinoma has been recently proposed. 9 In this system, the unique difference from the previous classification is that each grade was separated with a cutoff value of 20% of high‐grade patterns showing a clear difference in terms of prognosis, and we applied this cutoff value in our study for the proportion of the lepidic subtype. There were significant differences in SUVmax, GGO and differentiation. For other combined histological subtypes, papillary and solid patterns are more common in the less than 20% lepidic subtype group. However, there was no significant difference in tumor size or LVI between the two groups.
There are well established prognoses between genetic features and high‐grade patterns. However, there is little evidence of an association between genetic factors and intermediate lung adenocarcinoma. Kim et al. investigated prognostic factors in intermediate lung adenocarcinoma. 16 They found that STAS and PD‐L1 expression indicated a worse prognosis in intermediate lung adenocarcinoma.
In our study, the acinar‐predominant subtype with the most lepidic subtype (≥20%) had a favorable prognosis according to the Kaplan–Meier method for DFS and OS (p = 0.007, p = 0.047). Multivariate analysis revealed that less than 20% lepidic subtype (p = 0.036) and LVI (p = 0.006) were independent prognostic factors for DFS. However, the lepidic proportion did not reach significance for OS in univariate analysis (p = 0.056). Despite of this result, we believed that lepidic proportion is a valuable factor in acinar predominant adenocarcinoma. First, this might be a consequence of the small sample size and large proportion of noncancer‐related deaths. A total of 21 patients died, including 11 patients with other causes of death (52.4%). Second, recurrence is more common than death in stage IA lung cancer and there are many adjuvant treatments to increase survival for the patients with recurrence after curative resection so prognostic factor for recurrence is also important for appropriate treatment in patients with recurrence.
We also investigated using the second most predominant subtype. The lepidic subtype was identified as the second most predominant subtype in 120 patients, but this variable did not show significance for DFS or OS in univariate and multivariate analyses. We believe that grading classification using a cutoff value is a more valuable prognostic factor than using a predominant pattern.
There were several limitations in this study. First, this study was conducted with a retrospective, nonrandomized design with a relatively small sample size for analyzing DFS and OS. Second, this study included many noncancer‐related deaths so we were unable to determine that lepidic proportion is associated with overall survival. Third, we included only anatomical lung resection in stage IA adenocarcinoma. MLND was not performed in some patients according to the complete resection guidelines. However, MLND could be omitted for GGO‐dominant lesions in early‐stage lung cancer. Finally, this study was not from multiple centers; thus, selection bias may be inevitable.
In conclusion, acinar‐predominant adenocarcinoma has a variety regarding heterogeneity and proportions. Prognostic factor analysis may be difficult. However, the lepidic proportion in acinar‐predominant adenocarcinoma may be a valuable prognostic factor. We believe that large‐scale data analysis will be required to demonstrate this issue.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Jeon HW, Kim Y‐D, Sim SB, Moon MH. Prognostic impact according to the proportion of the lepidic subtype in stage IA acinar‐predominant lung adenocarcinoma. Thorac Cancer. 2021;12:2072–2077. 10.1111/1759-7714.14013
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Tsao MS, Marguet S, Le Teuff G, Lantuejoul S, Shepherd FA, Seymour L, et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol. 2015;33:3439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rami‐Porta R, Wittekind C, Goldstraw P, International Association for the Study of Lung Cancer (IASLC) Staging Committee . Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49:25–33. [DOI] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, Rusch VW, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–62. [DOI] [PubMed] [Google Scholar]
- 5. Park JK, Kim JJ, Moon SW, Lee KY. Lymph node involvement according to lung adenocarcinoma subtypes: lymph node involvement is influenced by lung adenocarcinoma subtypes. J Thorac Dis. 2017;9:3903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015;33:2877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol. 2013;8:612–8. [DOI] [PubMed] [Google Scholar]
- 8. Ito M, Miyata Y, Yoshiya T, Tsutani Y, Mimura T, Murakami S, et al. Second predominant subtype predicts outcomes of intermediate‐malignant invasive lung adenocarcinoma. Eur J Cardiothorac Surg. 2017;51:218–22. [DOI] [PubMed] [Google Scholar]
- 9. Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the study of lung cancer pathology committee. J Thorac Oncol. 2020;15:1599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am. 2016;25:447–68. [DOI] [PubMed] [Google Scholar]
- 11. Zhao ZR, Xi SY, Li W, Situ DR, Chen KM, Yang H, et al. Prognostic impact of pattern‐based grading system by the new IASLC/ATS/ERS classification in Asian patients with stage I lung adenocarcinoma. Lung Cancer. 2015;90:604–9. [DOI] [PubMed] [Google Scholar]
- 12. Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64. [DOI] [PubMed] [Google Scholar]
- 13. Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. [DOI] [PubMed] [Google Scholar]
- 14. Tsubokawa M, Mimae Y, Miyata S, Sasada S, Yoshiya T, Kushitani K, et al. Prognostic significance of vascular invasion in intermediate grade subtype of lung adenocarcinoma. Jpn J Clin Oncol. 2016;46:1015–21. [DOI] [PubMed] [Google Scholar]
- 15. Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Pääkkö P, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology. 2017;71:425–36. [DOI] [PubMed] [Google Scholar]
- 16. Kim M, Chung YS, Kim KA, Shim HS. Prognostic factors of acinar‐ or papillary‐predominant adenocarcinoma of the lung. Lung Cancer. 2019;137:129–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


