Abstract
The global burden of children and young people (CYP) with bronchiectasis is being recognised increasingly. They experience a poor quality of life and recurrent respiratory exacerbations requiring additional treatment, including hospitalisation. However, there are no published data on patient-driven clinical needs and/or research priorities for paediatric bronchiectasis.
Parent/patient-driven views are required to understand the clinical needs and research priorities to inform changes that benefit CYP with bronchiectasis and reduce their disease burden. The European Lung Foundation and the European Respiratory Society Task Force for paediatric bronchiectasis created an international roadmap of clinical and research priorities to guide, and as an extension of, the clinical practice guideline.
This roadmap was based on two global web-based surveys. The first survey (10 languages) was completed by 225 respondents (parents of CYP with bronchiectasis and adults with bronchiectasis diagnosed in childhood) from 21 countries. The parent/patient survey encompassed both clinical and research priorities. The second survey, completed by 258 health practitioners from 54 countries, was limited to research priorities.
The two highest clinical needs expressed by parents/patients were: having an action management plan for flare-ups/exacerbations and access to physiotherapists. The two highest health practitioners’ research priorities related to eradication of airway pathogens and optimal airway clearance techniques. Based on both surveys, the top 10 research priorities were derived, and unanimous consensus statements were formulated from these priorities.
This document addresses parents'/patients' clinical and research priorities from both the parents'/patients' and clinicians' perspectives and will help guide research and clinical efforts to improve the lives of people with bronchiectasis.
Short abstract
This document is an international roadmap on parents’/patients’ clinical and research priorities from both the parents’/patients’ and clinicians’ perspectives to help guide research and clinical efforts to improve the lives of people with bronchiectasis https://bit.ly/3xoonwi
Introduction
Bronchiectasis unrelated to cystic fibrosis (CF) is no longer considered rare [1–3] and is associated with a high disease burden for children and young people (CYP) as well as in adults, their families and health systems, resulting in substantial economic costs [4, 5]. Nevertheless, substantial knowledge gaps exist, and while there are published research priorities for adults with bronchiectasis [6, 7], research priorities considered important by parents of children with bronchiectasis and the clinical needs of people with bronchiectasis are unknown.
Paediatric-specific data are important, as although bronchiectasis in CYP and adults share some clinical similarities (e.g. wet/productive cough and superimposed exacerbations [4]), there are substantial disparities in several key areas. CYP require developmental-appropriate parental care and support. Important biological differences include altered pathogen profiles (bacteria [8] and microbiota [9]), age-related immunological responses [10] and treatment outcomes [1]. Diagnostic [1] and treatment methods, such as airway clearance techniques (ACTs), may also differ according to age [11, 12]. Additionally, comorbidities and underlying aetiologies are substantially different between adults and children [4]. Finally, while bronchiectasis was once thought irreversible and progressive, disease progression in children with mild disease can be halted and sometimes even reversed with optimal clinical management [1].
To address the lack of published research priorities and patient-derived “needs assessment” data for CYP with bronchiectasis, we undertook this project as an extension of the European Respiratory Society (ERS) clinical practice guideline (CPG) for managing children and adolescents with bronchiectasis [12]. We sought to develop a roadmap to guide health services and research priorities pertinent to the needs of CYP (and their parents/carers) with bronchiectasis, in order to reduce their disease burden and improve their short- and long-term outcomes.
Methods
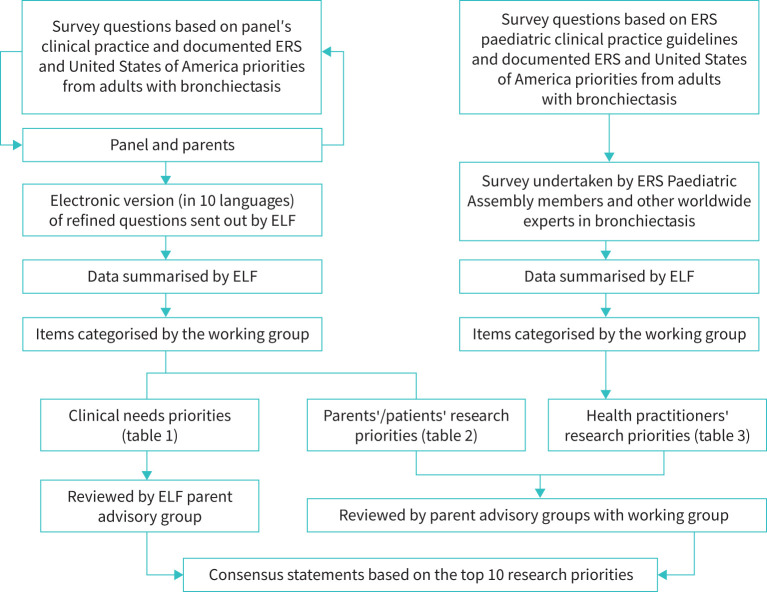
The European Lung Foundation (ELF) led two web-based surveys with the working group as detailed in the supplementary material. The first involved parents of CYP with bronchiectasis and adults with bronchiectasis as a child (henceforth called parents/patients group) and focused on priorities relating to parent/patient-based clinical needs and research. The second survey involved health practitioners focused on research priorities only (figure 1).
FIGURE 1.
Overview of the project methodology. ELF: European Lung Foundation; ERS: European Respiratory Society.
Parent/patient survey on quality of life, clinical needs and research priorities
Survey questions were formulated in English by the working group and consisted of de novo questions (from clinical experience) in addition to those adapted from the adult-based documents from the ERS [6] and United States [7]. The survey was professionally translated into nine languages and distributed widely (SurveyMonkey platform) between July 2019 and January 2020. It consisted of: 1) demographics; 2) effects of items on the child's and respondent's quality of life (QoL); and 3) other questions referring to clinical and research priorities, framed around “concerns about your child's/your health”. For each non-demographic question, there were four possible answers: 1=no effect, 2=minor, 3=moderate and 4=major effect.
The working group grouped the summarised participants’ priorities into research and/or clinical entities and categorised them into themes. The results, groupings and themes were reiteratively discussed with the two parent advisory groups (PAG): CPG and an Australian-based PAG.
Health practitioner survey for research priorities
The 49 items for the health practitioners survey were generated by the working group based on: 1) research questions raised from our CPG [12]; and 2) items adapted from adult data [6, 7]. After several reiterations among the working group members, the link to the web-based survey (only in English) was sent to members of the ERS paediatrics assembly. Working group members also sent the link to non-Europeans/ERS collaborators. The survey was open between 28 July 2020 and 30 September 2020 and utilised a five-point scale (1=unimportant, 2=slightly important, 3=moderately important, 4=very important, 5=essential). As with the parent/patient survey, the working group then grouped the priorities into research themes, matching the parent/patient-based themes.
Selection of the top 10 research priorities and formulating consensus statements
Research priorities from both surveys were collated, condensed and summarised to identify the top 10 priorities. We did not rank them but planned a voting process if consensus (PAG and working group) could not be obtained. The top 10 priorities were also reviewed by the Australian-based PAG (https://crelungs.org.au/cre-parent-and-community-advisory-group). Consensus statements were then formulated by the working group based on these research priorities.
Results
Parent/patient survey
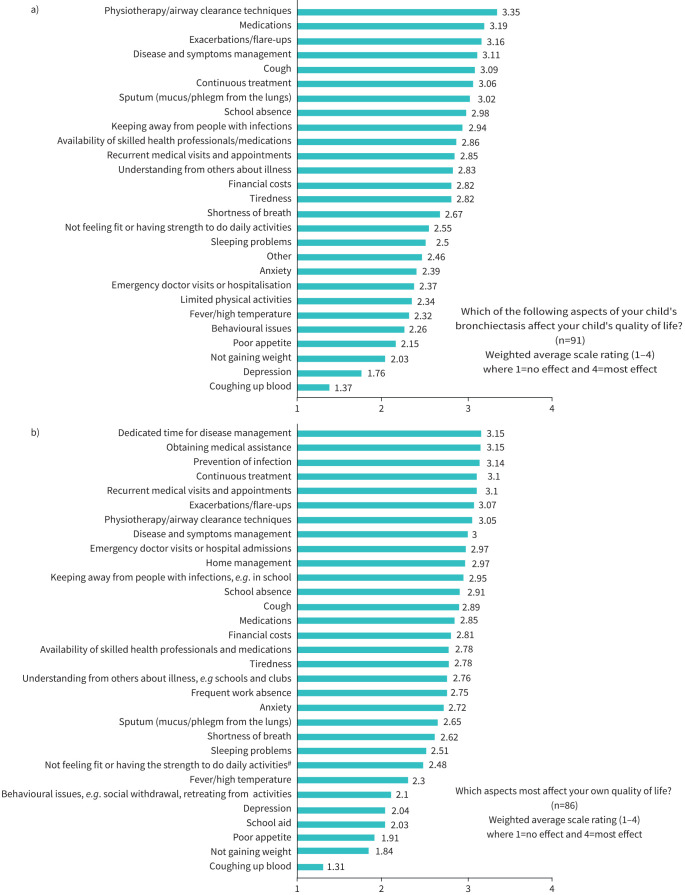
Overall, 225 participated; 70 (31%) were adults with bronchiectasis diagnosed during childhood and 155 (69%) parents/carers of a CYP with bronchiectasis. Seven items rated by parents affecting their child's QoL (figure 2a) had mean scores >3; the top five items related to physiotherapy/airway clearance, medications, exacerbations, disease and symptoms management, and cough. Aspects most affecting the parent's own QoL were dedicated time for disease management, obtaining medical assistance and preventing infection (figure 2b).
FIGURE 2.
Results from the parent/patient survey relating to aspects of their child's bronchiectasis on their quality of life. a) Mean scores of items rated by parents affecting their child's quality of life. b) Mean scores of items rated by parents affecting their own quality of life. #: e.g. walking far, swimming.
The mean item scores for clinical needs and research priorities are presented in tables 1 and 2, respectively. The three highest clinical needs were “Having an action management plan for flare-ups/exacerbations”, “Having access to physiotherapy and being taught the techniques and how to use the equipment at home” and “Good communication between healthcare professionals and each person with bronchiectasis” (table 1).
TABLE 1.
Parent/patient survey: clinical needs themes and priorities
| Theme | Items (verbatim from survey questions) | Mean score |
| Awareness and diagnosis | To find ways to diagnose bronchiectasis earlier, such as by local doctors# | 3.86 |
| To improve awareness of bronchiectasis in community care services, e.g. among community-based nurses and physiotherapists | 3.82 | |
| To identify the cause(s) of bronchiectasis | 3.80 | |
| Education and support for parents and families | Knowing more about the role of physiotherapy and pulmonary rehabilitation (a short course of regular exercise sessions and education sessions) | 3.80 |
| Having access to reliable, easy to understand information about different aspects of living with bronchiectasis | 3.78 | |
| Providing each person with copies of their test results so they can keep a useful history of the progress of their own condition | 3.74 | |
| Develop better ways of teaching people to use their medicines | 3.71 | |
| Improving access to quality care | Having access to physiotherapy and being taught the techniques and how to use the equipment at home | 3.94 |
| Good communication between healthcare professionals and each person with bronchiectasis | 3.91 | |
| Testing new techniques for managing bronchiectasis in real world environments, such as at home and community | 3.86 | |
| Better access to tests and experts on bronchiectasis | 3.80 | |
| Using peer support forums and social media to exchange information with others | 3.52 | |
| Managing exacerbations | Having an action management plan for flare-ups/exacerbations | 3.94 |
| Having a self-management programme and care plan designed with each person to help them have greater control over their condition and recognise/manage an exacerbation | 3.92 | |
| Finding triggers of exacerbation | 3.86 | |
| Educating primary care doctors to prescribe the same dose/length of antibiotic therapy for exacerbations as used in cystic fibrosis¶ | 3.81 | |
| Improving treatment | To improve how children with bronchiectasis are treated through using longer-term antibiotic therapy when a person's condition is stable+ | 3.71 |
| Being able to identify people at increased risk of poor outcomes or needing urgent treatment for their bronchiectasis | 3.85 | |
| Being able to monitor and treat the coughing up of blood | 3.77 | |
| Improving monitoring | Having regular lung function testing to help notice changes or increased risk of an exacerbation | 3.76 |
| Being able to monitor cough | 3.68 | |
| Regular sputum examinations when a person is stable and during an exacerbation to learn more about how the condition changes | 3.64 | |
| Having the equipment at home to monitor symptoms | 3.58 | |
| Ensuring each person has access to a home intravenous antibiotic service to avoid unnecessary hospital admissions | 3.53 | |
| Having regular computed tomography scans to look for changes or increased risk of an exacerbation§ | 3.45 |
#: refers to parent's/patient's experience of delayed referral from a lack of awareness of the symptoms of bronchiectasis and dismissing children's chronic wet cough; importantly, we do not expect primary care doctors to undertake computed tomography (CT) scans in young children. ¶: refers to parent's/patient's experience that they are often given a shorter antibiotic course (e.g. 3 days as opposed to current guideline recommendations of 14 days [12]) and/or lower doses than what is generally considered optimal; doses for children with CF are generally higher than for those without CF as children with CF have higher volume of distribution and renal clearance mechanisms; thus, we do not suggest prescribing antibiotics using CF-based dosing regimens, as it can be potentially dangerous for some agents. +: refers to identifying when and in whom long-term antibiotics should be used to induce clinical stability. §: the ERS Clinical Practice Guidelines [12] state that “In children/adolescents with bronchiectasis, we suggest the decision to repeat chest CT scans is individualised based on the clinical status and setting. Remarks: Repeat chest CT scans should be considered to answer a question which will change management.”
TABLE 2.
Parent/patient survey: research priorities and themes
| Research theme | Items (verbatim from survey questions) | Mean score |
| Understanding mechanisms and biology of bronchiectasis | Finding ways to prevent bronchiectasis | 3.89 |
| Identifying how bronchiectasis develops and continues | 3.87 | |
| Identifying the cause(s) of bronchiectasis | 3.80 | |
| Identifying how often and why bronchiectasis occurs in certain groups of people across the world | 3.74 | |
| Finding new ways to improve diagnosis and treatment | Finding ways to diagnose bronchiectasis earlier, such as by local doctors# | 3.86 |
| Testing new techniques for managing bronchiectasis in real world environments, such as at home and community settings (not in the laboratory or in hospitals) | 3.86 | |
| Finding new medicines to treat bronchiectasis | 3.84 | |
| Finding new physiotherapy/airway clearance techniques | 3.79 | |
| Using longer-term antibiotic therapy when a person's condition is stable¶ | 3.71 | |
| Developing medicines that can be taken in different ways, such as for inhaled or nebulised | 3.67 | |
| Improving knowledge and treatment of exacerbations | Identifying triggers for an exacerbation | 3.86 |
| Identifying people at increased risk of poor outcomes or needing urgent treatment for their bronchiectasis | 3.85 | |
| Using vaccines to prevent exacerbations | 3.78 | |
| Exploring the link between getting a cold (for example rhinovirus) and having an exacerbation | 3.74 | |
| Improving monitoring and how to identify predictors of disease progression | Identifying what makes some patients’ bronchiectasis get worse | 3.92 |
| Understanding the relationship between bronchiectasis and other medical conditions, e.g. asthma, “acid” reflux | 3.80 | |
| Being able to monitor and treat the coughing up of blood | 3.77 | |
| Having regular lung function testing to help notice changes or increased risk of an exacerbation | 3.76 | |
| Being able to monitor cough | 3.68 |
#: refers to parent's/patient's experience of delayed referral due to lack of awareness of the symptoms of bronchiectasis and dismissing children's chronic wet cough; we do not expect primary care doctors to undertake computed tomography scans in young children. ¶: refers to identifying when and in whom long-term antibiotics should be used to induce clinical stability.
The three highest rated research priorities (table 2) were “Identifying what makes some patients’ bronchiectasis get worse”, “Finding ways to prevent bronchiectasis” and “Identifying how bronchiectasis develops and continues”.
Health practitioner survey
The mean scores of the 258 health practitioners (from 54 countries) were 3.19–4.41 with little variation between practitioners grouped by number of patients with bronchiectasis seen regularly in their clinics (supplementary figure S1). Of the top six items (two items ranked equal 5th), four related to antibiotics (table 3), one to airway clearance and the other to factors associated with rapid progression and poor outcomes. Supplementary table S1 ranks items by mean scores.
TABLE 3.
Health practitioner survey: research priorities and themes
| Theme | In children and young people with bronchiectasis (unrelated to cystic fibrosis) | Mean score |
| Finding new ways to improve treatment | When and how (antibiotic, dose, regimen, route (intravenous, oral or inhaled/nebulised) and duration) should pathogens other than Pseudomonas aeruginosa be eradicated, and do patient outcomes improve afterwards? | 4.27 |
| What are the optimal and most cost-effective airway clearance techniques? | 4.26 | |
| When and how (antibiotic, dose, regimen, route (intravenous, oral or inhaled/nebulised) and duration) should P. aeruginosa be eradicated, and do patient outcomes improve afterwards? | 4.18 | |
| What is the best antibiotic, dose, regimen and duration for long-term oral antibiotic therapy in patients with bronchiectasis (according to the presence or absence of P. aeruginosa or other pathogens)? | 4.17 | |
| What are the indications of oral versus inhaled/nebulised long-term suppressive antibiotic treatment? | 4.14 | |
| When should airway clearance techniques be started in patients with bronchiectasis, and how often should it be done during the stable state and for exacerbations? | 4.09 | |
| What is the impact of long-term antibiotic therapy on anti-microbial resistance? | 4.03 | |
| What is the role of different mucoactive agents (e.g. inhaled hypertonic or isotonic saline, mannitol, oral erdosteine or N-acetyl cysteine)? | 4.03 | |
| What are the simple, reliable microbiological tests for determining lower airway infection? | 3.90 | |
| What are the most efficient clinical trial designs and measurable outcomes? | 3.83 | |
| What are the key factors leading to P. aeruginosa acquisition and infection? | 3.78 | |
| Which clinical and microbiological factors affect macrolide efficacy? | 3.71 | |
| What is the role of surgery (segmentectomy, lobectomy or pneumonectomy) and when should it be undertaken? | 3.62 | |
| What is the role of long-term inhaled corticosteroids? | 3.53 | |
| What is the role of non-pharmacological, non-airway clearance technique-based therapeutics, such as singing exercise, wind instruments and yoga during stable states and acute exacerbations? | 3.45 | |
| What is the best model/approach for transferring an adolescent with bronchiectasis to adult services? | 3.42 | |
| Finding new ways to improve diagnosis | What are the baseline investigations to identify underlying aetiologies of bronchiectasis? | 3.98 |
| What clinical factors should be present to trigger referring a child for a chest computed tomography scan to diagnose bronchiectasis? | 3.87 | |
| Improving knowledge and treatment of exacerbations | What is the optimal antibiotic therapy (dosage, how many antibiotics, type, oral versus intravenous versus inhaled/nebulised and length of therapy) for an exacerbation of bronchiectasis? | 4.41 |
| What are the most important factors to prevent acute exacerbations? | 4.12 | |
| How to define acute exacerbations that require additional treatment? | 4.05 | |
| What are the causes of an exacerbation of bronchiectasis? | 3.84 | |
| Which are the best systemic (e.g. blood) or local (e.g. sputum) inflammatory markers for the diagnosis, management and follow-up for an exacerbation? | 3.82 | |
| How should the severity of an exacerbation of bronchiectasis be assessed and what is its impact on long-term outcomes? | 3.78 | |
| What types of biomarker(s) can be used for predicting bronchiectasis exacerbation? | 3.68 | |
| Improving prevention and monitoring | What are the risk factors and causes of rapid progression of lung disease and poor outcomes (e.g. hospitalisation, lung transplantation and mortality)? | 4.17 |
| How best to prevent development of bronchiectasis? | 4.16 | |
| Should there be paediatric-focused patient registries? | 4.06 | |
| What are the best and most pragmatic functional tests (such as carbon monoxide diffusing capacity, 6-min walk test, lung clearance index, endurance shuttle walk, incremental exercise tests or accelerometers) as markers for severity of the disease, outcomes and end-points for the clinic? | 4.00 | |
| What are the risk or protective factors for lung function decline in patients with bronchiectasis? | 3.91 | |
| What are the factors that predict radiographic reversibility (on a high-resolution computed tomography scan)? | 3.84 | |
| What is the best approach/score to evaluate radiographic severity? | 3.82 | |
| What types of specific patient education packages, self-management plans and patient support groups improve outcomes? | 3.81 | |
| Should a severity and prognostic score for children that is useful in clinical practice be developed? | 3.70 | |
| What comorbidities are present and how do they influence bronchiectasis severity? | 3.59 | |
| Is cross-infection important, what are the best strategies and is strict patient segregation required? | 3.59 | |
| What types of biomarker(s) can be used to monitor bronchiectasis severity during stable state, so as to define the subgroup who will benefit from more intensive treatment? | 3.58 | |
| Can endo-phenotyping predict severity and outcomes in children? | 3.54 | |
| Understanding mechanisms and biology of bronchiectasis | Do different aetiologies and/or comorbidities of bronchiectasis predetermine microbiological characteristics and affect severity, patients’ quality of life and disease progression? | 3.92 |
| What is the role of viruses, fungi and anaerobes (as single agents and/or polymicrobial infections), during both the stable state and exacerbation, and what is their impact upon patient severity and outcomes? | 3.85 | |
| Is there an increased rate of primary immune defects (e.g. mannose-binding lectin deficiency, common variable immunodeficiency, IgM or IgA deficiency, or complement deficiency)? | 3.78 | |
| What is the incidence and prevalence of different aetiologies of bronchiectasis across the world? | 3.55 | |
| What is the relationship between paediatric and adult bronchiectasis? | 3.54 | |
| What is the importance of host–pathogen–environment interactions? | 3.53 | |
| What are the molecular and cellular mechanisms and pathobiological pathways of bronchiectasis development, exacerbations and progression? | 3.52 | |
| What is the composition and function of the host microbiome, both during the stable state and exacerbations, and does it impact directly disease severity and progression? | 3.48 | |
| What are the genetic and epigenetic findings in patients with bronchiectasis compared to healthy controls, and what is their role in acquisition of specific pathogens and patients’ outcomes? | 3.39 | |
| What is the best experimental model system of bronchiectasis? | 3.24 | |
| Other | What are the healthcare costs of bronchiectasis management across the world? | 3.19 |
Top 10 research priorities and consensus statements
Full consensus (table 4) was obtained. From these, the consensus statements below (no priority order) were developed and summarised (further detailed in the supplementary material).
Identifying risk and protective factors for bronchiectasis
TABLE 4.
Derivation of the top 10 research priorities#
| Consensus priorities (parent/patient and health practitioners) and confirmed with two parent advisory groups | Parent/patient survey (verbatim from survey questions) | Health practitioner survey (verbatim from survey questions) |
| Theme: Understanding mechanisms and biology of bronchiectasis | ||
| Identifying risk and protective factors for bronchiectasis | Identifying what makes some patients’ bronchiectasis get worse | How best to prevent development of bronchiectasis? |
| Finding ways to prevent bronchiectasis | ||
| Identifying the underlying aetiologies of bronchiectasis | Identifying the cause(s) of bronchiectasis | What are the baseline investigations to identify underlying aetiologies of bronchiectasis? |
| Theme: Diagnosis | ||
| Discovering ways to diagnose bronchiectasis earlier, including ways to increase health practitioner awareness and to facilitate earlier referrals | Discovering ways to diagnose bronchiectasis earlier, such as by local doctors¶ | |
| Theme: Improving knowledge and treatment of exacerbations | ||
| Identifying triggers/prevention factors and optimal antibiotic treatment for acute exacerbations | Identifying triggers for an exacerbation | What are the most important factors at preventing acute exacerbations? |
| Exploring the link between getting a cold (for example rhinovirus) and having an exacerbation | What is the optimal antibiotic therapy (dosage, how many antibiotics, type, oral versus intravenous versus inhaled/nebulised and length of therapy) for an exacerbation of bronchiectasis? | |
| Using vaccines to prevent exacerbations | ||
| Theme: Finding new ways to improve treatment | ||
| Finding new and optimal airway clearance techniques | Finding new physiotherapy/airway clearance techniques | What are the optimal and most cost-effective airway clearance techniques? |
| When should airway clearance techniques be started in patients with bronchiectasis, and how often should it be done during the stable state and for exacerbations? | ||
| Defining optimal antibiotic therapy for eradicating specific pathogens (e.g. Pseudomonas aeruginosa) and for suppressing bacteria once chronic infection is established | Using longer-term antibiotic therapy when a person's condition is stable+ | When and how (antibiotic, dose, regimen, route (intravenous, oral or inhaled/nebulised) and duration) should P. aeruginosa be eradicated, and do patient outcomes improve afterwards? |
| When and how (antibiotic, dose, regimen, route (intravenous, oral or inhaled/nebulised) and duration) should pathogens other than P. aeruginosa be eradicated, and do patient outcomes improve afterwards? | ||
| What are the indications of oral versus inhaled/nebulised long-term suppressive antibiotic treatment? | ||
| What is the best antibiotic, dose, regimen and duration for long-term oral antibiotic therapy in patients with bronchiectasis (according to the presence or absence of P. aeruginosa or other pathogens)? | ||
| Finding new medications and/or techniques for managing bronchiectasis | Testing new techniques for managing bronchiectasis in real world environments, such as at home and community | What is the role of different mucoactive agents (e.g. inhaled hypertonic or isotonic saline, mannitol, oral erdosteine or N-acetyl cysteine)? |
| Finding new medicines to treat bronchiectasis | ||
| Theme: Improving monitoring and how to identify predictors of disease progression | ||
| Identifying lung function tests/indices that predict outcomes | Having regular lung function testing to help notice changes or increased risk of an exacerbation | What are the best and most pragmatic functional tests (such as carbon monoxide diffusing capacity, 6-min walk test, lung clearance index, endurance shuttle walk, incremental exercise tests or accelerometers) as markers for severity of the disease, outcomes and end-points for the clinic? |
| Understanding the relationship between causes and comorbidities of bronchiectasis with clinical outcomes | To know how bronchiectasis affects other body parts/organs in addition to the lung | Do different aetiologies and/or comorbidities of bronchiectasis predetermine microbiological characteristics, and affect severity, patients’ quality of life and disease progression? |
| To understand the relationship between bronchiectasis and other medical conditions e.g. asthma, “acid” reflux | ||
| Identifying factors associated with worse bronchiectasis outcomes | Identifying what makes some patients’ bronchiectasis get worse | What are the risk factors and causes of rapid progression of lung disease and poor outcomes (e.g. hospitalisation, lung transplantation and mortality)? |
| Identifying people at increased risk of poor outcomes or needing urgent treatment for their bronchiectasis | What are the risk or protective factors for lung function decline in patients with bronchiectasis? | |
#: the list is not in order of priority, i.e. all are considered equal. ¶: refers to parent's/patient's experience of delayed referral due to lack of awareness of the symptoms of bronchiectasis and dismissing children's chronic wet cough; we do not expect primary care doctors to undertake computed tomography scans in young children. +: refers to identifying when and in whom long-term antibiotics should be used to induce clinical stability.
There are currently limited data and evidence for factors important for reversibility and/or prevention of bronchiectasis [12].
Consensus statement: Intervention studies that potentially reduce and/or prevent bronchiectasis are required. Examples include novel and/or maternal vaccinations to prevent early and/or severe childhood pneumonia and long-term azithromycin for recurrent protracted bacterial bronchitis. Additionally, long-term observational studies that delineate currently unidentified risk and protective factors for developing bronchiectasis are required.
Identifying the underlying aetiologies of bronchiectasis
Bronchiectasis has varying aetiologies [4]. The ERS CPG recommended undertaking a minimum panel of tests to identify underlying causes that may alter treatment approaches [12]. However, an underlying aetiology often cannot be ascertained. Little new data have been generated recently with recurrent protracted bacterial bronchitis (>3 episodes within 12 months of initial diagnosis) [13] being an exception. Identifying the underlying aetiologies of bronchiectasis may lead to new prevention and/or treatment approaches.
Consensus statement: Studies that further define a standard panel for identifying and/or predicting bronchiectasis are required. Examples include using novel technologies e.g. genomics, breathnomics and blood/airway gene expression signatures. Additionally, long-term observational studies to delineate new aetiologies associated with bronchiectasis developing are needed.
Discovering ways to diagnose bronchiectasis earlier, including ways to increase health practitioner awareness and to facilitate earlier referrals
Published literature supports the PAG's experience of long delays in diagnosing bronchiectasis in children [14, 15]. Earlier diagnosis requires recognition and treatment of chronic cough (predominant symptom of bronchiectasis [1]), increased patient and health professional awareness, suitable educational resources and health systems that support early and appropriate referrals to specialists with access to diagnostic capability. These capabilities include adopting the ERS CPG recommendation of using multi-detector chest computed tomography (CT) routinely with high-resolution computed tomography (HRCT) scans rather than HRCT scans alone and employing the paediatric-derived broncho-arterial ratio to define abnormality [12].
Consensus statement: Implementation science-based studies are needed to increase: 1) health practitioner awareness and 2) use of guidelines that facilitate earlier bronchiectasis diagnosis and referrals. We encourage research on novel diagnostic techniques, including those that can reduce human variability in diagnosis (e.g. artificial intelligence) and/or provide alternate/complementary methods to current standards relying upon chest CT scans. Additionally, research is required to identify and promote the early symptoms and signs of underlying bronchiectasis and develop simple biomarkers to allow local physicians and paediatricians to recognise this disorder promptly and reduce the risk of irreversible damage to the developing airways of young children.
Identifying triggers/prevention factors and optimal antibiotic treatment for acute exacerbations
Few data exist on finding triggers of exacerbations [16, 17] with the only trigger studied in CYP with bronchiectasis being viral-associated infections [18].
While bronchiectasis guidelines recommend antibiotics for respiratory exacerbations in both CYP and adults, data from a randomised controlled trial (RCT) on antibiotics for non-hospitalised exacerbations in children [19] suggest that a subset do not require these agents.
Consensus statement: Large prospective studies are needed to identify triggers of respiratory exacerbations and those at risk of these episodes. It is also important to generate novel data to differentiate between children with exacerbations requiring antibiotics from those where they are unnecessary. Such studies should include identifying systemic and/or airway biomarkers that may predict exacerbation outcomes.
Finding new and optimal ACTs
Parents/PAG give high value to airway clearance as a therapeutic modality and highlighted the lack of access to high-quality therapists. Several ACTs are suitable for various developmental/cognitive stages. Most ACTs are indicated empirically, usually based on the skills of physiotherapists. Since ACTs was the highest rated aspect affecting the children's QoL (figure 2a), new and optimal ACTs are needed for both stable and exacerbations states.
Consensus statement: We advocate multicentre studies of CYP with bronchiectasis to determine the efficacy of ACTs based on their frequency and utility of various methods. Innovative techniques that increase the efficacy of ACTs and reduce the time burden on patients and their carers are needed.
Defining optimal antibiotic therapy for eradicating specific pathogens (e.g. Pseudomonas aeruginosa) and for suppressing bacteria once chronic infection is established
Although P. aeruginosa is uncommon in CYP with bronchiectasis, it usually signals multi-lobar disease and/or serious underlying comorbidities [8]. Health practitioners rated highly the importance of eradicating bacterial pathogens, especially P. aeruginosa, and how this is achieved. Currently, no published paediatric data exist, while three small studies in adults were limited to P. aeruginosa infections [20–22].
As eradication is standard practice in CYP with CF [23], it is unlikely that placebo-controlled P. aeruginosa eradication trials will take place. Therefore, studies comparing various eradication regimens (e.g. oral versus inhaled anti-pseudomonal antibiotics, alone or in combination, or parenteral antibiotics as single or dual agents, and varying treatment course duration) are required. Trials are also needed to determine if eradicating other respiratory bacterial pathogens is possible and beneficial.
The ERS CPG recommends oral macrolides for at least 6 months in those with >1 hospitalised or ≥3 non-hospitalised exacerbations in the previous 12 months [12]. However, there are limited data and substantial knowledge gaps remain. RCTs are required to identify CYP with bronchiectasis most likely to benefit from long-term antibiotics (e.g. number of exacerbations/year), as well as to define which antibiotic (e.g. macrolide versus inhaled antibiotics) to prescribe, the optimum duration of treatment, describe how long these beneficial effects persist and establish the clinical significance of antibiotic-resistant respiratory bacterial pathogens.
Consensus statement: RCTs are needed to identify those benefiting from long-term antibiotics as well as the optimal antibiotic regimens (including oral or inhaled formulations) and their duration for eradicating bacterial pathogens (e.g. P. aeruginosa) and reducing exacerbations. Both clinical and patient-focused benefits and harm should be identified in these trials. Studies on the safety and efficacy of inhaled antibiotics in CYP and determining risk factors and clinical impact of anti-microbial resistance are also required.
Finding new medications and/or techniques for managing bronchiectasis
There are still no licensed long-term therapies available for bronchiectasis [4, 24]. Current treatments are mostly extrapolated from the CF literature, sometimes with adverse results when later subjected to high-quality RCTs [25].
Consensus statement: Investment in developing and/or assessing new therapeutics that can improve the lives of CYP with bronchiectasis are needed. This includes investment from both commercial and research funding bodies to accelerate the conduct of basic scientific studies and multicentre RCTs to allow the identification and licensing of beneficial therapeutic agents.
Identifying lung function tests/indices that predict outcomes
Tools that can monitor disease and predict outcomes are important for managing chronic diseases. Such prognostic/severity tools have been developed in adults with bronchiectasis but are not applicable in CYP [1].
Consensus statement: We require tools and indices that can be used to effectively monitor disease severity and predict outcomes for CYP with bronchiectasis. Longitudinal studies should be conducted in CYP with bronchiectasis to monitor lung function and other indices to identify specific monitoring parameters that can predict outcomes. The impact of reduced lung function upon respiratory and general health should be considered.
Understanding the relationship between causes and comorbidities of bronchiectasis with clinical outcomes
Bronchiectasis is the “final common pathway” of a pathobiological process involving impaired airway clearance, chronic lower airway infection and inflammation. The various aetiologies and comorbidities (e.g. concurrent asthma, gastro-oesophageal disease, airway malacia) potentially represent paediatric phenotypes and “treatable traits” [1]. However, limited data on paediatric bronchiectasis phenotypes or treatable traits exist.
Consensus statement: Well-characterised large cohorts of CYP with bronchiectasis that advance the concept of phenotypes and “treatable traits” are required. This includes utilising mathematical modelling methods and multiparametric immunophenotyping.
Identifying factors associated with worse bronchiectasis outcomes
Bronchiectasis, described previously as irreversible bronchial dilatation [26], is now defined as abnormal bronchial dilatation on chest CT scans with the phenotype of chronic or recurrent wet/productive cough and pulmonary exacerbations [1, 4]. Outcomes of bronchiectasis are varied, and mild radiographic bronchiectasis in children is potentially reversible when optimal treatment commences early in the disease [1]. Identifying factors influencing illness progression are important as these represent potential intervention points, i.e. secondary prevention, and may lead to new pharmacological agents.
Consensus statement: Long-term observational studies in large cohorts across multiple settings to further delineate factors influencing outcomes of CYP with bronchiectasis are required. These include genetic and environmental factors that predispose some patients to chronic infections (e.g. with P. aeruginosa) and/or frequent exacerbations. Studies with innovative treatment protocols based on patient phenotypes are also needed.
Discussion
We present the first document on the clinical needs of CYP with bronchiectasis and their carers, and highlight issues that most affect their QoL. Additionally, the 10 consensus research priorities outlined in our roadmap were developed conjointly by parents/patients, patient advocates with a multi-disciplinary panel of expert clinicians and clinician-researchers in the field. These topics were across four themes: understanding mechanisms and biology; diagnosis; improving knowledge and treatment of exacerbations; and finding new ways to improve treatment.
Bronchiectasis remains relatively under-researched and under-serviced [27], where the unmet needs of people with bronchiectasis are huge and there are relatively few RCTs [1, 4, 28]. A focus on CYP is advantageous as mild radiographic bronchiectasis is potentially reversible if treated early, thereby avoiding the later progressive decline in lung function while prevention remains the ultimate goal [1]. Novel and concerted approaches are required, and this document highlights both clinical gaps and research priorities.
Using these priorities should lead to better clinical services and research questions, thereby improving the lives of CYP with bronchiectasis and their families. Indeed, data from several different longitudinal paediatric cohorts demonstrate lung function in bronchiectasis can be improved [29–33] and remain within the normal range over the long term [34]. Furthermore, while still contentious, recent prospective data suggest that catch-up lung function in children can occur, leading to normal lung function in adulthood [35]. Although such observations need further confirmation, as lung trajectories were once thought to remain unchanged for life, this is particularly important as low spirometric lung function impairment even in the clinically normal range, is an independent prognostic marker of respiratory and cardiovascular disease mortality across a broad range of socioeconomic backgrounds and environmental settings [36–38].
The document has several strengths. Firstly, we engaged parents and patients (with ELF support) so as to bring relevance and increase the value of treatment by ensuring that care is responsive to societal changes and public expectations, elements which had been missing in CYP with bronchiectasis. Such data are essential for guiding research and clinical efforts to improve the lives of people with bronchiectasis. Secondly, the surveys were international, across high- and low–middle-income countries. Thirdly, the work accompanied the first international CPG on managing children and adolescents with bronchiectasis [12]. The CPG [12] provided up-to-date data from their systematic reviews on existing knowledge gaps in paediatric bronchiectasis.
Nevertheless, there are important limitations to consider. The proportion of parents/patients responding to the survey is unknown and some responses were incomplete. The survey that required access to the web-based technology and the internet (i.e. we did not have a paper-based option) may have limited participation in some sections of the community. This work was supported by an ERS taskforce, with limited reach to Asia, Africa and the Americas. Also, only ∼20% of members of the paediatrics assembly of the ERS responded, but this is not unexpected as not all assembly members are clinicians and/or manage CYP with bronchiectasis. Nevertheless, it is possible that the views of parents/patients and health practitioners may not be representative of the broader community of those with bronchiectasis and their families or the health practitioners caring for such patients. Additional points are raised in the supplementary material. Finally, we could not ascertain how views might change with the age of the child; it is possible that parents of a newly diagnosed child may have different views (e.g. focusing on early diagnosis) compared to a young person with established disease (e.g. more concerned about the detection and management of pulmonary exacerbations).
Conclusions
This document highlights the clinical needs of CYP with bronchiectasis and their carers, in addition to providing an international roadmap for research priorities as a consensus statement. The roadmap was developed by parents/patients, patient advocates and expert clinicians in the field. It should act as a valuable resource for health services, grant funding bodies, clinicians and researchers to stimulate better health service and research for improving the lives and outcomes of CYP with bronchiectasis and their families. In so doing, it will not only address this relatively under-researched and under-serviced [27] chronic disorder, but it will also lead to improved lung health for the patients as adults by reversing the disease and/or curtailing its high illness burden.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00122-2021.SUPPLEMENT (556KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: A.B. Chang reports grants from the National Health and Medical Research Council, Australia; and other fees to her institution from work relating to being a IDMC Member of an unlicensed vaccine (GSK) and an advisory member of study design for unlicensed molecule for chronic cough (Merck) outside the submitted work.
Conflict of interest: J. Boyd is an employee of the European Lung Foundation.
Conflict of interest: L. Bell has nothing to disclose.
Conflict of interest: V. Goyal has nothing to disclose.
Conflict of interest: I.B. Masters has nothing to disclose.
Conflict of interest: Z. Powell has nothing to disclose.
Conflict of interest: C. Wilson has nothing to disclose.
Conflict of interest: A. Zacharasiewicz reports financial compensation for participation in advisory boards for Vertex, Novartis, Chiesi, Gilead and AOP; and lecture fees from Hagleitner Hygiene, AstraZeneca and Heine und Löwenstein.
Conflict of interest: E. Alexopoulou has nothing to disclose
Conflict of interest: A. Bush has nothing to disclose.
Conflict of interest: J.D. Chalmers reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Insmed, and personal fees from Chiesi, Gilead Sciences, Novartis and Zambon, outside the submitted work.
Conflict of interest: R. Fortescue has nothing to disclose.
Conflict of interest: A.T. Hill has nothing to disclose.
Conflict of interest: B. Karadag has nothing to disclose.
Conflict of interest: F. Midulla has nothing to disclose.
Conflict of interest: G.B. McCallum has nothing to disclose.
Conflict of interest: D. Snijders has nothing to disclose.
Conflict of interest: W-J. Song has nothing to disclose.
Conflict of interest: T. Tonia reports acting as an ERS methodologist.
Conflict of interest: K. Grimwood reports various project grants and a Centre of Research Excellence relating to bronchiectasis in children from the Australian National Health and Medical Research Council and the Australian Medical Research Future Fund during the conduct of the study.
Conflict of interest: A. Kantar has nothing to disclose.
Support statement: This study was supported by the European Respiratory Society (part of the project was related to taskforce 2018-03). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chang AB, Bush A, Grimwood K. Bronchiectasis in children: diagnosis and treatment. Lancet 2018; 392: 866–879. doi: 10.1016/S0140-6736(18)31554-X [DOI] [PubMed] [Google Scholar]
- 2.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. doi: 10.1016/S0140-6736(18)31767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal V, Grimwood K, Masters IB, et al. State of the art: pediatric bronchiectasis. Pediatr Pulmonol 2016; 51: 450–469. doi: 10.1002/ppul.23380 [DOI] [PubMed] [Google Scholar]
- 4.Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nature Rev Dis Primers 2018; 4: 45. doi: 10.1038/s41572-018-0042-3 [DOI] [PubMed] [Google Scholar]
- 5.Goyal V, McPhail SM, Hurley F, et al. Cost of hospitalisation for bronchiectasis exacerbations in children. Respirology 2020; 25: 1250–1256. doi: 10.1111/resp.13828 [DOI] [PubMed] [Google Scholar]
- 6.Aliberti S, Masefield S, Polverino E, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. Eur Respir J 2016; 48: 632–647. doi: 10.1183/13993003.01888-2015 [DOI] [PubMed] [Google Scholar]
- 7.Henkle E, Aksamit TR, Daley CL, et al. US patient-centered research priorities and roadmap for bronchiectasis. Chest 2018; 154: 1016–1023. doi: 10.1016/j.chest.2018.06.032 [DOI] [PubMed] [Google Scholar]
- 8.Kapur N, Grimwood K, Masters IB, et al. Lower airway microbiology and cellularity in children with newly diagnosed non-CF bronchiectasis. Pediatr Pulmonol 2012; 47: 300–307. doi: 10.1002/ppul.21550 [DOI] [PubMed] [Google Scholar]
- 9.van der Gast CJ, Cuthbertson L, Rogers GB, et al. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann Am Thorac Soc 2014; 11: 1039–1048. doi: 10.1513/AnnalsATS.201312-456OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzutto SJ, Yerkovich ST, Upham JW, et al. Children with chronic suppurative lung disease have a reduced capacity to synthesize interferon-gamma in vitro in response to non-typeable Haemophilus influenzae. PLoS One 2014; 9: e104236. doi: 10.1371/journal.pone.0104236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AL, Button BM, Tannenbaum EL. Airway clearance techniques in children and adolescents with chronic suppurative lung disease and bronchiectasis. Front Pediatr 2017; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang AB, Fortescue R, Grimwood K, et al. European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur Respir J 2021; in press [ 10.1183/13993003.02990-2020]. [DOI] [PubMed] [Google Scholar]
- 13.Wurzel DF, Marchant JM, Yerkovich ST, et al. Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest 2016; 150: 1101–1108. doi: 10.1016/j.chest.2016.06.030 [DOI] [PubMed] [Google Scholar]
- 14.Eralp EE, Gokdemir Y, Atag E, et al. Changing clinical characteristics of non-cystic fibrosis bronchiectasis in children. BMC Pulm Med 2020; 20: 172. doi: 10.1186/s12890-020-01214-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santamaria F, Montella S, Pifferi M, et al. A descriptive study of non-cystic fibrosis bronchiectasis in a pediatric population from central and southern Italy. Respiration 2009; 77: 160–165. doi: 10.1159/000137510 [DOI] [PubMed] [Google Scholar]
- 16.Amati F, Simonetta E, Gramegna A, et al. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev 2019; 28: 190055. doi: 10.1183/16000617.0055-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell SC, Elborn JS, Byrnes CA. Bronchiectasis: treatment decisions for pulmonary exacerbations and their prevention. Respirology 2018; 23: 1006–1022. doi: 10.1111/resp.13398 [DOI] [PubMed] [Google Scholar]
- 18.Kapur N, Mackay IM, Sloots TP, et al. Respiratory viruses in exacerbations of non-cystic fibrosis bronchiectasis in children. Arch Dis Child 2014; 99: 749–753. doi: 10.1136/archdischild-2013-305147 [DOI] [PubMed] [Google Scholar]
- 19.Goyal V, Grimwood K, Ware RS, et al. Efficacy of oral antibiotics for non-severe exacerbations of bronchiectasis in children (BEST 1): a multi-centre, double-blind, double-dummy, randomised placebo-controlled trial. Lancet Respir Med 2019; 7: 791–801. doi: 10.1016/S2213-2600(19)30254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 356–360. doi: 10.1016/j.rmed.2011.11.018 [DOI] [PubMed] [Google Scholar]
- 21.Blanco-Aparicio M, Saleta Canosa JL, Valino LP, et al. Eradication of Pseudomonas aeruginosa with inhaled colistin in adults with non-cystic fibrosis bronchiectasis. Chron Respir Dis 2019; 16: 1479973119872513. doi: 10.1177/1479973119872513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orriols R, Hernando R, Ferrer A, et al. Eradication therapy against Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respiration 2015; 90: 299–305. doi: 10.1159/000438490 [DOI] [PubMed] [Google Scholar]
- 23.Jackson L, Waters V. Factors influencing the acquisition and eradication of early Pseudomonas aeruginosa infection in cystic fibrosis. J Cyst Fibros 2021; 20: 8–16. doi: 10.1016/j.jcf.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Sapru K, Hill AT. Advances in bronchiectasis. Clin Med (Lond) 2019; 19: 230–233. doi: 10.7861/clinmedicine.19-3-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998; 113: 1329–1334. doi: 10.1378/chest.113.5.1329 [DOI] [PubMed] [Google Scholar]
- 26.Chang AB, Bell SC, Torzillo PJ, et al. Bronchiectasis and chronic suppurative lung disease (CSLD) in children and adults in Australia and New Zealand: Thoracic Society of Australia and New Zealand Guideline: an update. Med J Aust 2015; 202: 21–23. doi: 10.5694/mja14.00287 [DOI] [PubMed] [Google Scholar]
- 27.Prentice BJ, Wales S, Doumit M, et al. Children with bronchiectasis have poorer lung function than those with cystic fibrosis and do not receive the same standard of care. Pediatr Pulmonol 2019; 54: 1921–1926. doi: 10.1002/ppul.24491 [DOI] [PubMed] [Google Scholar]
- 28.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 29.Bastardo CM, Sonnappa S, Stanojevic S, et al. Non-cystic fibrosis bronchiectasis in childhood: longitudinal growth and lung function. Thorax 2009; 64: 246–251. doi: 10.1136/thx.2008.100958 [DOI] [PubMed] [Google Scholar]
- 30.Collaro AJ, Chang AB, Marchant JM, et al. Culturally appropriate outreach specialist respiratory medical care improves the lung function of children in regional and remote Queensland. Lung 2020; 198: 361–369. doi: 10.1007/s00408-020-00332-7 [DOI] [PubMed] [Google Scholar]
- 31.Collaro AJ, Chang AB, Marchant JM, et al. Pediatric patients of outreach specialist Queensland clinics have lung function improvement comparable to that of tertiary pediatric patients. Chest 2020; 158: 1566–1575. doi: 10.1016/j.chest.2020.03.084 [DOI] [PubMed] [Google Scholar]
- 32.Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non-CF bronchiectasis: what influences lung function stability? Chest 2010; 138: 158–164. doi: 10.1378/chest.09-2932 [DOI] [PubMed] [Google Scholar]
- 33.Haidopoulou K, Calder A, Jones A, et al. Bronchiectasis secondary to primary immunodeficiency in children: longitudinal changes in structure and function. Pediatr Pulmonol 2009; 44: 669–675. doi: 10.1002/ppul.21036 [DOI] [PubMed] [Google Scholar]
- 34.McCallum GB, Singleton RJ, Redding GJ, et al. A decade on: follow-up findings of indigenous children with bronchiectasis. Pediatr Pulmonol 2020; 55: 975–985. doi: 10.1002/ppul.24696 [DOI] [PubMed] [Google Scholar]
- 35.Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535–544. doi: 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 36.Duong M, Islam S, Rangarajan S, et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health 2019; 7: e613–e623. doi: 10.1016/S2214-109X(19)30070-1 [DOI] [PubMed] [Google Scholar]
- 37.Agusti A, Noell G, Brugada J, et al. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017; 5: 935–945. doi: 10.1016/S2213-2600(17)30434-4 [DOI] [PubMed] [Google Scholar]
- 38.Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019; 7: 358–364. doi: 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00122-2021.SUPPLEMENT (556KB, pdf)