A short look to epidemiological and historical data
Primary diffuse large B-cell lymphoma (DLBCL) of the central nervous system (CNS) (PCNSL) is a rare and aggressive lymphoproliferative disease that was recently recognized as a distinct entity by the 2017 World Health Organization classification of hematopoietic and lymphoid tumors.1 PCNSL accounts for 4% of primary CNS neoplasms and 4%-6% of extranodal lymphomas. New cases are generally diagnosed in the fifth or sixth decade of life, and with a slightly higher frequency among males.2 Except for immunosuppression status, no other risk factors predisposing the development of PCNSL are known.
PCNSL first look: a multidisciplinary approach
Management of patients with newly diagnosed PCNSL is complex and requires a multidisciplinary approach (Table 1). Clinical presentation of PCNSL is heterogeneous and depends on the involved CNS structures that could include brain, leptomeninges, eyes, cranial nerves, and spinal cord. Symptoms are mainly focal neurological deficits, personality changes, and increased intracranial pressure, and usually require immediate evaluation by neurologists and/or neurosurgeons, with a subsequent neuroimaging examination. Contrast-enhanced brain magnetic resonance imaging (MRI) (bMRI) is the best modality option for assessing PCNSL patients. Lesions are often isointense/hypointense on T2-weighted MRI, with surrounding edema and a homogeneous and strong pattern of enhancement. PCNSL presents as a solitary lesion in 60%-70% of cases, mostly located in the hemispheres, basal ganglia, corpus callosum, and periventricular regions. It tends to infiltrate the subependymal tissues and disseminates through the cerebrospinal fluid (CSF) to the meninges, which is often asymptomatic and detectable by cytology/immunophenotypic analysis on CSF in 16% of cases. Some patients (10%-20%) experience visual complaints (i.e. floaters, blurred vision, visual field defects, and decrease in vision) as initial and often exclusive symptoms. This ‘alternative’ presentation needs to be taken into account in the differential diagnosis of the ‘masquerade syndromes’ from an expert ophthalmologist who will confirm the presence of intraocular disease. An accurate staging disease aims both to define the extension of disease in different CNS structures and to rule out concomitant systemic lymphoma. Conventional staging with [18F]2-fluoro-2-deoxy-D-glucose-positron emission tomography (18FDG-PET), bone marrow biopsy/aspiration and testicular ultrasound, detects extra-CNS disease in 4%-12% of patients with presumptive diagnosis of PCNSL. Although it is used as a fast and sensitive procedure to exclude systemic disease during staging, 18FDG-PET plays a limited role to assess PCNSL lesions and response to treatment, mostly due to physiologic tracer accumulation in the brain. Recently, the novel PET tracer [68Ga]Ga-PentixaFor, used for the visualization of C-X-C chemokine receptor 4 (CXCR4)-positive malignancies, showed interesting applicability in both visualization and treatment response assessment of CNS lymphoma.3 Prospective assessment of this new theranostic agent in PCNSL is warranted.
Table 1.
Diagnostic and staging work up in PCNSL
| Test | Aims |
|---|---|
| Diagnostic approaches | |
| Radiology assessment: Brain MRI; spinal MRI (only in symptomatic cases or with CSF positivity) |
To assess CNS involvement and prognostic factors (such as deep lesions) |
| Stereotactic brain lesion biopsy (more rarely open brain biopsy): Morphology; IHC; molecular/cytogenetic analysis |
To carry out histopathological diagnosis on the primary CNS lesion |
| Vitrectomy and/or vitreous and/or humor aspirate (in cases of suspected ocular involvement): Morphology; immunophenotype/IHC |
To carry out cytological diagnosis on primary vitreous-retina lesion |
| CSF analysis: Cytology Immunophenotype Molecular assessment: MYD88 mutational status and IL-10 levels in CSF |
To assess leptomeningeal dissemination To confirm the PCNSL suspicion in cases of CNS lesions not suitable for biopsy |
| Staging approaches | |
| Radiometabolic assessment: 18FDG-PET/CT total body and testis ultrasound |
To exclude extra-CNS disease: 18FDG-PET and testicular ultrasound demonstrate the presence of extraneural disease in 4%-12% of patients with presumptive diagnosis of PCNSL |
| Bone marrow biopsy and aspirate: Morphology; immunophenotype/IHC |
To assess bone marrow reserve To exclude bone marrow involvement |
| CSF analysis: Physico-chemical exam Cytology Immunophenotype |
To assess leptomeningeal involvement: CSF sampling should be carried out in every patient with suspected or confirmed PCNSL. Increases in the leucocytes cell count and protein concentrations are often present, while glucose concentration is usually normal. Concurrent meningeal involvement, often asymptomatic, is detected by conventional CSF cytology in 16% of cases |
| Ophthalmologic evaluation: Fundoscopy and slit lamp examination, fluorescein angiography |
To assess ocular lymphomatous infiltration: asymptomatic ocular involvement is detected in 5% of cases |
| Fitness work-up | |
| Blood test: Full blood count; liver and renal function index; serology for HIV, HCV, HBV; pregnancy test |
To evaluate the bone marrow reserve To exclude liver and renal damage To evaluate supportive antiviral therapy |
| Cardiac assessment: Echocardiography, electrocardiogram Basal pro-BNP protein and troponin serum level |
To assess baseline cardiac function |
| Pulmonary assessment (only for patients with known lung disease): Thorax CT scan Pulmonary function test (spirometry test and diffuse capability of CO2) PS according to ECOG or Karnofsky scale |
To diagnose chronic obstruction pulmonary disease or other pulmonary comorbidities To define with standard tool the patient’s fitness and to consider the social and professional life before disease onset as well as comorbidities and eventual organ impairment secondary to them, in order to better define the choice of treatment |
18FDG-PET/CT, [18F]2-fluoro-2-deoxy-D-glucose-positron emission tomography/computerized tomography; CNS, central nervous system; CSF, cerebrospinal fluid; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency viruses; IHC, immunohistochemistry; IL-10, interleukin 10; MRI, magnetic resonance imaging; PCNSL, primary central nervous system lymphoma; pro-BNP, pro hormone B-type natriuretic peptide; PS, performance status.
At presentation, most PCNSL patients receive immediate steroid therapy with fast symptomatic improvement and radiographic regression in ~40% of patients. Mass shrinking can interfere with histopathological diagnosis; for this reason the use of steroids should be limited to cases of osmotherapy inefficacy and, if started, should be withheld at least 7-10 days before diagnostic biopsy. Importantly, a presumptive diagnosis of PCNSL should induce expert neurosurgeons to carry out stereotactic-guided biopsy of the mass to confirm diagnosis. Whereas stereotactic biopsy is a fast and safe procedure, wide tumor resection can induce permanent neurological deficits and treatment delay, without any survival benefits. In selected patients who require a timely control of neurological deterioration due to brain herniation or ventricle dilation, however, indication to tumor resection should be considered.
At our institution, MYD88 mutational status and interleukin-10 level are assessed on CSF in patients with low PCNSL suspicion or with unsuitable lesions for biopsy, because the combined analysis is able to distinguish PCNSL from other CNS entities with a sensitivity and specificity of 94% and 98%, respectively.4
At consultation, our attention is focused on the general conditions of the patient, which implies a careful collection of information and clinical evaluation in order to assess the need of additional health facilities and services to improve the initial assistance. It is also important to identify the caregiver before to share the therapeutic choice with the interlocutors.
Criteria conditioning the choice of treatment
Patient stratification
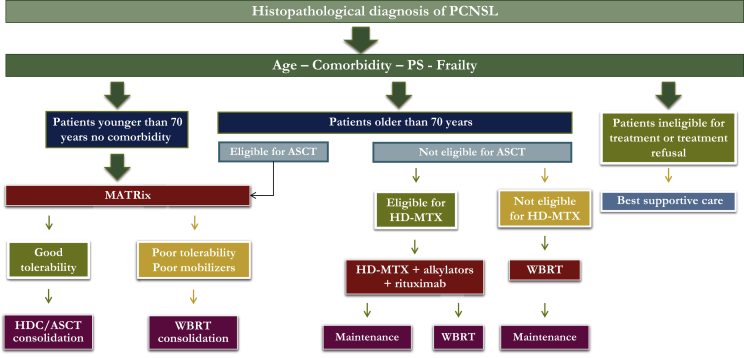
The multidisciplinary team devoted to the management of PCNSL patients at our institution has extensive experience in clinical research, leading several clinical studies in this field. Accordingly, when faced with a patient with a new diagnosis of PCNSL, our first thought is to enroll him/her in a prospective trial. The following concepts regard our therapeutic choices for PCNSL patients managed outside prospective trials (Figure 1).
Figure 1.
Therapeutic flow chart.
HDC/ASCT, high dose chemotherapy plus autologous stem cell transplantation; HD-MTX, high dose methotrexate; MATRix, high dose methotrexate, cytarabine, thiotepa, rituximab; PCNSL, primary central nervous system lymphoma; PS, performance status; WBRT, whole brain radiotherapy.
Age and performance status (PS) are important prognostic factors; however, several other details, like comorbidity, organ function, frailty, and risk of neurotoxicity, should be considered in the choice of the better treatment. In prospective trials, age cut-off used to define young patients varied between 60 and 75 years. In routine practice, patients aged between 60 and 75 years often receive personalized treatment because of large variability in comorbidity and neurological conditions. Thus, in this population, we consider suitability to receive high dose chemotherapy plus autologous stem cell transplantation (HDC/ASCT) as the main parameter to distinguish ‘young-fit’ and ‘elderly-frail’ patients.
How we treat young, fit patients
The modern treatment of PCNSL includes two phases: induction and consolidation. Thus, based on the results of the IELSG32 trial,5 polychemotherapy with four cycles of the MATRix regimen [methotrexate (MTX), cytarabine, thiotepa, rituximab] followed by HDC/ASCT is the preferred treatment option for young patients without severe comorbidities. Peripheral autologous hematopoietic stem cells (HSC) are usually collected after the second cycle. Patients with responsive disease according to International Primary CNS Lymphoma Collaborative Group criteria6 are consolidated with ASCT using a conditioning regimen with carmustine and thiotepa.5 Our standard choice of MTX dose and administration schedule, that is 3.5 g/m2 in a 3-h infusion, is based on well-documented higher response rate and CSF levels. In the case of dose reductions due to toxicity, we try to deliver a dose of 3 g/m2 (85%) to not compromise CNS penetration, maintaining tumoricidal levels in the brain parenchyma and CSF. MATRix includes rituximab, the role of which in PCNSL treatment is a matter of debate. At the time to design the IELSG32 study, the results of the HOVON 105/ALLG NHL 24 randomized trial were not available. The latter was focused on the role of rituximab in PCNSL patients aged 18-70 years, demonstrating a near significant benefit in patients younger than 60 years.7 Conversely, an effect in patients older than 60 years was not demonstrated, probably because these patients did not receive consolidation radiotherapy, with consequent disappointing survival figures, which probably hampered a benefit from this antibody. Moreover, the long-lasting results of the IELSG32 trial (after a median follow-up of 88 months) that demonstrate a significantly better progression-free survival (PFS) and overall survival in patients treated with rituximab,8 and the recent report that this drug does not affect cognitive functions9 prompt us to confirm our choice to keep rituximab as part of the MATRix combination.
Disease evaluation with bMRI is carried out after the second and fourth cycle of MATRix as well as after 2-3 months from ASCT. Eye and CSF assessments are carried out at the same time points in case of ocular and/or meningeal involvement at diagnosis, otherwise they are done at the end of the whole treatment or in case of clinical suspicion. In case of disease persistence in the vitreo-retinal compartment at the end of systemic therapy, indication to intravitreal treatment with rituximab 1 mg/0.1 ml and MTX 0.4 mg/0.1 ml is discussed with the ophthalmologist. Leptomeningeal relapse is an uncommon event in PCNSL patients treated with a modern approach. Accordingly, and in line with recently reported and ongoing international trials, we do not use intrathecal chemotherapy as part of first-line treatment.
Whole brain radiotherapy (WBRT) is an alternative to ASCT in case of HSC harvest failure or complications during induction. WBRT is not our first choice as consolidation therapy due to the increased risk of impairment of cognitive functions demonstrated in the IELSG324 and the PRECIS trials.10 The rehabilitative program is an important support for the recovery of these patients; thus, it is planned starting from the beginning of the induction therapy. Neuropsychological tests are usually carried out at diagnosis and at the end of first-line therapy, and used as baseline data to monitor any potential cognitive decline during follow-up.
How we treat elderly or frail patients
Most patients older than 70 years are not eligible for ASCT, and should be stratified according to the suitability to receive HD-MTX-based chemotherapy (Figure 1). Patients with normal renal function, adequate bone marrow reserve, and preserved cardiac function (ventricular ejection fraction ≥45%, no recent acute coronary artery disease or uncontrolled arrhythmias) are good candidates for this treatment. A combined treatment with HD-MTX, an alkylating agent (i.e. procarbazine, temozolomide) and rituximab is recommended.11,12 In responding patients, low-dose WBRT (23.4 Gy) consolidation13 or maintenance with oral alkylating agents14 should be considered. According to multicenter retrospective experience,15 selected patients older than 70 years with good PS and suitable organ function may be candidates for HDC/ASCT. For these patients, we consider MATRix at 25% reduction dose as induction before carmustine-thiotepa-conditioned ASCT (Figure 1).
Primary WBRT is an option for unfit patients not eligible for HD-MTX, with a 2-year PFS of 30%. As for high-grade gliomas, some investigators suggested adding concomitant and maintenance temozolomide, but this remains an experimental approach. Occasionally, unfit patients are referred to exclusive palliative care and, in this case, indication to brain biopsy should be discussed with caregivers.
How we treat patients with relapsed or refractory disease
A standard of care for patients with relapsed or refractory PCNSL remains to be established. Clinical trials should be the preferred treatment option because this is the best strategy to identify new active drugs and strategies that could be subsequently investigated as part of first-line therapy. When a prospective trial is not available, therapeutic choice should be based on published phase II trials. In case of late relapse (>24 months) and previous response to HD-MTX-based regimens, HD-MTX re-challenge is a safe and effective strategy. In earlier relapses, we prefer to use high-dose ifosfamide-containing regimens, in particular the R-IE (rituximab/ifosfamide/etoposide) regimen, which is associated with a 38% overall response rate (ORR) and a 2-year survival after relapse of 25%. In both early and late relapses, we recommend a subsequent consolidation or maintenance therapy, the choice of which depends on previous treatments, availability of autologous HSC, comorbidity, and the patient’s general condition.
Besides chemotherapy combinations, new drugs are being investigated with promising results. The mechanistic target of rapamycin (mTOR) inhibitor temsirolimus has been demonstrated to confer an ORR of 54% in a heavily pretreated, elderly population; however, its use is limited by a considerable toxicity and treatment-related mortality of 13.5%. The first-in-class Bruton tyrosine kinase inhibitor ibrutinib has been investigated as a single agent or in combination with chemotherapy in patients with CNS lymphoma revealing fast and impressive responses. At a flat dose of 560 mg daily, ibrutinib has been associated with an ORR of 50%, and dose escalation until 840 mg resulted in a 77% ORR.16,17 Ibrutinib achieved adequate CSF concentration with a safe profile; however, an increased incidence of invasive aspergillosis has been reported (up to 39% of patients), but a timely interruption or omission of steroid therapy might prevent this severe complication. Immunomodulatory drugs (lenalidomide and pomalidomide) have been investigated in patients with relapsed or refractory PCNSL or primary vitreous-retina lymphoma achieving an objective response in half of the patients, but the response was usually short lived.18,19 Lenalidomide, used as single agent at low dose (5-10 mg) as maintenance therapy and in combination with rituximab, deserves to be further investigated in this setting. At the moment, we do not use anti-programmed cell death protein 1 antibodies because results of phase II trials are pending.
Anti-CD19 chimeric antigen receptor (CAR)-T cells were recently approved for the treatment of relapsed/refractory DLBCL, with promising results in other B-cell lymphomas. This approach deserves to be addressed in patients with relapsed or refractory PCNSL considering its encouraging preliminary results reported in patients with secondary CNS DLBCL.20
Acknowledgements
We are indebted to our patients and their families for their generous commitment. We particularly thank hematologists, oncologists, neuroradiologists, pathologists, neurosurgeons, ophthalmologists, radiation oncologists, and psychologists at IRCCS San Raffaele Scientific Institute for the sustained clinical and scientific collaboration. We thank all the nurses for the clinical assistance and appreciate the excellent technical support of our study coordinator office.
Funding
None declared.
Disclosure
TC: funding from Roche; other: Sandoz. AJMF: speaker fee from Adienne; research grants from Bristol-Myers Squibb, Beigene, Pharmacyclics, Hutchison Medipharma, Amgen, Genmab, ADC Therapeutics, Gilead, Novartis, and Pfizer; advisory boards from Gilead, Novartis, Juno, and PletixaPharm; inventor of patents on NGR-hTNF/RCHOP in relapsed or refractory PCNSL and SNGR-hTNF in brain tumors. All other authors have declared no conflicts of interest.
References
- 1.Kluin P.M., Deckert M., Ferry J.A. Primary diffuse large B-cell lymphoma of the CNS. In: Swerdlow S.H., Campo E., Harris N.L., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research in Cancer; Lyon, France: 2017. pp. 300–302. [Google Scholar]
- 2.Shiels M.S., Pfeiffer R.M., Besson C. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417–424. doi: 10.1111/bjh.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herhaus P., Lipkova J., Lammer F. CXCR4-targeted PET imaging of central nervous system B-cell lymphoma. J Nucl Med. 2020;61(12):1765–1771. doi: 10.2967/jnumed.120.241703. [DOI] [PubMed] [Google Scholar]
- 4.Ferreri A.J.M., Calimeri T., Lopedote P. MYD88 L265P mutation and interleukin-10 detection in cerebrospinal fluid are highly specific discriminating markers in patients with primary central nervous system lymphoma: results from a prospective study. Br J Haematol. 2021;193(3):497–505. doi: 10.1111/bjh.17357. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri A.J.M., Cwynarski K., Pulczynski E. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510–e523. doi: 10.1016/S2352-3026(17)30174-6. [DOI] [PubMed] [Google Scholar]
- 6.Abrey L.E., Batchelor T.T., Ferreri A.J. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg J.E.C., Issa S., Bakunina K. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019;20(2):216–228. doi: 10.1016/S1470-2045(18)30747-2. [DOI] [PubMed] [Google Scholar]
- 8.Ferreri A.J.M., Cwynarski K., Pulczynski E. MATRIX induction followed by autologous stem cell transplant or whole-brain irradiation in Primary CNS Lymphoma. 7-year results of the IELSG32 randomized trial. Abstract 047_16th ICML. Haemat Oncol. 2021;39(Supp 2) doi: 10.1002/hon.47_2879. [DOI] [Google Scholar]
- 9.van der Meulen M., Dirven L., Habets E.J.J. Neurocognitive functioning and radiologic changes in primary CNS lymphoma patients: results from the HOVON 105/ALLG NHL 24 randomised controlled trial. Neuro Oncol. 2021:noab021. doi: 10.1093/neuonc/noab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houillier C., Taillandier L., Dureau S. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37(10):823–833. doi: 10.1200/JCO.18.00306. [DOI] [PubMed] [Google Scholar]
- 11.Omuro A., Chinot O., Taillandier L. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–e259. doi: 10.1016/S2352-3026(15)00074-5. [DOI] [PubMed] [Google Scholar]
- 12.Fritsch K., Kasenda B., Schorb E. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study) Leukemia. 2017;31(4):846–852. doi: 10.1038/leu.2016.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris P.G., Correa D.D., Yahalom J. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulczynski E.J., Kuittinen O., Erlanson M. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by the Nordic Lymphoma Group. Haematologica. 2015;100(4):534–540. doi: 10.3324/haematol.2014.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorb E., Fox C.P., Fritsch K. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant. 2017;52(8):1113–1119. doi: 10.1038/bmt.2017.23. [DOI] [PubMed] [Google Scholar]
- 16.Grommes C., Pastore A., Palaskas N. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. doi: 10.1158/2159-8290.CD-17-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soussain C., Choquet S., Blonski M. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi: 10.1016/j.ejca.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein J.L., Geng H., Fraser E.J. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595–1607. doi: 10.1182/bloodadvances.2017014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tun H.W., Johnston P.B., DeAngelis L.M. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018;132(21):2240–2248. doi: 10.1182/blood-2018-02-835496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frigault M.J., Dietrich J., Martinez-Lage M. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–866. doi: 10.1182/blood.2019001694. [DOI] [PMC free article] [PubMed] [Google Scholar]