Abstract
The development of anticancer vaccines as a pillar of cancer immunotherapy has been hampered by the scarcity of suitable tumor-specific antigens. While response to immune checkpoint inhibitors is driven by T cells recognizing mutated antigens, the vast majority of these neoantigens are patient-specific, mandating personalized approaches. In addition, neoantigens are often subclonal present in only a fraction of tumor cells resulting in immune evasion of neoantigen-negative tumor cells. Isocitrate dehydrogenase (IDH)1 mutations, most frequently encoding for the neomorphic protein IDH1R132H, are frequent driver mutations found in the majority of diffuse World Health Organization grade 2 and 3 gliomas. In addition, IDH1R132H generates a shared clonal neoepitope that is recognized by mutation-specific T-helper cells. A recent phase 1 trial (NOA-16, NCT02454634) demonstrated safety and immunogenicity of IDH1-vac, a long IDH1R132H peptide vaccine in patients with newly diagnosed astrocytoma and provided evidence of biological efficacy based on imaging parameters. In addition, vaccine-induced IDH1R132H-reactive tumor-infiltrating T cells were identified. Here we discuss clinical and scientific implications and future developments of IDH-directed immunotherapies.
Key words: glioma, isocitrate dehydrogenase, neoantigen, vaccine, immunotherapy
Highlights
-
•
IDH1R132H is a rare example of a shared driver mutation generating a clonal neoepitope.
-
•
NOA16 trial shows that active immunization against IDHR132H is safe and induces mutation-specific glioma-homing T cells.
-
•
Single-cell sequencing analyses open the opportunity for adoptive T-cell therapy using TCR-transgenic T cells.
-
•
Further developments include combination therapies with immune checkpoint inhibitor and IDH inhibitors.
Neoantigens as vaccine targets in cancer immunotherapy
The discovery that response to immune checkpoint inhibitors (ICIs) is correlated to tumor mutational burden (TMB) across cancer entities1 has provided a mechanistic basis for targeting mutated antigens, so-called neoantigens, by vaccines. As most neoantigens generated by non-synonymous mutations are private,2 personalized approaches using messenger RNA (mRNA) and peptide vaccines have been developed and translated into phase 1 clinical trials, also in gliomas.3,4 Due to tumor-specificity of neoantigens, both side-effects and efficacy are favorable compared with vaccines targeting tumor-associated antigens (TAAs), which have previously been tested in patients with cancer including gliomas, most likely due to insufficient breach of endogenous tolerance to TAAs. Most personalized vaccine approaches have focused on the induction of cytotoxic CD8+ T-cell responses against neoepitopes presented on major histocompatibility complexes (MHC) class I. The majority of neoantigens, however, are presented on MHC class II molecules stimulating CD4+ T-helper cell responses.5 The efficacy of ICIs critically depends on CD4+ T-helper cell responses in experimental glioma models6 and mutation-specific CD4+ T-helper cells are capable of eradicating large tumors in patients.7 Personalized neoepitope-specific vaccines induce CD4+ rather than CD8+ T-cell responses in cancer patients8,9 and specific strategies to target MHCII-restricted patient-specific neoepitopes by vaccines may be developed with improving accuracy of currently available algorithms to predict presentation by MHCII allelotypes.10,11 In addition to the absolute number of neoantigens, their clonality or clonal representation within the tumor has been shown to be a key determinant of response to ICIs.12 Subclonal neoantigens are prone to immune evasion due to clonal selection.13 In addition to a comparatively low TMB, gliomas are signified by a low neoantigen clonality, particularly in the recurrent situation, which is also a reason why trials with ICI as a monotherapy in this disease have been unsuccessful in unselected patient populations thus far.14 Hence, recurrent driver mutations represent an attractive target for specific immunotherapies, as neoantigens derived from them are clonal and provide an opportunity for an off-the-shelf vaccine. Such off-the-shelf vaccines are also more cost-effective than personalized neoantigen vaccines with respect to patient identification, regulatory processes, and manufacturing.
MUtant IDH as a neoantigen
Mutations in the gene for isocitrate dehydrogenase type 1 (IDH1) resulting in an exchange of histidine to arginine at position 132 of the amino acid sequence (IDH1R132H) are frequent events in diffuse gliomas. About 70% of World Health Organization (WHO) grade 2 and 3 gliomas harbor these disease-defining clonal driver mutations. Preclinical animal studies have provided evidence that neoepitopes are generated from IDH1R132H and presented on MHC to elicit a mutation-specific T-cell response effective in controlling tumor growth.15,16 In an MHC-humanized mouse model transgenic for the human MHCII allele HLA-DR1, a long peptide vaccine covering the hotspot mutation R132H generated CD4+ T-helper cells which are capable of infiltrating syngeneic tumors and controlling their growth, most likely by releasing proinflammatory cytokines such as interferon-γ and tumor necrosis factor-α. In some patients with recurrent IDH1R132H-positive gliomas, spontaneous CD4+ T-cell responses can be detected.15 Detection of T-cell responses depends on assay sensitivity,17 and sequencing-based methodologies to improve this sensitivity are being developed.18,19 In addition to mutation-specific T-cell responses, IDH1R132H-specific antibody responses can be elicited by IDH1-vac in MHC-humanized mice and can also be detected in some patients with recurrent IDH1R132H-mutant gliomas. Of note, there is currently no evidence, that any of the rarer IDH1 or IDH2 mutations elicit similar mutation-specific immune responses. Both in preclinical mouse models and in patients, there is evidence that IDH1R132H is naturally processed and presented on MHCII, albeit with similar affinity and avidity as the wild-type peptide.15 In situ proximity ligation assays indicated that IDH1R132H is presented in human gliomas, both on MHCII+ tumor cells and on tumor-infiltrating myeloid cells.20 As IDH1R132H is expressed only in tumor but not in normal cells, thus representing a true tumor-specific antigen, mutation-specific T-cell responses will only target tumor cells. Indeed, there is no evidence of off-target toxicity of an IDH1R132H-specific vaccine from preclinical or clinical studies. The antigenic function of IDH1R132H in principle applies to other types of tumors with this type of mutation, such as cholangiocarcinoma, osteosarcoma, and acute myeloid leukemia, although this has not been formally proven.
Immunomodulatory function of mutant IDH
Why do gliomas with IDH1R132H develop despite the evidence that this driver mutation generates an immunogenic neoantigen? One potential explanation is the location of gliomas in an immune sanctuary site. Another reason could be the specific enzymatic function of mutant IDH and its oncometabolite R-2-hydroxyglutarate (R-2-HG). Strikingly, when compared with IDH-wild-type gliomas, IDH-mutant gliomas display reduced and not enhanced CD4+ and CD8+ T-cell infiltration.21, 22, 23 This is due to a dual immunomodulatory function of R-2-HG. This metabolite alters the intrinsic chemokine profile of tumor cells to repel rather than attract T cells.24 In addition, R-2-HG secreted from tumor cells is taken up by tumor-infiltrating T cells and represses the activation of CD4+ and CD8+ T cells by blocking T-cell receptor (TCR) signaling and altering the cytokine profile.23,25 In addition to its impact on T cells, there is profound skewing of the tumor-infiltrating myeloid cell phenotype in IDH-mutant compared with IDH-wild-type gliomas, resulting in an immunosuppressive myeloid phenotype.26, 27, 28, 29 Also in myeloid cells, this skewing is mediated by direct and indirect effects of R-2-HG. As a result, IDH-mutant gliomas may be more resistant to immunotherapeutic approaches beyond specific vaccines, including ICIs. In fact, inhibitors of the enzymatic function of IDHs revert the immunosuppressive microenvironment in gliomas and sensitize these tumors to ICIs in preclinical models. On a mechanistic level, IDH inhibitors reinvigorate TCR activation by enhancing nuclear factor of activated T cells (NFAT) signaling in (vaccine-induced) antigen-specific T cells and revert the immunosuppressive phenotype of tumor-infiltrating myeloid cells by normalizing tryptophan metabolism.23,30 These data support clinical trials combining immunotherapies with IDH inhibitors in IDH-mutant gliomas.
Development of immunotherapies targeting mutant IDH
Three different IDH1-directed mutation-specific peptide vaccines have been, or are currently being tested in four clinical trials (Tables 1 and 2). Available data from the multicenter first-in-man phase trial of the Neurooncology Working Group (NOA) of the German Cancer Society (NOA-16, NCT02454634), that met its primary endpoints, show that a long peptide vaccine covering the mutated region is safe and immunogenic,31 with vaccine-induced immune responses in 93.3% of patients across multiple MHC alleles. This trial included 33 patients with newly diagnosed grade 3 and grade 4 IDH1R132H-positive astrocytomas. Patients with an oligodendroglial phenotype signified by allelic losses on chromosomes 1p and 19q and persistence of nuclear ATRX expression were excluded. All patients in the trial were treated with radiotherapy and/or chemotherapy. To avoid the inclusion of patients with standard of care treatment-related pseudoprogression (PsPD), patients were screened and enrolled 4 weeks after completion of radiotherapy followed by an exclusion of patients with PsPD. Upon enrolment, patients received eight vaccines in total over a period of 6 months, integrated into adjuvant temozolomide chemotherapy. More than 90% of patients received all eight vaccines. More than 90% of patients had treatment-related adverse events, none of which was severe. Some 66% and 47% of the adverse events classified as possibly related to IDH1-vac were local administration site conditions (injection site induration or erythema, respectively), which is in the order of what was expected from subcutaneous peptide/protein vaccines administered with these adjuvants. Current follow-up data in this single-arm trial show 3-year progression-free survival (PFS) of 63% [95% confidence interval (CI) 44% to 77%] and overall survival of 84% (95% CI 67% to 93%). Patients with immune responses showed a 2-year PFS of 82% (95% CI 62% to 92%). While these data do not prove clinical efficacy, several important findings recapitulate preclinical data and provide evidence of biological efficacy. (i) There was a strong positive correlation of intratumoral IDH1R132H peptide presentation in the tumor tissue, as assessed by proximity ligation assay at baseline, with the magnitude and sustainability of specific peripheral T-cell responses as quantified by a newly established mutation specificity score. These data support the concept that IDH1R132H is processed and presented in the tumor tissue and supports mutation-specific T-cell responses. (ii) There was a high frequency of PsPD, indicating intratumoral inflammatory reactions. In the safety dataset, PsPD occurred in 37.5% of patients compared with 16.7% of patients in a molecularly matched control cohort. Importantly, PsPDs were associated with increased vaccine-induced peripheral T-cell responses and were not detected in patients without immune responses. (iii) Tumor-infiltrating IDH1R132H-reactive T cells were detected in a patient with PsPD. Combined single-cell TCR and RNA sequencing of these T cells revealed clonal expansion and an activated gene expression signature of cells bearing a TCR reactive to IDH1R132H. These data not only provide the rationale for a phase II clinical trial, but also form the basis for rational combination strategies to enhance the infiltration and intratumoral activity of IDH1R132H-reactive T cells induced by vaccines.
Table 1.
IDH1-directed vaccines in clinical trials
| Name of the compound | Mechanism of action | Phase of clinical trial development | Company |
|---|---|---|---|
| IDH1-vac | Activation of IDH1R132H-specific T cells | I | German Cancer Research Center, Heidelberg University |
| PEPIDH1M | Activation of IDH1R132H-specific T cells | I | Duke University |
| IDH1R132H-DC vaccine | Activation of IDH1R132H-specific T cells | I | Beijing Tiantan Hospital |
IDH, isocitrate dehydrogenase.
Table 2.
Current on-going clinical trials using IDH1-directed vaccines
| Clinicaltrial.gov identifier | Tumor type | Setting | Phase | Treatment arms | Target accrual |
|---|---|---|---|---|---|
| NCT02193347 | Gliomas, WHO grade 2, IDH-mutanta | Recurrent | 1 | Single arm: PEPIDH1M vaccine + tetanus-diphtheria toxoid (Td) + temozolomide |
24 |
| NCT02454634 | Astrocytoma, WHO grade 3 and 4, IDH-mutanta | Newly diagnosed | 1 | Single arm: IDH1-vac Radiotherapy and/or temozolomide |
39 |
| NCT03893903 | Glioma, IDH-mutanta | Recurrent | 1 | Arm 1: IDH-vac Arm 2: IDH-vac + avelumab Arm 3: avelumab |
60 |
| NCT02771301 | Glioma, IDH-mutanta | Newly diagnosed | 1 | Single arm: IDH1R132H-DC vaccine + radiotherapy + temozolomide | 30 |
IDH, isocitrate dehydrogenase; WHO, World Health Organization.
Only IDH1R132H-mutant tumors eligible.
Combination of IDH1 vaccine and ICI
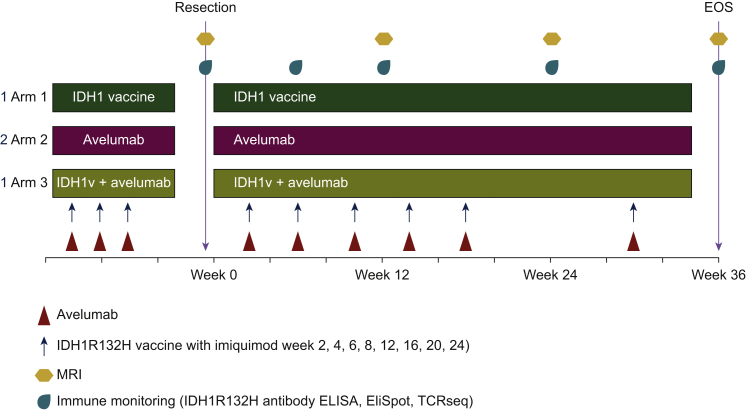
In addition to combinations with IDH inhibitors, ICIs may provide an opportunity to amplify vaccine-induced T-cell responses. Combining the IDH1 peptide vaccine with ICIs may enhance IDH1R132H-specific T-cell responses and may inhibit immune evasion of tumor cells due to counteracting inhibition of function of PD1-expressing T cells in the glioma microenvironment. Several clinical trials in patients with cancer including gliomas are currently underway to investigate the safety and efficacy of therapeutic combinations of cancer vaccines with immune checkpoint blockade. First results indicated clinical benefit of such combinations and demonstrated that their toxicity profile is acceptable and similar to safety profiles of monotherapy with ICIs. The ongoing NOA21 trial (Figure 1) evaluates, in a randomized phase 1 window-of-opportunity design, the safety and immunogenicity of IDH1-vac in combination with the programmed death-ligand 1 (PD-L1)-blocking ICI avelumab (NCT03893903).
Figure 1.
NOA21 window-of-opportunity trial enrolling patients with recurrent IDH1R132H-mutated gliomas eligible for resection.
Patients are randomized to receive neoadjuvant IDH vaccine, avelumab, or combined IDH vaccine and avelumab.
ELISA, enzyme-linked immunosorbent assay; EliSpot, enzyme-linked immunosorbent spot; EOS, end of study; IDH, isocitrate dehydrogenase; MRI, magnetic resonance imaging; TCRSeq, T-cell receptor-sequencing.
Conclusion
IDH1R132H is a rare example of a shared driver mutation generating a clonal neoepitope. Preclinical data and the NOA16 trial provide evidence that active immunization using a long peptide is safe and induces mutation-specific proinflammatory CD4+ T-helper cell responses capable of infiltrating IDH1R132H-positive tumors. Single-cell sequencing analyses, TCR repertoire analyses, and TCR cloning not only provide a much more in-depth assessment of vaccine-induced T-cell responses, but also open the opportunity for adoptive T-cell therapy using TCR-transgenic T cells. Further developments include combination therapies with ICIs and IDH inhibitors.
Acknowledgments
Funding
This work was supported by the Dr Rolf M. Schwiete Foundation (no grant number), the Sonderförderlinie ‘Neuroinflammation’ of the Ministry of Science of Baden Württemberg (no grant number), the Joint Funding Program MGH-Heidelberg Alliance in Neuro-Oncology (no grant number), the Wilhelm Sander Foundation (2012.118.1), the NCT 3.0 program ‘Cancer immunotherapy program’ program ‘genetically modified cells for cancer immune therapy’ (no grant number), the Baden-Württemberg Stiftung [grant number BWST_ISF2018-046], German Cancer Aid [grant number 70112399], the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 404521405, SFB 1389—UNITE Glioblastoma.
Disclosure
MP and WW are inventors and patent-holders on ‘Peptides for use in treating or diagnosing IDH1R132H positive cancers’ (EP2800580B1). LB has declared no conflicts of interest.
References
- 1.Rizvi N.A., Hellmann M.D., Snyder A. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 3.Hilf N., Kuttruff-Coqui S., Frenzel K. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 4.Keskin D.B., Anandappa A.J., Sun J. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platten M., Offringa R. Cancer immunotherapy: exploiting neoepitopes. Cell Res. 2015;25(8):887–888. doi: 10.1038/cr.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslan K., Turco V., Blobner J. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat Commun. 2020;11(1):931. doi: 10.1038/s41467-020-14642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran E., Turcotte S., Gros A. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott P.A., Hu Z., Keskin D.B. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin U., Derhovanessian E., Miller M. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 10.Racle J., Michaux J., Rockinger G.A. Robust prediction of HLA class II epitopes by deep motif deconvolution of immunopeptidomes. Nat Biotechnol. 2019;37(11):1283–1286. doi: 10.1038/s41587-019-0289-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen B., Khodadoust M.S., Olsson N. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol. 2019;37(11):1332–1343. doi: 10.1038/s41587-019-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao D., Margolis C.A., Vokes N.I. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271–1281. doi: 10.1038/s41588-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milo I., Bedora-Faure M., Garcia Z. The immune system profoundly restricts intratumor genetic heterogeneity. Sci Immunol. 2018;3(29):eaat1435. doi: 10.1126/sciimmunol.aat1435. [DOI] [PubMed] [Google Scholar]
- 14.Touat M., Li Y.Y., Boynton A.N. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. doi: 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher T., Bunse L., Pusch S. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 16.Pellegatta S., Valletta L., Corbetta C. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun. 2015;3:4. doi: 10.1186/s40478-014-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weenink B., van Brakel M., Wijers R., Sillevis Smitt P.A.E., French P.J., Debets R. Lack of B and T cell reactivity towards IDH1(R132H) in blood and tumor tissue from LGG patients. J Neurooncol. 2019;144(1):79–87. doi: 10.1007/s11060-019-03228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunse L., Green E.W., Platten M. High-throughput discovery of cancer-targeting TCRs. Methods Enzymol. 2019;629:401–417. doi: 10.1016/bs.mie.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Green E.W., Bunse L., Bozza M., Sanghvi K., Platten M. TCR validation toward gene therapy for cancer. Methods Enzymol. 2019;629:419–441. doi: 10.1016/bs.mie.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Bunse L., Schumacher T., Sahm F. Proximity ligation assay evaluates IDH1R132H presentation in gliomas. J Clin Invest. 2015;125(2):593–606. doi: 10.1172/JCI77780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao R., Stapor D., Luke J.J. Molecular correlates and therapeutic targets in T cell-inflamed versus non-T cell-inflamed tumors across cancer types. Genome Med. 2020;12(1):90. doi: 10.1186/s13073-020-00787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Sorensen M.D., Kristensen B.W., Reifenberger G., McIntyre T.M., Lin F. D-2-Hydroxyglutarate is an intercellular mediator in IDH-mutant gliomas inhibiting complement and T cells. Clin Cancer Res. 2018;24(21):5381–5391. doi: 10.1158/1078-0432.CCR-17-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunse L., Pusch S., Bunse T. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24(8):1192–1203. doi: 10.1038/s41591-018-0095-6. [DOI] [PubMed] [Google Scholar]
- 24.Kohanbash G., Carrera D.A., Shrivastav S. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127(4):1425–1437. doi: 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottcher M., Renner K., Berger R. D-2-hydroxyglutarate interferes with HIF-1alpha stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization. Oncoimmunology. 2018;7(7):e1445454. doi: 10.1080/2162402X.2018.1445454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amankulor N.M., Kim Y., Arora S. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 2017;31(8):774–786. doi: 10.1101/gad.294991.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friebel E., Kapolou K., Unger S. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181(7):1626–1642.e1620. doi: 10.1016/j.cell.2020.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Klemm F., Maas R.R., Bowman R.L. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643–1660.e1617. doi: 10.1016/j.cell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedrich M., Sankowski R., Bunse L. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat Cancer. 2021 doi: 10.1038/s43018-021-00201-z. [DOI] [PubMed] [Google Scholar]
- 30.Kadiyala P., Carney S.V., Gauss J.C. Inhibition of 2-hydroxyglutarate elicits metabolic reprogramming and mutant IDH1 glioma immunity in mice. J Clin Invest. 2021;131(4):e139542. doi: 10.1172/JCI139542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platten M., Bunse L., Wick A. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021;592(7854):463–468. doi: 10.1038/s41586-021-03363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]