Abstract
Background
Abdominal lymph node metastasis (ALNM) is common in patients with metastatic non‐small‐cell lung cancer (NSCLC). However, its mechanism of spread remains to be elucidated. We investigated whether thoracic duct has the role as a pathway for ALNM in NSCLC using clinical data.
Methods
We classified ALNM into subgroups by their location and evaluated its prevalence and association with clinical characteristics in 892 patients with metastatic NSCLC. The abdominal lymph nodes were classified into direct or indirect groups depending on whether they drain directly into the trunk (intestinal trunk or lumbar trunks) connected to the cisterna chyli.
Results
One hundred‐five patients (11.8%) had ALNM. The paraaortic lymph node was most commonly involved, followed by the aortocaval, left gastric, paracaval, and celiac lymph nodes. After grouping the patients by location of ALNM, 56 patients (53.3%) with ALNM were in the “direct only” group, only seven patients (6.7%) were in the “indirect only” group, and 42 patients (40.0%) were in “both” groups. In patients whose intrathoracic lesions were limited to the right thorax, there was a significantly lower prevalence of ALNM (3.4% vs. 14.3%, p < 0.001). On multivariate logistic regression analysis of clinical variables, higher N category was associated with increased risk of ALNM.
Conclusions
This study suggests that the thoracic duct is one of the potential routes of lymphatic spread to the abdominal lymph nodes. Clinicians should assess for the presence of ALNM during staging work‐up and follow‐up for NSCLC patients with intrathoracic lesion in left thorax and with high N category.
Keywords: abdominal lymph nodelymphatic spreadmetastasisnon‐small‐cell lung cancerthoracic duct
ALNM was more common in the direct than indirect lymph nodes. ALNM was more common in patients with left thorax lesion compared to those with right thorax lesion only. ALNM risk was increased for patients with higher N category. These results indicate that ALNM occurs by lymphatic spread through the thoracic duct in patients with metastatic NSCLC.

INTRODUCTION
Cancer metastasis is the main cause of cancer‐related death. Unfortunately, ~40% of patients with non‐small‐cell lung cancer (NSCLC) present with metastasis at the time of diagnosis. 1 The prevalence, mechanism of metastasis, and clinical significance have been extensively investigated in bone, lung, brain, liver, and the adrenal glands, which are each common metastatic sites of lung cancer. 2 , 3 , 4 , 5 , 6 , 7 However, although abdominal lymph node metastasis (ALNM) is also relatively common, it remains comparatively poorly understood.
Metastasis develops in an unpredictable manner, but the general mechanisms for metastasis have been extensively investigated. 8 , 9 The spread of lung cancer via the lymph is a topic of major clinical interest. Recently, a finding of metastasis in any lymph node other than a regional node was incorporated as an element of the M descriptor of the eighth TNM staging system. 10 However, the precise mechanism of lymphatogenous metastasis remains elusive. 11
Our current understanding of the mechanism of ALNM is largely based on postmortem analyses or hypothetical models. 6 , 7 , 8 , 9 The presence of tributaries from the thoracic duct and their relationship with the intrathoracic, celiac, and paraaortic lymph nodes have been suggested as possibly related, providing the opportunity for carriage of malignant cells through the thoracic duct. Indeed, malignant cells that have accumulated in the thoracic duct can increase intralymphatic pressure and change the direction of lymph flow. 5 , 10 The retrograde spread of malignant cells through lymph nodes was previously identified in the axillary lymph nodes of patients with lung cancer and the abdominal lymph nodes of patients with ovarian cancer. 12 , 13 However, no prior work has investigated whether ALNM occurs through a retrograde pattern of lymphatic spread the thoracic duct in cancer patients.
In this work, we investigated whether thoracic duct have the role as a pathway for abdominal lymph node metastasis in NSCLC using clinical data.
METHODS
Study population
A total of 974 patients with histologically confirmed NSCLC who were diagnosed between January 2005 and December 2018 at Inha University Hospital (Incheon, Republic of Korea) were initially considered for this study (Figure S1). All patients were diagnosed with stage IV at the time of diagnosis. Patients who did not undergo positron emission tomography (PET) (n = 28) or who did not undergo brain imaging (n = 23) or a whole‐body bone scan (n = 5) were excluded. In addition, patients who were found to also have malignancy below the diaphragm (n = 16) were excluded. Patients who had a history of thoracic or abdominal surgery including lymph node dissection (n = 10) were also excluded. All patients underwent a standard work‐up at the hospital. Information such as sex, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status, histology, epidermal growth factor receptor (EGFR) mutation status, T category, N category, and organs of metastasis were analyzed. The stage of all patients was estimated according to the eighth edition of the TNM classification system. 10 All information was collected prospectively from the electronic medical records of the Lung Cancer Cohort of Inha University Hospital. 14 This study was approved by the Institutional Review Board of Inha University Hospital, which waived the requirement of obtaining written informed consent from patients.
Identification of abdominal lymph node metastasis
ALNM was identified based on 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (FDG‐PET/CT) scan results. All patients fasted for a minimum of 6 hours, and fasting blood glucose levels were <150 mg/dL before the FDG‐PET/CT scan. PET/CT scans were performed on a PET/CT scanner (Discovery PET/CT 690 and a 64‐slice CT, GE Healthcare) and a Biograph Duo (Siemens Medical Solutions USA, Inc.) Sixty minutes after the injection of FDG, whole‐body emission scans were acquired using a PET camera. The CT attenuation correction acquisition parameters for the GE scanner were as follows: voltage, 120 kV; tube current, 100–200 mA Auto mA: ASiR; and slice thickness, 3.75 mm. The parameter settings for the Siemens device were: voltage, 130 kV; CARE Dose 4D mA tube current; and slice thickness, 5.00 mm. PET data were acquired using 2 min/bed position with a 128 × 128 matrix. Images were reconstructed using an iterative reconstruction algorithm. All PET images were corrected for attenuation using the acquired CT data.
All FDG‐PET/CT images for initial staging were reviewed by two experienced nuclear medicine physicians (I.Y.H. and M.L.) who were blinded and did not have knowledge of either the clinical data or any other imaging results. The presence of abnormal FDG uptake was indicated when accumulation of the radiotracer moderately‐to‐markedly increased relative to the expected uptake in normal structures or surrounding tissue, with the exclusion of physiologic bowel and urinary activity. The classification of lymph nodes on PET/CT images as cancer‐positive was based on the presence of focally increased FDG uptake on the PET images in a location that corresponded to the lymph node chains on the CT images. 15 All lymph nodes identified on the CT portion of PET/CT were also recorded.
Classification of metastatic abdominal lymph node and laterality of intrathoracic lesion
The metastatic abdominal lymph nodes were categorized by their location as followings: paraaortic, aortocaval, left gastric, paracaval, celiac, retroaortic, superior mesenteric, retrocaval, hepatic, mesenteric, common iliac, external iliac, internal iliac, peripancreatic, pericholedochal, splenic hilar, and renal hilar lymph nodes. 16 To investigate pattern of ALNM, the abdominal lymph nodes were classified into “direct node” or “indirect node” depending on whether they are drained directly into the trunk (intestinal trunk or lumbar trunks) connected to the cisterna chyli. Patients with ALNM were classified into “direct only” (patient whose ALNM in only direct node), “indirect only” (patient whose ALNM in only indirect node), or “both” (patient whose ALNM in both direct and indirect node) groups by location of ALNM.
To evaluate the potential association of ALNM with laterality of the intrathoracic lesion, patients were categorized by location of intrathoracic lesion as “right thorax only” (patient whose intrathoracic lesion in only right thorax), or “left thorax” (patient whose intrathoracic lesion in the left thorax or both thorax). To exclude the possibility that ALNM has occurred from the abdominal organ harboring metastasis, we also performed a subgroup analysis among patients who had no metastasis in abdominal organ.
Statistical analysis
The association between ALNM and clinical variables and the association between ALNM and laterality of the intrathoracic lesion were assessed using χ2 tests. The effect of ALNM on survival was estimated by the Kaplan–Meier method and log‐rank testing. A binary logistic regression analysis was also performed to identify the association of ALNM with clinical variables along with odds ratios (ORs) and 95% confidence intervals (CIs). Variables that were found to have a p value of 0.1 or less in univariate analysis were included in a multivariate logistic regression. All statistical tests were two sided, and a p value <0.05 was considered to be statistically significant. Analyses were performed using a statistical software package (SPSS version 19.0, SPSS).
RESULTS
Patient characteristics
The median age of the 892 patients with metastatic NSCLC at time of diagnosis was 69 years (range, 34–96). Of these, 628 patients (70.4%) had adenocarcinoma and 185 (20.8%) had squamous cell carcinoma. By organ of metastasis, bone was most common (388 patients, 43.5%), followed by lung (326 patients, 36.5%), brain (238 patients, 26.7%), adrenal gland (120 patients, 13.5%), ALNM (105 patients, 11.8%), and liver (105 patients, 11.8%) (Table 1). Of the patients with ALNM, 12 patients (11.4%) had ALNM as the only distant metastasis. Forty‐two patients (40.0%) had ALNM in one region; 20 patients (19.0%) in two regions; 13 patients (12.4%) in three regions; 13 patients (12.4%) in four regions; 17 patients (16.2%) in five or more regions. The distribution of sex, smoking history, ECOG performance status, histology, EGFR activating mutation, and T category were not significantly different between patients with and without ALMN (Table 2). However, ALNM was common in patients over 69 years of age or among those with high N category (p = 0.019 and p < 0.001, respectively). Patients with ALNM had significantly worse overall survival than those without ALNM (median survival time: 6.8 months vs. 12.1 months, log‐rank p < 0.001) (Figure S2).
TABLE 1.
Organ of metastasis in patients with non‐small‐cell lung cancer
| Patient, n (%) | |
|---|---|
| Bone | |
| Yes | 388 (43.5) |
| No | 504 (56.5) |
| Lung | |
| Yes | 326 (36.5) |
| No | 566 (63.5) |
| Brain | |
| Yes | 238 (26.7) |
| No | 654 (73.3) |
| Adrenal gland | |
| Yes | 120 (13.5) |
| No | 772 (86.5) |
| Liver | |
| Yes | 105 (11.8) |
| No | 787 (88.2) |
| Abdominal lymph node | |
| Yes | 105 (11.8) |
| No | 787 (88.2) |
| Others | |
| Yes | 99 (11.1) |
| No | 793 (88.9) |
TABLE 2.
Characteristics of patients with metastatic non‐small‐cell lung cancer by abdominal lymph node metastasis
| Abdominal lymph node metastasis | |||
|---|---|---|---|
| Variables | Yes (n = 105) | No (n = 787) | p value |
| Age | |||
| >69 | 40 (38.1) | 396 (50.3) | 0.019 |
| ≤69 | 65 (61.9) | 391 (49.7) | |
| Gender | |||
| Male | 74 (70.5) | 498 (63.3) | 0.149 |
| Female | 31 (29.5) | 289 (36.7) | |
| Smoking history | |||
| Ever | 73 (70.2) | 524 (67.4) | 0.573 |
| Never | 31 (29.8) | 253 (32.6) | |
| ECOG PS | |||
| 0–1 | 58 (56.3) | 484 (62.1) | 0.261 |
| ≥2 | 45 (43.7) | 296 (37.9) | |
| Histology | |||
| SQC | 16 (15.2) | 169 (21.5) | 0.250 |
| ADC | 77 (73.3) | 551 (70.0) | |
| Others | 12 (11.4) | 67 (8.5) | |
| EGFR mutation | |||
| Positive | 28 (26.7) | 255 (32.4) | 0.236 |
| Negative | 77 (73.3) | 532 (67.6) | |
| T category | |||
| Tx | 2 (1.9) | 15 (1.9) | 0.136 |
| T1 | 7 (6.7) | 38 (4.8) | |
| T2 | 13 (12.4) | 136 (17.3) | |
| T3 | 15 (14.3) | 177 (22.5) | |
| T4 | 68 (64.8) | 421 (53.5) | |
| N category | |||
| N0 | 12 (11.4) | 214 (27.2) | <0.001 |
| N1 | 3 (2.9) | 78 (9.9) | |
| N2 | 14 (13.3) | 176 (22.4) | |
| N3 | 76 (72.4) | 319 (40.5) | |
Abbreviations: ADC, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; SQC, squamous cell carcinoma.
Distribution of abdominal lymph node metastasis
Involvement of the paraaortic lymph node (54 patients, 51.4%) was most commonly observed, followed by aortocaval (46 patients, 43.8%), left gastric (28 patients, 26.7%), paracaval (27 patients, 25.7%), and celiac lymph nodes (27 patients, 25.7%) (Table 3 and Figure 1). By abdominal lymph node grouping, 56 patients (53.3%) with ALNM were in the “direct only” group; only seven patients (6.7%) were in the “indirect only” group; 42 patients (40.0%) were in the “both” group (Table 4). All seven patients of the “indirect only” group had solitary ALNM, and five of them had ALNM in the left gastric node. When analyzing patients with ALNM who have not metastasis in the abdominal organ (n = 57), 28 patients (49.1%) were in the “direct only” group; only three patients (5.3%) were in the “indirect only” group; and 26 patients (45.6%) were in the “both” group.
TABLE 3.
The distribution of metastatic lymph nodes in non‐small‐cell lung cancer patients with abdominal lymph node metastasis
| Location | No. of patients (%) |
|---|---|
| Direct nodes | |
| Paraaortic | 54 (51.4) |
| Aortocaval | 46 (43.8) |
| Paracaval | 27 (25.7) |
| Celiac | 27 (25.7) |
| Retroaortic | 17 (16.2) |
| Superior mesenteric | 15 (14.3) |
| Retrocaval | 13 (12.4) |
| Indirect nodes | |
| Left gastric | 28 (26.7) |
| Hepatic | 10 (9.5) |
| Common iliac | 9 (8.6) |
| Mesenteric | 7 (6.7) |
| External iliac | 6 (5.7) |
| Internal iliac | 5 (4.8) |
| Peripancreatic | 5 (4.8) |
| Pericholedochal | 1 (1.0) |
| Splenic hilar | 1 (1.0) |
| Renal hilar | 1 (1.0) |
FIGURE 1.
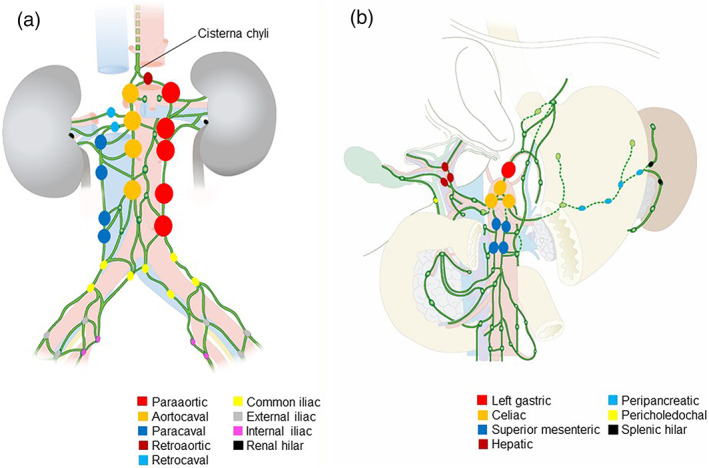
Distribution of metastatic lymph nodes in the abdomen. Larger symbols indicate higher frequency of metastasis. (a) Lymph nodes surrounding abdominal aorta and vena cava (b) celiac lymph nodes with associated lymph nodes
TABLE 4.
Abdominal lymph node metastasis by location in patients with metastatic non‐small‐cell lung cancer
| Group | Number of patients (%) |
|---|---|
| With ALNM (n = 105) | |
| Direct only | 56 (53.3) |
| Indirect only | 7 (6.7) |
| Both | 42 (40.0) |
| With ALNM/without metastasis in abdominal organ (n = 57) | |
| Direct only | 28 (49.1) |
| Indirect only | 3 (5.3) |
| Both | 26 (45.6) |
Abbreviations: ALNM, abdominal lymph node metastasis.
Association of abdominal lymph node metastasis with laterality of intrathoracic lesion or clinical variables
Intrathoracic lesions of 207 patients (23.2%) were in “right thorax” only in which the lymph fluid would expect to be drained into the right lymphatic duct. In contrast, 685 patients (76.8%) had intrathoracic lesion in left thorax in which the lymph fluid would expect to be drained into the thoracic duct. Interestingly, ALNM was significantly more common in patients with “left thorax” lesions compared to those with “right thorax” lesions only (14.3% vs. 3.4%, p < 0.001, Table 5(a)). A comparable result was observed after analyzing patients without metastasis to the abdominal organs (n = 663, 10.8% vs. 1.8%, p < 0.001, Table 5(b)). There were no differences in clinical variables between patients with “right thorax only” and “left thorax” lesions (data not shown). The association of abdominal lymph node metastasis with clinical variables in the 892 patients with metastatic NSCLC is shown in Table 6. In univariate logistic regression analysis, higher N category was significantly associated with increased risk of having ALNM (p < 0.001). N category remained significantly associated with the risk of ALNM after adjustment for potential confounding by other clinical variables (p < 0.001).
TABLE 5.
Abdominal lymph node metastasis by laterality of intrathoracic lesion in patients with metastatic non‐small‐cell lung cancer
| Abdominal lymph node metastasis | |||
|---|---|---|---|
| (a) Entire patients (n = 892) | p value <0.001 | ||
| Laterality | Yes (n = 105) | No (n = 787) | |
| Right thorax only | 7 (3.4) | 200 (96.6) | |
| Left thorax | 98 (14.3) | 587 (85.7) | |
| (b) Patients without metastasis in abdominal organ (n = 663) | p value <0.001 | ||
| Laterality | Yes (n = 57) | No (n = 606) | |
| Right thorax only | 3 (1.8) | 162 (98.2) | |
| Left thorax | 54 (10.8) | 444 (89.2) | |
TABLE 6.
Association of abdominal lymph node metastasis with clinical variables in patients with metastatic non‐small‐cell lung cancer: univariate and multivariate analysis
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p value | OR (95% CI) | p value |
| Age | 0.019 | 0.086 | ||
| >69 | reference | reference | ||
| ≤69 | 1.65 (1.08–2.50) | 1.46 (0.95–2.24) | ||
| Sex | 0.150 | |||
| Male | reference | |||
| Female | 0.72 (0.46–1.13) | |||
| Smoking history | 0.573 | |||
| Ever | reference | |||
| Never | 0.88 (0.56–1.37) | |||
| ECOG PS | 0.262 | |||
| 0–1 | reference | |||
| ≥2 | 1.27 (0.84–1.92) | |||
| Histology | 0.255 | |||
| SQC | reference | |||
| ADC | 1.48 (0.84–2.60) | |||
| Others | 1.89 (0.85–4.21) | |||
| EGFR mutation | 0.237 | |||
| Positive | reference | |||
| Negative | 1.32 (0.83–2.08) | |||
| T category | 0.083 | 0.195 | ||
| Tx‐T1 | reference | reference | ||
| T2 | 0.56 (0.23–1.40) | 0.64 (0.25–1.64) | ||
| T3 | 0.50 (0.21–1.21) | 0.39 (0.56–0.98) | ||
| T4 | 0.95 (0.45–2.02) | 0.67 (0.30–1.47) | ||
| N category | <0.001 | <0.001 | ||
| N0 | reference | reference | ||
| N1 | 0.57 (0.19–2.50) | 0.73 (0.20–2.66) | ||
| N2 | 1.42 (0.64–3.15) | 1.47 (0.66–3.29) | ||
| N3 | 4.25 (2.26–8.00) | 4.35 (2.26–8.35) | ||
Abbreviations: ADC, adenocarcinoma; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; OR, odds ratio; SQC, squamous cell carcinoma.
DISCUSSION
This study demonstrates that ALNM occurs by lymphatic spread through the thoracic duct in patients with metastatic NSCLC. The cisterna chyli, distal part of the thoracic duct, receives lymph flow from the intestinal and lumbar trunks. 17 We classified metastatic abdominal lymph nodes into direct and indirect nodes and evaluated the pattern of lymphatic spread. We found that most of the patients with ALNM (93.3%) had metastatic lesion in the direct nodes. This finding suggests that ALNM develops by lymphatic spread through thoracic duct involvement in patients with NSCLC.
ALNM was more frequently observed in patients with “left thorax” (lymph drain into the thoracic duct) lesions compared to those with “right thorax” (lymph drain into the right lymphatic duct) lesions only (p < 0.001). This finding consistently supports the role of the thoracic duct as a pathway for ALNM. In addition, the risk for ALNM was increased with higher N category (adjusted OR: 4.35, p < 0.001). This result indicates that the lymphatic pathway is involved in the process of metastasis to the abdominal lymph node in NSCLC. The National Comprehensive Cancer Network (NCCN) guidelines do not recommend that abdomen CT should be routinely performed during follow‐up of NSCLC patients. 18 Our results suggest that abdomen CT should be considered during follow‐up of NSCLC patients with intrathoracic lesion in left thorax and with high N category.
Most of the patients in the “indirect only” group had a single metastasis to the left gastric node. Consistently, most of the indirect nodes with ALNM were left gastric nodes. Although the left gastric node is included as an indirect node, its location is relatively adjacent to the direct node (celiac node) and cisterna chyli compared to the other indirect nodes. To exclude the potential occurrence of ALNM from metastatic abdominal organs, we performed a subgroup analysis including just those patients who did not have metastasis to abdominal organs. The result of that subgroup analysis strongly supported the presence of a metastatic pathway through the thoracic duct. In this study, the abdominal lymph node was the fifth most common metastasis site among the lung cancer patients. Indeed, abdominal lymph node was the only distant metastatic site in 11.4% of patients with ALNM. This result suggests that clinicians should carefully assess for the potential presence of ALNM during staging work‐up for lung cancer patients.
There are some limitations in the present study. First, this study was conducted at single center, calling the generalizability of these results until external confirmatory studies are performed. However, clinical information was obtained from Inha Lung Cancer Cohort in which the data was prospectively obtained. Second, the presence of ALNM was not confirmed by pathological examination in this study. Instead, we used PET/CT scans to define ALNM presence and location. PET has previously been shown to be highly accurate for the detection of lymph node metastasis (sensitivity: 70%–93%, specificity: 81%–100%). 19 The prevalence of ALNM was 11.8% among patients with metastatic NSCLC, in line with previous studies. 20
In conclusion, this study suggests that the thoracic duct is one of the potential routes of lymphatic spread to the abdominal lymph nodes. Clinicians should assess for the presence of ALNM using abdomen CT during staging work‐up and follow‐up for NSCLC patients with intrathoracic lesion in left thorax and with high N category. Further studies are required to further explore the pathways of lymphatic spread in lung cancer.
CONFLICT OF INTEREST
The authors declare that they have no competing interests to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization: Jun Hyeok Lim, Jeong‐Seon Ryu.
Data curation: Wookyung Ryu, Myoung Kyu Lee, Mi Hwa Park.
Formal analysis: Wookyung Ryu, Myoung Kyu Lee, Mi Hwa Park, Jun Hyeok Lim, Jeong‐Seon Ryu.
Investigation: In Young Hyun, Minkyung Lee, Eun‐Ji No, Seok Joong Yong, Jung Soo Kim.
Methodology: Wookyung Ryu, Myoung Kyu Lee, Mi Hwa Park, Jun Hyeok Lim, Jeong‐Seon Ryu.
Project administration: Jun Hyeok Lim, Jeong‐Seon Ryu.
Supervision: Jun Hyeok Lim, Jeong‐Seon Ryu.
Validation: In Young Hyun, Minkyung Lee, Eun‐Ji No, Seok Joong Yong, Jung Soo Kim.
Writing – original draft: Wookyung Ryu, Myoung Kyu Lee, Mi Hwa Park, Jun Hyeok Lim, Jeong‐Seon Ryu.
Writing – review & editing: Wookyung Ryu, Myoung Kyu Lee, Mi Hwa Park, In Young Hyun, Minkyung Lee, Eun‐Ji No, Seok Joong Yong, Jung Soo Kim, Jun Hyeok Lim, Jeong‐Seon Ryu.
Supporting information
FIGURE S1 Study flow chart. PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography
FIGURE S2 Overall survival of patients by ALNM. ALNM, abdominal lymph node metastasis
ACKNOWLEDGEMENTS
This work was supported by INHA UNIVERSITY Research Grant.
Ryu W, Lee MK, Park MH, et al. Abdominal lymph node metastasis by lymphatic spread through the thoracic duct in patients with non‐small‐cell lung cancer. Thorac Cancer. 2021;12:2078–2084. 10.1111/1759-7714.14014
REFERENCES
- 1. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–7. [DOI] [PubMed] [Google Scholar]
- 2. Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katakami N, Kunikane H, Takeda K, Takayama K, Sawa T, Saito H, et al. Prospective study on the incidence of bone metastasis (BM) and skeletal‐related events (SREs) in patients (pts) with stage IIIB and IV lung cancer—CSP‐HOR 13. J Thorac Oncol. 2014;9:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johung KL, Yeh N, Desai NB, Williams TM, Lautenschlaeger T, Arvold ND, et al. Extended survival and prognostic factors for patients with ALK‐rearranged non–small‐cell lung cancer and brain metastasis. J Clin Oncol. 2016;34:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression‐free survival in patients with non–small cell lung cancer treated with nivolumab. J Thorac Oncol. 2017;12:e140–e1. [DOI] [PubMed] [Google Scholar]
- 6. Bazhenova L, Newton P, Mason J, Bethel K, Nieva J, Kuhn P. Adrenal metastasis in lung cancer: clinical implications of a mathematical model. J Thorac Oncol. 2014;9:442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu S, Tan KS, Kadota K, Eguchi T, Bains S, Rekhtman N, et al. Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer–specific death in squamous cell carcinoma. J Thorac Oncol. 2017;12:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. [DOI] [PubMed] [Google Scholar]
- 9. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. [DOI] [PubMed] [Google Scholar]
- 10. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 11. Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang H‐C, Hang J‐F, Wu M‐H, Chou T‐Y, Chiu C‐H. Lung adenocarcinoma with ipsilateral breast metastasis: a simple coincidence? J Thorac Oncol. 2013;8:974–9. [DOI] [PubMed] [Google Scholar]
- 13. Chang T‐C, Changchien C‐C, Tseng C‐W, Lai C‐H, Tseng C‐J, Lin S‐E, et al. Retrograde lymphatic spread: a likely route for metastatic ovarian cancers of gastrointestinal origin. Gynecol Oncol. 1997;66:372–7. [DOI] [PubMed] [Google Scholar]
- 14. Ryu J‐S, Ryu HJ, Lee S‐N, Memon A, Lee S‐K, Nam H‐S, et al. Prognostic impact of minimal pleural effusion in non–small‐cell lung cancer. J Clin Oncol. 2014;32:960–7. [DOI] [PubMed] [Google Scholar]
- 15. Gallamini A, Zwarthoed C, Borra A. Positron emission tomography (PET) in oncology. Cancer. 2014;6:1821–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harisinghani MG. Atlas of Lymph Node Anatomy. New York, NY: Springer Science & Business Media; 2012. [Google Scholar]
- 17. Martini F, Timmons MJ, Tallitsch RB, Ober WC, Garrison CW, Welch KB, et al. Human Anatomy. San Francisco, CA: Pearson/Benjamin Cummings; 2006. [Google Scholar]
- 18. Network NCC. NCCN Non‐Small Cell Lung Cancer Guidelines, Version 4.2021. March 3, 2021.
- 19. Eschmann S, Friedel G, Paulsen F, Budach W, Harer‐Mouline C, Dohmen B, et al. FDG PET for staging of advanced non‐small cell lung cancer prior to neoadjuvant radio‐chemotherapy. Eur J Nucl Med Mol Imaging. 2002;29:804–8. [DOI] [PubMed] [Google Scholar]
- 20. Karyagar S, Koc ZP, Karyagar SS, Öztürk I, Cengiz E, Sayc Y, et al. Abdominal lymph node metastasis in patients with non‐small‐cell lung cancer as shown by PET/CT. Clin Nucl Med. 2013;38:691–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Study flow chart. PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography
FIGURE S2 Overall survival of patients by ALNM. ALNM, abdominal lymph node metastasis


