Abstract
Cardiovascular adverse events induced by immune checkpoint inhibitors (ICIs) have gained significant interest over the past decade due to their impact on short- and long-term outcomes. They were initially thought to be rare, but the increasing use of ICIs in the treatment of both advanced and early stages of various malignancies has resulted in a substantial increase in their incidence. Different guidelines have proposed screening measures for ICI-induced myocarditis by incorporating troponin measurements at baseline and during the first few weeks of treatment. However, no specific guidelines have been developed yet regarding the interpretation of an asymptomatic rise in troponins. This state-of-the art review aims to provide an overview of the clinical relevance of elevated troponins during checkpoint inhibition and recommendations on how to manage elevated troponin levels during ICI therapy.
Key words: cardiac troponin, cardiotoxicity, myocarditis, cancer, immunotherapy, biomarker
Highlights
-
•
The clinical interpretation and implication of troponin levels remain unclear.
-
•
Many mechanisms can contribute to an asymptomatic increase in troponinemia.
-
•
Conditions are urgently needed to correctly interpret troponin results.
-
•
Prospective studies are needed before recommending troponin surveillance as a standard of care.
Introduction
The development of immune checkpoint inhibitors (ICIs) has altered the field of oncology remarkably by achieving durable antitumor responses in many advanced malignancies with previously poor prognosis. ICIs are monoclonal antibodies that block inhibitory ligand–receptor interactions, essential for both immunological homeostasis and self-tolerance, in order to enhance the activity of the patient's immune system to fight cancer. In 2011, ipilimumab, an inhibitor of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), was the first ICI to gain approval by the United States Food and Drug Administration. In the following years, other ICIs became clinically available, that is, inhibitors of programmed cell death protein-1 (PD-1; nivolumab, pembrolizumab, cemiplimab) and its ligand, programmed cell death ligand-1 (PD-L1; atezolizumab, avelumab, durvalumab; Table 1).
Table 1.
Overview of the currently approved ICIs by the United States Food and Drug Administration
| Type of ICI | Drug | Indications | |
| Anti-CTLA-4 | Ipilimumab (YERVOY) | Melanoma NSCLC RCC HCC Colorectal cancer Malignant pleural mesothelioma |
|
| Anti-PD-1 | Pembrolizumab (KEYTRUDA) | Melanoma NSCLC SCLCcHL Urothelial cancer HNSCC RCC HCC cSCC |
Primary mediastinal large B-cell lymphoma Gastric cancer Esophageal cancer Cervical cancer Merkel cell carcinoma MSI-H or dMMR (colorectal) cancer Endometrial carcinoma Triple-negative breast cancer |
| Nivolumab (OPDIVO) | Melanoma NSCLC SCLC cHL Urothelial cancer HNSCC RCC HCC |
Esophageal cancer Gastric cancer Gastroesophageal junction cancer Malignant pleural mesothelioma MSI-H or dMMR colorectal cancer |
|
| Cemiplimab (LIBTAYO) | Cutaneous squamous cell carcinoma Basal cell carcinoma NSCLC |
||
| Anti-PD-L1 | Avelumab (BAVENCIO) | Merkel cell carcinoma RCC Urothelial cancer |
|
| Atezolizumab (TECENTRIQ) | Melanoma NSCLC SCLC Urothelial cancer HCC Triple-negative breast cancer |
||
| Durvalumab (IMFINZI) | NSCLC SCLC |
||
cHL, classical Hodgkin's lymphoma; cSCC, cutaneous squamous cell carcinoma; CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; dMMR, deficient mismatch repair; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; MSI-H, microsatellite instability-high; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1; RCC, renal cell carcinoma; SCLC, small-cell lung cancer.
Despite their important oncological benefit, the increasing use of ICIs led to the discovery of a distinct set of adverse events [AEs; i.e. immune-related adverse events (irAEs)]. The most commonly diagnosed irAEs include dermatologic, endocrine, and gastrointestinal AEs. Cardiovascular, pulmonary, hematological, renal, and neurological irAEs are usually more severe, although are less frequently seen.1
As survival increases, cardiovascular diseases have become more prominent both during and after cancer treatment which in turn led to the development of a new subspecialty, that is, ‘cardio-oncology’. Immune-related cardiovascular AEs (Table 2) were initially thought to be scarce, although a substantial increase in incidence has been reported over the past few years.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 A rare but potentially life-threatening cardiovascular irAE is myocarditis. There is a growing interest in this specific irAE as it has the highest mortality rate (up to 50%) among all cardiac irAEs.8 Especially ICI-induced myocarditis is thought to be underreported due to the wide varieties in clinical presentation, ranging from subclinical disease to a fulminant presentation. The lack of routine monitoring for cardiac events in immunotherapy trials has most likely contributed to the underreporting of ICI-induced cardiotoxicities. The detection of increased troponin levels might lead to a temporary discontinuation of ICI therapy until further investigations are performed, which may increase the risk of disease progression by withholding life-saving therapy. Therefore some institutions have started to determine cardiac troponin (cTn) levels in an attempt to detect subclinical forms of ICI-induced myocarditis and introduce prompt initiation of therapy potentially mitigating cardiac morbidity and mortality. cTn measurements are most often conducted at baseline and regular intervals up to 12 weeks (first three to four doses) after treatment initiation, covering the relatively short timeframe during which ICI-induced myocarditis is most likely to occur.12
Table 2.
Possible cardiovascular irAEs in patients treated with ICIs
| ICI-related cardiovascular toxicities | |
|---|---|
| Myocarditis | |
| Pericardial disease |
|
| Perimyocarditis | |
| Vasculitis |
|
| LVD |
|
| Acute coronary syndromes (e.g. myocardial infarction, angina pectoris) | |
| Arrhythmias and cardiac conduction abnormalities |
|
ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; LVD, left ventricular dysfunction.
Cardiac biomarkers have become a cornerstone in the diagnosis of cardiovascular diseases. However, their role in cardio-oncology, more specifically in the active surveillance of ICI-mediated cardiotoxic effects and to identify patients at increased risk, is not clear. Previous research has focused on the interpretation of cardiac troponin levels upon conventional anticancer therapies (i.e. anthracyclines, HER2-targeted therapies, and antivascular endothelial growth factor therapy) and their prognostic value in therapy-induced cardiotoxicity.13, 14, 15 On the contrary, data regarding the relationship between troponins and ICI therapy are scarce and inconsistent. Nevertheless, numerous cancer centers have already incorporated serial troponin assessment in their ICI treatment programs. However, in the absence of evidence-based guidelines, this strategy may lead to clinical challenges, inadequate steroid use, and early cessation of life-prolonging therapy. In this review, we present an overview of the current evidence regarding troponin assessments in patients with cancer treated with ICIs and stress the need for further research before recommending troponin surveillance as a standard of care in all of these patients. Moreover, possible mechanisms contributing to asymptomatic troponin elevations will be discussed together with future recommendations.
Troponin
The use of troponin T (TnT) and troponin I (TnI) as serum markers of cardiomyocyte injury was first described in the late 1970s and 1980s.16,17 They have become indispensable in the diagnosis of acute coronary syndromes. The development of a new generation of highly sensitive assays has allowed us to detect minimal troponin levels with high precision. However, the availability of multiple vendor-specific assays causes an impediment upon comparison of studies due to the varying 99th percentiles and limits of detection.18, 19, 20, 21, 22, 23 Nevertheless, the increase in sensitivity resulted in the detection of elevated troponin values in conditions other than myocardial infarction, also referred to as myocardial injury.24
Baseline troponin measurement prior to ICI therapy
Aside from performing a baseline electrocardiogram (ECG), echocardiogram, and cardiovascular risk assessment in all patients prior to ICI treatment, there is a growing consensus to perform troponin measurements.1,6,9,13,25, 26, 27, 28 Recently, the Heart Failure Association Cardio-Oncology Study Group and the International Cardio-Oncology Society published risk stratification guidelines for different cardiotoxic anticancer therapies where they highly recommended pretreatment troponin determination.29 On the contrary, no recommendations for or against baseline troponin measurements were mentioned in the American Society of Clinical Oncology 2017 guidelines nor in the European Society for Medical Oncology of 2017 and 2020 recommendations.30, 31, 32 It should also be noted that clinical trials with ICIs have not yet incorporated baseline troponin evaluation. We only found a small number of studies investigating baseline troponin levels prior to ICI therapy.
Petricciuolo et al.33 was the first study to investigate the prognostic value of high-sensitivity TnT (hs-TnT) levels in 30 patients prior to anti-PD-1/PD-L1 treatment. At 3 months, the primary endpoint of cardiovascular death, stroke or transient ischemic attack, pulmonary embolism, nonfatal myocardial infarction, and new-onset heart failure was met in seven patients. All of these patients had baseline TnT levels ≥14 ng/l. Furthermore, 13 patients experienced the secondary endpoint (i.e. progression of cardiac involvement based on the CARDIOTOX classification). Interestingly, 9 out of 13 patients had baseline troponin levels ≥14 ng/l.34 The authors suggested that 14 ng/l was the best cutoff value to predict the primary and secondary endpoints at 3 months.33 Lee Chuy et al.35 performed an ECG and TnI measurement in 76 patients with advanced melanoma at baseline and weekly until the second dose of combination ICI therapy (ipilimumab and nivolumab). Minimal baseline elevations were seen in five patients (0.02 ng/ml). Four out of five patients remained asymptomatic, while one patient experienced symptoms of pneumonia, although all patients remained hemodynamically stable over a median follow-up of 198 days. A thorough description was given of the detectable TnI cases at baseline (≥0.01 ng/ml and <0.06 ng/ml); however, this was not listed for the other patients. Although the authors stated that complementary testing was performed for each of these patients and did not show abnormalities, this was not further specified.35 Baseline TnI measurements were also performed in 59 patients with advanced non-small-cell lung cancer who had received at least one previous line of systemic therapy. Patients were divided into three groups according to the estimated risk of troponin release prior to nivolumab treatment. Patients with a history of cardiac disease were allocated to the very-high-risk group. The high-risk group consisted of patients with an extracardiac target organ disease (e.g. stage III-IV chronic kidney disease or diabetes). Patients in the low-risk group had none of the aforesaid diseases. Baseline TnI appeared to be elevated (>0.015 ng/ml) in only three patients, which the authors attributed to the presence of pre-existing cardiovascular disease (i.e. chronic heart failure, paroxysmal atrial fibrillation, aortic valve stenosis, and coronary artery disease). None of these patients developed cardiovascular events. It is important to note that other patients that were part of the very-high-risk/high-risk group did not have elevated troponin levels at baseline. Furthermore, 86% of the patients had received up to three lines of treatment prior to nivolumab initiation. No details on the prior treatment regimens were provided, although these could also have contributed to baseline elevations. One patient, allocated to the low-risk group, with a normal TnI at baseline developed a sustained troponin increase shortly after treatment initiation, which was interpreted as a marker of nivolumab-related subclinical myocarditis.36 Waliany et al.37 determined baseline hs-TnI values in 101 patients, as they also included 113 patients who had already started ICI treatment before cardiotoxicity surveillance. They defined positive levels as hs-TnI ≥55 ng/l. Although the authors mention that patients with positive hs-TnI levels were evaluated by a multidisciplinary cardio-oncology team, no data were published yet regarding the specific baseline values and their interpretation (Table 3).37
Table 3.
Troponin and immune checkpoint inhibitor studies
| Authors | Patient number | ICI | Major findings concerning troponins |
|---|---|---|---|
| Waliany et al.37 | 214 | Not specified (PD-1, PD-L1, CTLA-4) | 24 patients had positive hs-TnI (≥55 ng/l) levels, whereas 3 had myocarditis. In the other 21 patients these values were attributed to type 2 NSTEMI secondary to other etiologies |
| Petricciuolo et al.33 | 30 | Pembrolizumab, nivolumab, atezolizumab, durvalumab | A baseline hs-TnT ≥ 14 ng/l was found to be a good predictor for cardiovascular death, stroke or transient ischemic attack, pulmonary embolism, nonfatal myocardial infarction, new-onset heart failure, and also progression of cardiac involvement at 3 months |
| Lee Chuy et al.35 | 76 | Ipilimumab + nivolumab | Minimally detectable nondiagnostic TnI levels (≥0.01 ng/ml to <0.06 ng/ml) were seen in 13 patients. None developed clinical or subclinical myocarditis or MACE |
| Sarocchi et al.36 | 59 | Nivolumab | Hs-TnI levels above the ULN (0.046 ng/ml) were seen in seven patients, one at baseline and six during treatment. In only one patient this was interpreted as subclinical ICI-induced myocarditis |
CTLA-4, cytotoxic T-lymphocyte-associated antigen-4; hs-TnT, high-sensitivity troponin T; ICI, immune checkpoint inhibitor; MACE, major adverse cardiac events; NSTEMI, non-ST-elevation myocardial infarction; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1; ULN, upper limit of normal.
We can conclude that the data regarding baseline troponin measurements in patients prior to ICI treatment are limited and elevations are most often attributed to cardiac disease and/or comorbidities. Some guidelines have even recommended to withhold ICI therapy when abnormal biomarkers are detected in asymptomatic patients, although studies supporting this strategy are lacking.1,12 Therefore the value and clinical implication of baseline troponin levels remain unclear, although it might be useful as a reference value to interpret subsequent changes during ICI therapy.
Serial troponin determination during ICI therapy
The measurement of troponin levels upon signs and symptoms of cardiovascular toxicities during ICI therapy (chest pain, arrhythmia, palpitations, peripheral edema, progressive or acute dyspnea, pleural effusion, and fatigue) has remained unquestionable.1,32 Nevertheless, no consensus has been achieved regarding the timing and frequency of troponin measurement and its use to assess ICI-related cardiac injury among patients during treatment.
A prospective study, conducted by Lee Chuy et al.,35 obtained troponin levels every week until the second dose of dual checkpoint inhibition and found them to be minimally elevated in 11 out of 76 patients (≥0.01 and <0.06 ng/ml). None of the patients developed myocarditis nor major adverse cardiac events over a median of 198 days.35 However, the authors did not provide details on how they ruled out (subclinical) myocarditis in these patients. A total of 362 blood samples from 59 patients receiving nivolumab as treatment for advanced non-small-cell lung cancer were analyzed for TnI levels by Sarocchi et al.36 Samples were obtained prior to the first five administrations followed by a sample at every other infusion. Troponin levels were interpreted along with cardiac comorbidities, signs and symptoms, ECG, echocardiography, and disease progression, although the latter was not further specified. Normal, but detectable TnI levels (0.015-0.045 ng/ml) were observed in 14 patients, whereas six patients had at least one positive troponin level (≥0.046 ng/ml). One patient had persistently elevated troponin values from week 8 onward. Although there was no history of cardiac disease, symptoms, or echocardiographic wall-motion abnormalities, the authors interpreted this as a subclinical, self-limiting myocarditis. However, it should be noted that the diagnosis of myocarditis requires at least two criteria (significant changes in cTn, ECG, echocardiography, or cardiovascular magnetic resonance imaging) to be fulfilled in asymptomatic patients.38 On the contrary, the positive troponin levels in the other five patients were noted at different time points. Consistently elevated levels were seen in a patient with chronic heart failure who also had an elevated baseline TnI. The other four patients had elevated levels in the last samples right before nivolumab was discontinued, which the authors attributed to the deterioration of the patient’s clinical status and/or cancer progression.36 However, this study shows major methodological weaknesses and the results should be interpreted with caution. Another prospective study implemented active surveillance for ICI-associated myocarditis during monotherapy as well as combination therapy for a total of 9 months. Measurements of hs-TnI were performed at baseline and after each ICI dose (up to 10 doses). A total of 1274 hs-TnI measurements were performed; 24 of 214 patients (11.2%) had a rise in TnI levels (≥55 ng/l). However, only three were defined as ICI-associated myocarditis, whereas the other 21 cases were linked to type 2 non-ST-segment elevation myocardial infarction secondary to other etiologies which were not further specified. None of these patients developed a decrease in left ventricular ejection fraction (LVEF; Table 3).37
Serial troponin measurements have mostly been carried out right before each cycle to avoid additional hospital visits. However, the appropriate time interval between tests as well as the cutoff values for clinically meaningful changes remain unknown and vary among studies. No evidence has been provided yet on the additional value of serial troponin measurements. Nevertheless, different guidelines and studies recommend serial biomarker sampling, especially in patients at high risk for cardiotoxic effects (i.e. an abnormal baseline assessment, a high cardiovascular risk profile, and/or receiving combination therapy).2,6,9,13,25,39, 40, 41
Asymptomatic troponin elevations during ICI therapy: possible underlying mechanisms
Manifestations of ICI-induced cardiotoxicities are highly variable. Especially in myocarditis, the presentation can range from subclinical disease to fatigue, chest pain, heart failure, cardiogenic shock, arrhythmias, and sudden death. The mortality rate remains high while the underlying pathophysiological mechanisms are still poorly understood.5 Therefore, various guidelines recommend screening of troponin levels for early suspicion, diagnosis, and management of subclinical ICI-induced cardiovascular toxicities. Many published myocarditis reports indeed feature elevated troponin levels.41,42 However, it is important to keep in mind that troponin is a nonspecific marker of myocardial injury. Hence, not all troponin elevations observed during ICI therapy necessarily imply that these patients have or will develop ICI-induced myocarditis. Moreover, the studies that investigated baseline and serial troponin levels in patients with cancer treated with ICIs often found elevations in asymptomatic patients who did not experience a clinically significant cardiac AE. The interpretation, diagnosis, and therapeutic strategies of elevated levels of cTn in these patients challenge both cardiologist and oncologist.
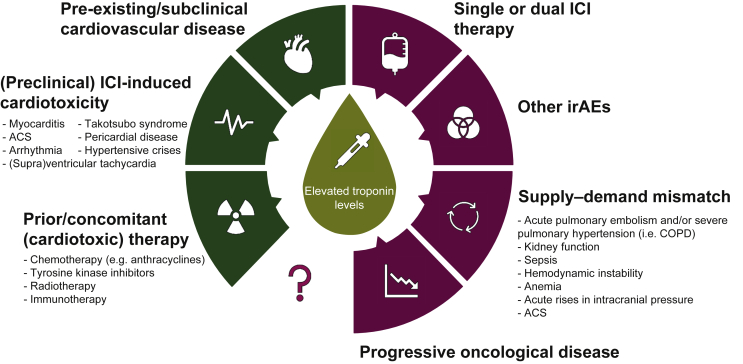
Different factors and circumstances have to be taken into consideration to interpret cTn elevations in patients with cancer treated with ICIs (Figure 1).
Figure 1.
Possible causes of elevated troponin levels during ICI therapy.
The increase in troponin levels seen in patients prior to and during ICI therapy can be caused by numerous factors ranging from prior/concomitant therapy to mechanisms that are not yet fully understood. The cardiotoxicity-related causes are illustrated in dark green (left) and the noncardiac causes are depicted in purple (right).
ACS, acute coronary syndrome; COPD, chronic obstructive pulmonary disease; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events.
First, it is important to realize that no definite conclusions can be drawn from a single aberrant value.26,43,44 Hence, re-evaluation should be performed within 24 h to determine the dynamics and extent of troponin elevation along with other laboratory parameters. Acute coronary syndromes are usually characterized by a rise and fall pattern in troponin levels while a steady increase is usually observed in myocarditis.2,12,23
Second, elevated levels have previously been reported in patients with cancer which were subsequently associated with worse clinical outcomes, a more advanced tumor stage, and all-cause mortality. Furthermore, the deterioration of the patient's clinical status and/or progressive oncological disease during ICI treatment may cause troponin abnormalities. An elevated cTn level could therefore act as a prognostic biomarker of disease progression rather than for an adverse cardiovascular event.36,45, 46, 47
Third, patients with cardiac disease (e.g. heart failure, coronary artery disease) sometimes have stable but mildly elevated troponin levels.48 ICIs often trigger a general increase in systemic inflammation which could also cause an acceleration or decompensation of pre-existing cardiac disease.6
Fourth, noncardiac disease (e.g. chronic kidney disease) and anemia, sepsis, or hemodynamic instability are commonly seen in patients with cancer and are known to cause elevated troponin levels.23,36,49
Fifth, some patients receiving ICIs have already been pretreated with other cancer therapies that are known to cause an increase in troponin levels (i.e. anthracycline-containing chemotherapy, various high-dose chemotherapy regimens with or without anthracyclines, myeloablative therapy, HER2 inhibitor therapy, and thoracic radiotherapy). Prior cardiotoxic therapy may cause subclinical cardiac problems that could possibly result in elevated troponin levels during ICI treatment.14 Furthermore, many ongoing clinical trials are investigating the combination of checkpoint blockade with non-ICI therapies to explore possible synergistic effects. For example, the combination of ICIs with targeted therapies, such as tyrosine kinase inhibitors, has already been investigated and resulted in an enhanced efficacy in advanced renal cell carcinoma and BRAF-mutant melanoma.50,51 However, drug-induced cardiotoxicity has been described for both therapeutic classes and might potentiate each other.52
Sixth, the risk of cardiac irAEs may vary according to the proposed regimen. The risk of cardiovascular complications seen upon PD-1/PD-L1 and combination therapy was much higher as opposed to CTLA-4 therapy.8,53 Furthermore, Tay et al.54 provided insights into cardiomyopathy resulting from PD-1-PD-L1 axis blockade, by using nivolumab in vitro in Rockefeller University embryonic stem cells (RUES)-derived cardiomyocytes and in vivo in melanoma tumor-bearing mice. They observed cardiomyocyte inflammation and apoptosis along with an increased expression of cardiac TnI and left ventricular dilatation.54 Thus, elevated troponin levels might indicate low-grade or scattered myocardial inflammation as a result of blocking the PD-1–PD-L1 axis which might not yet be visual on cardiac imaging.55,56 The best technique to diagnose subclinical cardiac dysfunction has not yet been established. For example, both global longitudinal strain and global circumferential strain can be measured with echocardiography or cardiac magnetic resonance imaging. Strain imaging is a more sensitive technique than the measurement of LVEF as a parameter of systolic function. It is important to note that little to no attention has been granted to diastolic function that might precede future clinical detectable cardiovascular AEs.57
Seventh, other irAEs, apart from cardiovascular irAEs, may also lead to elevated TnT levels. This was suggested by Sarocchi et al.,36 who noted troponin elevations with different irAEs (thyroid, liver, and gastrointestinal tract), although it remained unclear to which extent other causes were explored. This might be explained by a mismatch in myocardial oxygen demand–supply. Elevated troponin levels can also be detected in patients with myositis, myasthenia gravis, and/or myalgia. These irAEs often tend to overlap with cardiac dysfunction.26,58
Eighth, some patients with cancer might have an undiagnosed/pre-existing autoimmune disease (e.g. polymyositis, rheumatoid arthritis, systemic lupus erythematosus, and sarcoidosis) which could contribute to myocardial inflammation, elevated troponin levels, and possible future ICI-induced cardiotoxicities.38,59, 60, 61
Last, subclinical forms of myocarditis may initially present themselves by isolated abnormal troponin levels.62 In a case–control study conducted by Cautela et al.,63 15 out of 60 patients (25%) presented with an asymptomatic troponin elevation. Especially high troponin levels should warrant prompt investigation, as two different forms of ICI-induced myocarditis have been identified, namely, a high-grade and a low-grade from. Patients in the high-grade myocarditis group had higher serum TnT levels and a shorter interval to diagnosis as opposed to those in the low-grade group.64
Although several guidelines are recommending troponin measurements, there is a current lack of evidence to perform baseline and serial troponin measurements. However, in the absence of recommendations on how to manage elevated troponins in this population, oncologists and cardiologists are confronted with diagnostic and therapeutic challenges. Withholding or discontinuing ICIs can lead to early cessation of life-prolonging therapy. Moreover, inadequate steroid use can further stimulate tumor progression by compromising the efficacy of ICI therapy.
If troponin elevations are seen during baseline assessment, patients should be referred to a cardiologist to interpret these values along with a cardiovascular risk assessment (age, hypertension, tobacco use, hyperlipidemia, diabetes, overweight/obese, family history).65, 66, 67, 68, 69, 70, 71, 72, 73, 74 If a rise in troponins is observed during ICI therapy, it should be interpreted along with the patient's baseline value, current disease status, medical history, and a thorough clinical work-up, that is, symptoms, extensive blood analysis, and cardiovascular assessment (ECG, echocardiogram). We would also recommend determination of the fasting lipid profile in all patients prior to ICI therapy as ICIs have recently been associated with a threefold higher risk for atherosclerotic cardiovascular events. This could also result in atherosclerosis-related troponin elevation and potentially serious cardiovascular complications.25,75,76 Furthermore, echocardiography in patients receiving ICI therapy should perhaps pay more attention to the patient's diastolic function and strain imaging as this could depict abnormalities preceding alterations in the patient's LVEF. If minimal troponin elevations are observed, measurements should be repeated after a few days. High levels, in the absence of any other causes, should warrant immediate referral to a cardiologist.
However, we should note that our recommendations are currently based on anecdotal evidence and expert opinions. Multidisciplinary interaction between oncologists and cardiologists is imperative to develop further insights into the clinical relevance of elevated troponin levels. Moreover, there is an urgent need to identify which specific patients could potentially benefit from troponin surveillance during ICI treatment.
Conclusion and future perspective
The lack of routine monitoring for cardiac events in immunotherapy trials has most likely contributed to the underreporting of ICI-induced cardiotoxicities.77 Although cardiac irAEs seem rare, they often have a nonspecific clinical presentation and the potential to cause rapid clinical deterioration. Hence, there is an urgent need for biomarkers to predict cardiac irAEs and to identify patients at risk. Nevertheless, in the absence of robust evidence several guidelines have already started to recommend baseline and serial troponin assessment which led to the implementation of standard laboratory measurements during ICI treatment in multiple cancer centers.
In fact, both baseline and serial troponin measurements are widely available and can easily be performed with routine blood analyses, further making cTn an attractive biomarker. These blood analyses are considered to be noninvasive as they are linked to other scheduled blood tests which are carried out prior to each treatment cycle, minimizing hospital visits. Altogether, cTn assessment during treatment has found its way as a biomarker for cardiac irAEs in patients treated with ICI.39
In our opinion, this new approach has led to diagnostic and therapeutic challenges for both the oncologist and cardiologist. The detection of increased troponin levels might lead to a temporary discontinuation of ICI therapy until further investigations are performed, which may increase the risk of disease progression by withholding life-saving therapy. Currently, there is no good evidence to support the recommendation of several guidelines to assess cTn in all patients receiving ICI therapy. Moreover, inadequate steroid use could compromise the prognosis of many asymptomatic patients receiving ICI therapy even further. Nevertheless, it should be noted that some patients could potentially benefit from troponin surveillance, for example, patients receiving ICI combination therapy or ICIs with other agents with established toxicities, or with an abnormal baseline cardiac investigation, or with a previous cardiovascular history.
To our knowledge, this is the first review to provide an overview of studies that investigated troponin levels prior to and during ICI therapy. Nevertheless, the reported studies are limited and contain many methodological weaknesses.
In conclusion, the exact role of troponin in the early diagnosis of myocardial injury as well as in the prediction of cardiotoxicity among ICI therapy remains unknown. Guidelines should be established by both cardiologists and oncologists regarding the interpretation of aberrant troponin levels. However, based on the current evidence, it should be questioned whether troponin measurements can already be implemented as a standard of care in all patients treated with ICIs. Future prospective studies are needed to clarify the role of troponins in patients receiving ICI therapy and identify which patients may benefit from troponin surveillance.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D.Y., Okoye G.D., Neilan T.G., Johnson D.B., Moslehi J.J. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19:21. doi: 10.1007/s11886-017-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain V., Bahia J., Mohebtash M., Barac A. Cardiovascular complications associated with novel cancer immunotherapies. Curr Treat Options Cardiovasc Med. 2017;19:36. doi: 10.1007/s11936-017-0532-8. [DOI] [PubMed] [Google Scholar]
- 4.Michel L., Rassaf T., Totzeck M. Cardiotoxicity from immune checkpoint inhibitors. IJC Heart Vasc. 2019;25:100420. doi: 10.1016/j.ijcha.2019.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 6.Lyon A.R., Yousaf N., Battisti N.M.L., Moslehi J., Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 7.Pirozzi F., Poto R., Aran L. Cardiovascular toxicity of immune checkpoint inhibitors: clinical risk factors. Curr Oncol Rep. 2021;23:1–8. doi: 10.1007/s11912-020-01002-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem J.E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J.R., Florido R., Lipson E.J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–868. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daxini A., Cronin K., Sreih A.G. Vasculitis associated with immune checkpoint inhibitors—a systematic review. Clin Rheumatol. 2018;37:2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 11.Chen D.Y., Huang W.K., Chien-Chia Wu V. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: a review when cardiology meets immuno-oncology. J Formos Med Assoc. 2020;119:1461–1475. doi: 10.1016/j.jfma.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Spallarossa P., Sarocchi M., Tini G. How to monitor cardiac complications of immune checkpoint inhibitor therapy. Front Pharmacol. 2020;11:972. doi: 10.3389/fphar.2020.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pudil R., Mueller C., Čelutkienė J. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–1983. doi: 10.1002/ejhf.2017. [DOI] [PubMed] [Google Scholar]
- 14.Michel L., Mincu R.I., Mahabadi A.A. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22(2):350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale D., Sandri M.T., Martinoni A. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36(2):517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 16.Cummins P., McGurk B., Littler W.A. Possible diagnostic use of cardiac specific contractile proteins in assessing cardiac damage. Clin Sci. 1979;56(3):30P. [Google Scholar]
- 17.Katus H.A., Remppis A., Looser S., Hallermeier K., Scheffold T., Kübler W. Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol. 1989;21(12):1349–1353. doi: 10.1016/0022-2828(89)90680-9. [DOI] [PubMed] [Google Scholar]
- 18.Apple F.S., Collinson P.O. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 19.Boeddinghaus J., Twerenbold R., Nestelberger T. Clinical use of a new high-sensitivity cardiac troponin I assay in patients with suspected myocardial infarction. Clin Chem. 2019;65(11):1426–1436. doi: 10.1373/clinchem.2019.304725. [DOI] [PubMed] [Google Scholar]
- 20.Body R., Twerenbold R., Austin C. Diagnostic accuracy of a high-sensitivity cardiac troponin assay with a single serum test in the emergency department. Clin Chem. 2019;65(8):1006–1014. doi: 10.1373/clinchem.2018.294272. [DOI] [PubMed] [Google Scholar]
- 21.Boeddinghaus J., Nestelberger T., Twerenbold R. High-sensitivity cardiac troponin i assay for early diagnosis of acute myocardial infarction. Clin Chem. 2019;65(7):893–904. doi: 10.1373/clinchem.2018.300061. [DOI] [PubMed] [Google Scholar]
- 22.Sörensen N.A., Neumann J.T., Ojeda F. Diagnostic evaluation of a high-sensitivity troponin i point-of-care assay. Clin Chem. 2019;65(12):1592–1601. doi: 10.1373/clinchem.2019.307405. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan V.S., Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124(21):2350–2354. doi: 10.1161/CIRCULATIONAHA.111.023697. [DOI] [PubMed] [Google Scholar]
- 24.Sarkisian L., Saaby L., Poulsen T.S. Prognostic impact of myocardial injury related to various cardiac and noncardiac conditions. Am J Med. 2016;129(5):506–514.e1. doi: 10.1016/j.amjmed.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Puzanov I., Diab A., Abdallah K. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonaca M.P., Olenchock B.A., Salem J.E. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(1):80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson J.A., Schneider B.J., Brahmer J. 2021. NCCN Guidelines Version 2.2021 Management of Immunotherapy-Related Toxicities NCCN Guidelines Panel Disclosures Continue NCCN. [Google Scholar]
- 28.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 29.Lyon A.R., Dent S., Stanway S. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armenian S.H., Armstrong G.T., Aune G. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36:2135–2144. doi: 10.1200/JCO.2017.76.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haanen J.B.A.G., Carbonnel F., Robert C. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 32.Curigliano G., Lenihan D., Fradley M. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petricciuolo S., Delle Donne M.G., Aimo A., Chella A., De Caterina R. Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur J Clin Invest. 2020;51(4):e13400. doi: 10.1111/eci.13400. [DOI] [PubMed] [Google Scholar]
- 34.López-Sendón J., Álvarez-Ortega C., Zamora Auñon P. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41(18):1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 35.Lee Chuy K., Oikonomou E.K., Postow M.A. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center experience. Oncologist. 2019;24(5):e196–e197. doi: 10.1634/theoncologist.2019-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarocchi M., Grossi F., Arboscello E. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist. 2018;23(8):936–942. doi: 10.1634/theoncologist.2017-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waliany S., Neal J.W., Reddy S. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. JACC Cardio Oncol. 2021;3(1):137–139. doi: 10.1016/j.jaccao.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caforio A.L.P., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 39.Ganatra S., Neilan T.G. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23(8):879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller O.J., Spehlmann M.E., Frey N. Cardio-toxicity of checkpoint inhibitors. J Thoracic Disease. 2018;10:S4400–S4404. doi: 10.21037/jtd.2018.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradhan R., Nautiyal A., Singh S. Diagnosis of immune checkpoint inhibitor-associated myocarditis: a systematic review. Int J Cardiol. 2019;296:113–121. doi: 10.1016/j.ijcard.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaffe A.S., Vasile V.C., Milone M., Saenger A.K., Olson K.N., Apple F.S. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. 2011;58(17):1819–1824. doi: 10.1016/j.jacc.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Hughes M., Lilleker J.B., Herrick A.L., Chinoy H. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis. 2015;74:795–798. doi: 10.1136/annrheumdis-2014-206812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavo N., Raderer M., Hülsmann M. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 46.Lim E., Choy L.L., Flaks L. Detected troponin elevation is associated with high early mortality after lung resection for cancer. J Cardiothorac Surg. 2006;1(1):37. doi: 10.1186/1749-8090-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danese E., Montagnana M., Giudici S. Highly-sensitive troponin I is increased in patients with gynecological cancers. Clin Biochem. 2013;46(12):1135–1138. doi: 10.1016/j.clinbiochem.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 48.Westermann D., Neumann J.T., Sörensen N.A., Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol. 2017;14:472–483. doi: 10.1038/nrcardio.2017.48. [DOI] [PubMed] [Google Scholar]
- 49.Twerenbold R., Badertscher P., Boeddinghaus J. 0/1-Hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation. 2018;137(5):436–451. doi: 10.1161/CIRCULATIONAHA.117.028901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rini B.I., Plimack E.R., Stus V. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–11127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 51.Ascierto P.A., Ferrucci P.F., Fisher R. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med. 2019;25:941–946. doi: 10.1038/s41591-019-0448-9. [DOI] [PubMed] [Google Scholar]
- 52.Guo C.W., Alexander M., Dib Y. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur J Cancer. 2020;124:15–24. doi: 10.1016/j.ejca.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tay W.T., Fang Y.H., Beh S.T. Programmed cell death-1: programmed cell death-ligand 1 interaction protects human cardiomyocytes against T-cell mediated inflammation and apoptosis response in vitro. Int J Mol Sci. 2020;21(7):2399. doi: 10.3390/ijms21072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kindermann I., Barth C., Mahfoud F. Update on myocarditis. J Am College Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 56.Friedrich M.G., Sechtem U., Schulz-Menger J. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blaes A.H., Thavendiranathan P., Moslehi J. Cardiac toxicities in the era of precision medicine: underlying risk factors, targeted therapies, and cardiac biomarkers. Am Soc Clin Oncol Educ B. 2018;38:764–774. doi: 10.1200/EDBK_208509. [DOI] [PubMed] [Google Scholar]
- 58.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2):e013757. doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varricchi G., Galdiero M.R., Tocchetti C.G. Cardiac toxicity of immune checkpoint inhibitors: cardio-oncology meets immunology. Circulation. 2017;136:1989–1992. doi: 10.1161/CIRCULATIONAHA.117.029626. [DOI] [PubMed] [Google Scholar]
- 60.Johnson D.B., Sullivan R.J., Ott P.A. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2:234–240. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 61.Tison A., Quéré G., Misery L. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100–2111. doi: 10.1002/art.41068. [DOI] [PubMed] [Google Scholar]
- 62.Norwood T.G., Westbrook B.C., Johnson D.B. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5(1):91. doi: 10.1186/s40425-017-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cautela J., Zeriouh S., Gaubert M. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer. 2020;8(2):1887. doi: 10.1136/jitc-2020-001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Champion S.N., Stone J.R. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod Pathol. 2020;33:99–108. doi: 10.1038/s41379-019-0363-0. [DOI] [PubMed] [Google Scholar]
- 65.Blaes A., Prizment A., Koene R.J., Konety S. Cardio-oncology related to heart failure: common risk factors between cancer and cardiovascular disease. Heart Fail Clin. 2017;13:367–380. doi: 10.1016/j.hfc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter B.D., Abnet C.C., Feskanich D. Smoking and mortality—beyond established causes. N Engl J Med. 2015;372(7):631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 68.Stocks T., Van Hemelrijck M., Manjer J. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension. 2012;59(4):802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 69.Mendonça F.M., De Sousa F.R., Barbosa A.L. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64:182–189. doi: 10.1016/j.metabol.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Giovannucci E., Harlan D.M., Archer M.C. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 72.Dobbins M., Decorby K., Choi B.C.K. The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med. 2013;2013:1–16. doi: 10.5402/2013/680536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid D., Behrens G., Keimling M., Jochem C., Ricci C., Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30:397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 74.Cao Y., Keum N.N., Chan A.T., Fuchs C.S., Wu K., Giovannucci E.L. Television watching and risk of colorectal adenoma. Br J Cancer. 2015;112(5):934–942. doi: 10.1038/bjc.2014.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drobni Z.D., Alvi R.M., Taron J. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poels K., van Leent M.M.T., Boutros C. Immune checkpoint inhibitor therapy aggravates T cell–driven plaque inflammation in atherosclerosis. JACC CardioOncol. 2020;2(4):599–610. doi: 10.1016/j.jaccao.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agostinetto E., Eiger D., Lambertini M. Cardiotoxicity of immune checkpoint inhibitors: a systematic review and meta-analysis of randomised clinical trials. Eur J Cancer. 2021;148:76–91. doi: 10.1016/j.ejca.2021.01.043. [DOI] [PubMed] [Google Scholar]