Abstract
Introduction
The better survival rates after breast cancer allow for setting of long-term goals, such as Quality of Life (QoL) and aesthetic outcomes following breast reconstruction. Studies find a higher breast-related QoL and greater satisfaction with breasts following autologous breast reconstruction (ABR) compared to implant-based breast reconstruction (IBR). However, aesthetic results from donor sites can influence body image. This concern is little addressed in the literature. Therefore, the aim of this study was to compare the long-term breast-related and body-related QoL of women who underwent ABR to women who underwent IBR.
Material and methods
A multicenter, cross-sectional survey was conducted between November and December 2020 among women who underwent postmastectomy breast reconstruction between January 2015 and December 2018. A general questionnaire, the BREAST-Q, and the BODY-Q were used to collect data. Multivariable linear regression was performed to adjust differences in Q-scores for potential confounders.
Results
In total, 336 patients were included (112 IBR, 224 ABR). Autologous reconstruction resulted in significantly higher mean scores in all subdomains of the BREAST-Q. On the BODY-Q, IBR scored significantly higher on scars, while ABR scored moderately to significantly higher on all other scales. Despite a lower mean score on Hips & outer thighs in women with Lateral Thigh Perforator (LTP) flap reconstruction, no negative influence on body image was found in these women.
Conclusions
Long-term breast-related and body-related outcomes of ABR are superior to IBR. Donor site aesthetic does not adversely affect body image in women who underwent free flap breast reconstruction.
Keywords: Breast reconstruction, Body image, Donor site, Quality of life
Highlights
-
•
ABR patients reported better long-term breast-related QoL compared to IBR patients.
-
•
DIEP flap reconstruction patients report high satisfaction with abdomen appearance.
-
•
Donor site appearance does not affect body image in free flap reconstruction.
1. Introduction
In the Netherlands, one in seven women develops breast cancer during lifetime [1,2]. It is the most common cancer in women. The five-year survival rate continues to rise as a result of improved early detection and treatment [1]. This allows for setting of long-term goals, such as improving Quality of Life (QoL) and aesthetic outcomes [3]. As a result, breast reconstruction has become increasingly important in the therapeutic course after breast cancer. Furthermore, breast reconstruction can contribute to the restoration of QoL and body image in women undergoing prophylactic mastectomy because of familial risk of breast cancer [4,5].
The two main options for post-mastectomy breast reconstruction are implant-based breast reconstruction (IBR) and autologous breast reconstruction (ABR) [6]. Both types have their advantages and disadvantages [7]. For example, IBR requires a less invasive operation with a shorter recovery time, but ABR can achieve a more natural feeling, even more with the upcoming nerve coaptation for recovery of sensation [[8], [9], [10]]. Autologous breast reconstruction is more cost-effective than implants, especially in women with a longer life expectancy [[11], [12], [13]].
Studies using patient-reported outcome measures (PROMs) found a higher breast-related QoL and greater satisfaction with breasts following ABR compared to IBR [[14], [15], [16]]. However, donor site morbidity and aesthetic outcomes are major concerns in ABR, which are relatively little addressed in the existing literature [[17], [18], [19]]. In addition to satisfaction with breasts, aesthetic outcomes of donor sites may also play an important role in the subjective perception of the body. The concept of body image is becoming increasingly important in psycho-oncology, as an impaired body image due to breast cancer treatments can have long-term negative effects on psychological well-being and QoL in breast cancer survivors [[20], [21], [22]]. Assessment of body image in a broader sense could therefore be a valuable addition to the commonly used breast-related outcome measures, such as the BREAST-Q [23,24]. Women faced with a choice to undergo breast reconstruction should be adequately informed regarding both breast-related and body-related outcomes.
Little is known in the literature about the long-term (>2 years) breast-related and donor site-related patient-reported outcomes after ABR; IBR patients and a short follow-up duration often predominate [16,25]. Having completed cancer treatment for a longer period of time may allow women to view their breast reconstruction differently, conceivably more critically than in the short term. Therefore, the aim of this study was to compare the long-term breast-related and body-related QoL of women who have undergone ABR to women who have undergone IBR, reported two to five years after the reconstruction procedure.
2. Material and methods
2.1. Study population
This multicenter, cross-sectional survey was conducted between November and December 2020. Women 18 years or older who underwent a postmastectomy breast reconstruction in either Maastricht University Medical Centre (MUMC+) or Zuyderland Medical Centre between January 2015 and December 2018 were invited to participate. Women who underwent immediate or delayed IBR or ABR were eligible. Exclusion criteria were: bilateral reconstruction with unilateral IBR and contralateral ABR or a mixed timing, tertiary breast reconstruction (after failed reconstruction or unsatisfactory results), breast reconstruction by autologous fat transfer (AFT), currently no breast reconstruction after previously failed reconstruction, or currently distant metastases. All participants signed an online informed consent form. The study was approved by the Medical Ethics Committees of Maastricht University Medical Center (METC2020-2232) and Zuyderland MC (METCZ20200113).
2.2. Data collection
Patients were invited to participate in this study by means of an invitation letter by (e−)mail, including a personal link to the online questionnaire (Qualtrics, Provo, UT, USA). They were requested to complete the questionnaire within four weeks if they wanted to participate. A reminder was sent after 3 weeks to those who did not respond. A paper version was available on request.
2.3. Questionnaires
The online survey consisted of items on patient demographics, medical history, breast reconstruction, and the following patient-reported outcome measures (PROMs).
The BREAST-Q Version 1.0 (Dutch), Reconstruction module (postoperative scales), was used to assess breast-related satisfaction and QoL. This validated questionnaire consists of six domains: satisfaction with breasts, psychosocial well-being, sexual well-being, physical well-being chest, physical well-being abdomen and satisfaction with outcome.
The BODY-Q was used to measure satisfaction with the appearance of specific body parts that can be donor sites in ABR. The following scales of the BODY-Q were analyzed: abdomen, body, buttocks, hips & outer thighs, scars, and body image.
Additional medical information, such as tumor staging, was obtained from the electronic medical records.
2.4. Statistical analysis
Baseline demographics were analyzed with descriptive statistics. Continuous variables were reported as mean values, standard deviation (SD), and range, and were compared between IBR and ABR using the independent-samples t-test, categorical variables were reported as counts (%) and were compared using Pearson's chi square test. The Mann-Whitney U test was used to compare ordinal data.
BREAST-Q and BODY-Q scores were transformed using the Q-score software into scores from 0 to 100 with 0 meaning ‘worst’ and 100 meaning ‘best’.
Multivariable linear regression analysis was performed to adjust differences between IBR and ABR on Q-scores for confounding variables. For BREAST-Q results, we selected confounding variables a priori (i.e., age and pTx stage) and using backward stepwise elimination based on the Wald test (i.e., educational level, smoking, BMI, cup size, Nx staging, radiotherapy, hormone therapy, reconstruction timing, and follow-up duration). Next, we added an interaction between reconstruction type and timing to the model. In case of a significant interaction, indicating that the difference between IBR and ABR differed between immediate and delayed reconstructions, the model was subsequently analyzed stratified by reconstruction timing (immediate and delayed). For BODY-Q results, a priori selected confounders (age, BMI, smoking, reconstruction type, follow-up duration after reconstruction) were used to adjust between-group differences. The regression analysis was repeated for specific flap procedures in order to further analyze the influence of the donor site appearance on the body-related quality of life.
A p value ≤ 0.05 was considered statistically significant. All analyses were performed in IBM SPSS Statistics 25.
3. Results
3.1. Patient demographics and clinical characteristics
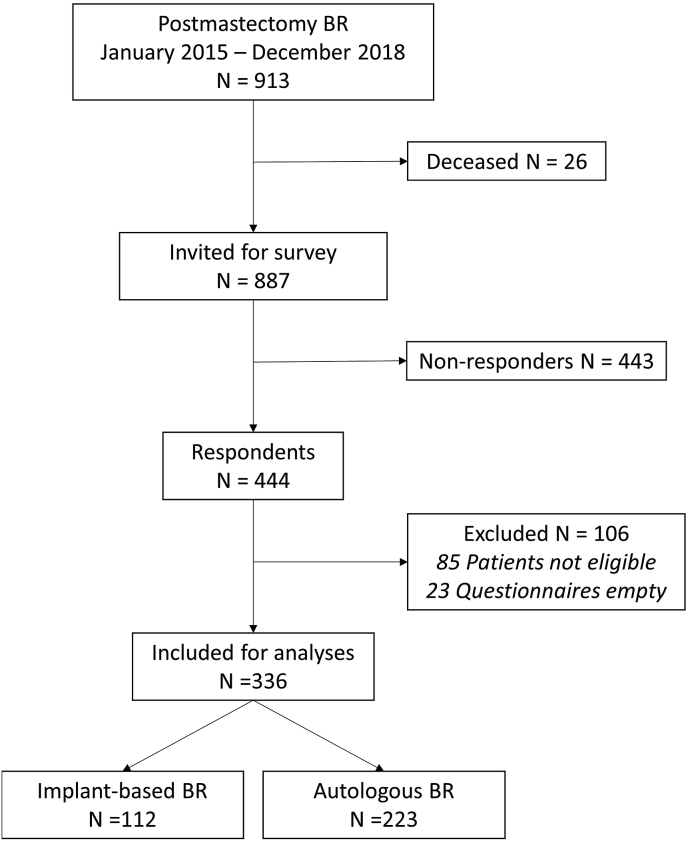
Between January 2015 and December 2018, 913 women underwent postmastectomy breast reconstruction. Of those women, 26 deceased. The survey yielded a response rate of 50.1% (444 of 887 patients). Eighty-five respondents were excluded based on the eligibility criteria, and 23 questionnaires were returned blank or mostly empty. In total, 336 patients were eligible for analysis, of which 224 women underwent autologous breast reconstruction (66.7%) and 112 women underwent two-staged IBR (33.3%). Of the 224 ABR patients, 191 (85.3%) underwent deep inferior epigastric perforator (DIEP) flap reconstruction, 30 (13.4%) underwent lateral thigh perforator (LTP) flap reconstruction, two women underwent both a DIEP reconstruction on one side and an LTP reconstruction on the other (0.9%), and one woman (0.4%) underwent a stacked hemi-abdominal extended perforator (SHAEP) flap reconstruction (Fig. 1).
Fig. 1.
Flowchart of patient inclusion.
The mean age of all included women was 55.5 years (SD 9.8, range 28–82) and mean BMI was 25.7 (SD 4.2, range 18.2–43.9). The mean follow-up after breast reconstruction was 46.7 months (SD 14.8, range 23–76). Patient characteristics per reconstruction type are presented in Table 1. On average, women with ABR had a higher BMI, a larger bra cup size, were less likely to be active smokers, and had a higher educational level compared to women with IBR. Women with IBR had on average a lower lymph node staging (N stage) at diagnosis, underwent radiotherapy relatively less often but more often hormone therapy. Implant-based reconstruction was performed relatively more often immediately than delayed, compared to autologous breast reconstruction.
Table 1.
Patient characteristics by reconstruction type.
| Characteristic | IBR (n = 112) | ABR (n = 224) | P value |
|---|---|---|---|
| Age (mean ± SD) | 56.4 ± 11.3 | 55.0 ± 8.9 | 0.256 |
| Body Mass Index (mean ± SD) | 24.2 ± 3.9 | 26.3 ± 4.2 | <0.001 |
| Breast cup size preoperatively (n, %) | <0.001 | ||
| AA | 2 (1.8) | 0 (0) | |
| A | 12 (10.7) | 9 (4) | |
| B | 49 (43.8) | 61 (27.2) | |
| C | 16 (14.3) | 64 (28.6) | |
| D | 20 (17.9) | 55 (24.6) | |
| E | 11 (9.8) | 24 (10.7) | |
| >E | 2 (1.8) | 10 (4.5) | |
| Smoking (n, %) | <0.001 | ||
| Yes | 21 (18.8) | 10 (4.5) | |
| No | 91 (81.3) | 214 (95.5) | |
| Allergies (n, %) | 0.146 | ||
| Yes | 28 (25.0) | 73 (32.6) | |
| No | 84 (75.0) | 150 (67.0) | |
| Chronic disease, self-reported (n, %) | 0.569 | ||
| Yes | 24 (21.4) | 54 (24.1) | |
| No | 88 (78.6) | 169 (75.4) | |
| Relationship (n, %) | 0.775 | ||
| Yes | 88 (78.6) | 179 (79.9) | |
| No | 24 (21.4) | 45 (20.1) | |
| Children (n, %) | 0.190 | ||
| Yes | 95 (84.8) | 201 (89.7) | |
| No | 17 (15.2) | 23 (10.3) | |
| Educational level | 0.001 | ||
| 1 – No education | 0 (0) | 0 (0) | |
| 2 – Elementary education | 5 (4.5) | 1 (0.4) | |
| 3 – Secondary education | 24 (21.4) | 25 (11.2) | |
| 4 – Middle-level vocational education/ | 43 (38.4) | 95 (42.4) | |
| 5 – Higher-level vocational education/college/university | 36 (32.1) | 77 (34.4) | |
| 6 – Academic/doctoral degree | 3 (2.7) | 25 (11.2) | |
| Reconstruction timing (n, %) | <0.001 | ||
| Primary | 88 (78.6) | 121 (54.0) | |
| Secondary | 24 (21.4) | 103 (46.0) | |
| Laterality (n, %) | 0.938 | ||
| Unilateral BR | 62 (55.4) | 125 (55.8) | |
| Bilateral BR | 50 (44.6) | 99 (44.2) | |
| Complications (n, %) | 0.173 | ||
| Yes | 32 (28.6) | 82 (36.6) | |
| No | 78 (69.6) | 142 (63.4) | |
| Follow-up duration after reconstruction in months (mean ± SD) | 51.5 ± 14.3 | 44.3 ± 14.6 | <0.001 |
| Mastectomy indication | |||
| Invasive carcinoma | 82 (73.2) | 170 (75.9) | 0.790 |
| In situ carcinoma/non-cancerous pathology | 17 (15.2) | 28 (12.5) | |
| Bilateral prophylactic mastectomy | 13 (11.6) | 26 (11.6) | |
| Tumor stage at diagnosisa | |||
| T | 0.822 | ||
| 1 | 43 (38.4) | 82 (36.6) | |
| 2 | 28 (25.0) | 63 (28.1) | |
| 3 | 5 (4.5) | 8 (3.6) | |
| N | <0.001 | ||
| 0 | 63 (56.3) | 92 (41.1) | |
| 1 | 12 (10.7) | 51 (22.8) | |
| 2 | 1 (0.9) | 8 (3.6) | |
| 3 | 0 (0) | 4 (1.8) | |
| M | 1.000 | ||
| 0 | 81 (100) | 168 (100) | |
| Bloom & Richardson [1] | 0.876 | ||
| Grade 1 | 15 (18.3) | 26 (15.3) | |
| Grade 2 | 35 (42.7) | 71 (41.8) | |
| Grade 3 | 17 (20.7) | 32 (18.8) | |
| Chemotherapy (%) | 0.151 | ||
| Yes | 54 (48.2) | 126 (56.3) | |
| No | 58 (51.8) | 97 (43.3) | |
| Radiotherapy (%) | 0.001 | ||
| Yes | 20 (17.9) | 81 (36.2) | |
| No | 92 (82.1) | 142 (63.4) | |
| Hormone therapy (n, %) | 0.040 | ||
| Yes | 40 (35.7) | 106 (47.3) | |
| No | 72 (64.3) | 117 (52.2) | |
| Immunotherapy (n, %) | 0.643 | ||
| Yes | 32 (14.3) | ||
| No | 191 (85.3) | ||
Only invasive tumors included.
3.2. Breast-related quality of life
Unadjusted and adjusted mean BREAST-Q scores for both IBR and ABR patients are presented in Table 2. Women who underwent ABR reported higher satisfaction with breast and outcome, as well as higher physical, psychosocial, and sexual well-being, compared to IBR patients. However, the subdomains psychosocial well-being (unadjusted between-group difference: 4.4, p = 0.068) and sexual well-being (unadjusted between-group difference: 5.0, p = 0.078) were not statistically significant. Adjusted for potential confounders, linear regression showed significantly higher mean scores in all subdomains of the BREAST-Q in ABR patients.
Table 2.
Regression model for BREAST-Q scores in IBR vs. ABR patients.
| Dependent variable | IBR (n = 112) | ABR (n = 224) | Unadjusted difference (95% CI) | P value | Adjusted difference (95% CI) | P value |
|---|---|---|---|---|---|---|
| Satisfaction with breast | 55.5 ± 18.4 | 68.3 ± 19.4 | 12.8 (8.4–17.2) | <0.001 | 14.7 (8.3–21.0) | <0.001 |
| Satisfaction with outcome | 60.0 ± 20.8 | 70.9 ± 21.6 | 10.9 (6.0–15.7) | <0.001 | 14.9 (8.0–21.9) | <0.001 |
| Psychosocial well-being | 68.8 ± 21.2 | 73.2 ± 20.3 | 4.4 (−0.3–9.1) | 0.068 | 7.6 (1.0–14.2) | 0.024 |
| Sexual well-being | 52.9 ± 24.6 | 57.8 ± 22.7 | 5.0 (−0.6–10.5) | 0.078 | 9.4 (1.6–17.2) | 0.019 |
| Physical well-being | 64.2 ± 17.6 | 68.7 ± 17.2 | 4.5 (0.5–8.4) | 0.027 | 6.2 (0.8–11.7) | 0.026 |
Independent variables computed in this model: age, BMI, cup size preoperatively, smoking, educational level, tumor classification (T stage, N stage), radiotherapy, hormone therapy, reconstruction type, reconstruction timing, follow-up duration after reconstruction.
When an interaction between reconstruction type and timing was added as an independent variable to the regression model for breast-related QoL scores, the interaction effect was significant. Therefore, the regression analyses were stratified by immediate and delayed reconstruction (Table 3). Stratification of the breast-related outcomes showed that the effect of the reconstruction type on satisfaction with breast was greater in patients that underwent delayed reconstruction. In immediate breast reconstruction, however, the effect of the reconstruction type on satisfaction with outcome was greater.
Table 3.
Regression model for BREAST-Q scores, stratified by reconstruction timing.
| Immediate reconstruction (n = 209) |
Delayed reconstruction (n = 127) |
|||
|---|---|---|---|---|
| Dependent variable | Adjusted difference (95% CI) | P value | Adjusted difference (95% CI) | P value |
| Satisfaction with breast | 14.1 (6.7–21.4) | <0.001 | 19.8 (6.7–32.9) | 0.003 |
| Satisfaction with outcome | 19.3 (11.7–26.9) | <0.001 | 10.7 (−4.0–25.4) | 0.150 |
| Psychosocial well-being | 9.2 (1.7–16.8) | 0.017 | 9.5 (−4.4–23.3) | 0.177 |
| Sexual well-being | 8.8 (−0.1–17.7) | 0.051 | 11.5 (−5.3–28.4) | 0.177 |
| Physical well-being | 9.3 (2.8–15.8) | 0.005 | 0.6 (−10.4–11.5) | 0.920 |
Independent variables computed in this model: age, BMI, cup size preoperatively, smoking, educational level, tumor classification (T stage, N stage), radiotherapy, hormone therapy, reconstruction type, reconstruction timing, follow-up duration after reconstruction.
3.3. Body-related quality of life
Compared to IBR patients, ABR patients scored a higher mean outcome on the BODY-Q scales Abdomen and Buttocks, but scored lower on Hips & outer thighs, Body, and Scars. The latter showed a significant difference in favor of IBR patients (unadjusted between-group difference: 6.5, p = 0.008). On Body image, both groups scored nearly the same mean score (mean difference: 0.2, p = 0.950). Multivariable regression analysis showed statistically significant mean differences on Abdomen in favor of the ABR patients and on Scars in favor of the IBR patients. The adjusted mean difference on Body image, however, did not reach statistical significance (mean difference: 2.3, p = 0.469). The outcomes of the regression analyses for BODY-Q scores are presented in Table 4.
Table 4.
Regression model for BODY-Q scores in IBR vs. ABR patients.
| Dependent variable | IBR (n = 112) | ABR (n = 224) | Unadjusted difference (95% CI) | P value | Adjusted difference (95% CI) | P value |
|---|---|---|---|---|---|---|
| Abdomen | 59.8 ± 28.7 | 65.9 ± 28.2 | 6.1 (−0.5–12.8) | 0.070 | 11.4 (4.6–18.1) | 0.001 |
| Buttocks | 64.7 ± 24.0 | 65.3 ± 27.1 | 0.6 (−5.5–6.7) | 0.843 | 3.4 (−3.0–9.9) | 0.296 |
| Hips & outer thighs | 66.7 ± 25.0 | 62.5 ± 26.6 | −4.3 (−10.3–1.8) | 0.169 | 0.4 (−6.0–6.7) | 0.912 |
| Body | 62.0 ± 20.9 | 60.9 ± 23.7 | −1,1 (−6.5–4.2) | 0.676 | 4.6 (−0.4–9.7) | 0.086 |
| Scars | 78.8 ± 19.6 | 72.3 ± 20.8 | −6.5 (−11.3–1.7) | 0.008 | −6.0 (−11.1–0.8) | 0.023 |
| Body image | 61.5 ± 27.2 | 61.7 ± 26.9 | 0.2 (−6.0–6.4) | 0.950 | 2.3 (−3.9–8.5) | 0.469 |
Independent variables computed in this model: age, BMI, smoking, reconstruction type, follow-up duration after reconstruction.
3.4. Influence of specific donor site appearance
Women who underwent DIEP flap reconstruction (n = 191) had a mean age of 55.9 years (SD 8.5, range 33–78) and a mean BMI of 26.7 (SD 4.2, range 18.7–43.9). They scored on average 6.7 points higher on Abdomen (p = 0.053) and 6.4 points lower on Scars (p = 0.012) of the BODY-Q, compared to IBR patients. Mean scores on Body and Body image were nearly equal in both groups (mean difference: 0.3, p = 0.919). Adjusted for potential confounders, DIEP flap patients scored significantly higher on both Abdomen (p=<0.001) and Body (p = 0.028), and significantly lower on Scars (p = 0.043) compared with IBR patients.
Women who underwent LTP flap reconstruction (n = 30) had a mean age of 49.9 years (SD 9.9, range 30–70) and a mean BMI of 24.2 (SD 3.4, range 19.6–34.7). On average, they reported a significantly lower outcome on Hips & outer thighs (mean difference: −20.4, p < 0.001) compared to IBR patients. Also on all other BODY-Q scales, LTP patients scored a lower mean outcome, although these differences were not statistically significant. Adjusted for potential confounders, mean scores of Hips & outer thighs (p < 0.001) and Scars (p = 0.037) were significantly lower in LTP patients compared to IBR patients, other scales did not differ significantly. Outcomes of the univariable and multivariable regression analyses for BODY-Q scores of DIEP and LTP patients are presented in Table 5.
Table 5.
Regression model for BODY-Q scores in IBR vs. DIEP patients and LTP patients.
| Dependent variable | IBR (n = 112) | DIEPa (n = 191) | Unadjusted difference (95% CI) | P value | Adjusted difference (95% CI) | P value | LTPb (n = 30) | Unadjusted difference (95% CI) | P value | Adjusted difference (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdomen | 59.8 ± 28.7 | 66.5 ± 28.2 | 6.7 (−0.1–13.5) | 0.053 | 13.9 (6.5–20.5) | <0.001 | 58.4 ± 28.2 | −1.3 (−14.4–11.7) | 0.839 | −0.3 (−6.0–5.4) | 0.921 |
| Buttocks | 64.7 ± 24.0 | 66.0 ± 27.2 | 1.3 (−4.0–7.6) | 0.672 | 5.1 (−1.6–11.8) | 0.139 | 59.7 ± 27.0 | −5.0 (−15.5–5.5) | 0.351 | −3.6 (−8.8–1.7) | 0.184 |
| Hips & outer thighs | 66.7 ± 25.0 | 65.1 ± 26.1 | −1.6 (−7.8–4.5) | 0.603 | 5.1 (−1.3–11.5) | 0.119 | 46.3 ± 24.5 | −20.4 (−30.6–10.3) | <0.001 | −9.7 (−14.8–4.6) | <0.001 |
| Body | 62.0 ± 20.9 | 61.2 ± 23.9 | −0.8 (−6.2–4.7) | 0.787 | 5.9 (0.6–11.2) | 0.028 | 56.3 ± 22.4 | −5.6 (−15.1–3.8) | 0.239 | −2.1 (−6.2–2.1) | 0.328 |
| Scars | 78.8 ± 19.6 | 72.4 ± 21.3 | −6.4 (−11.4–1.4) | 0.012 | −5.6 (−11.0–0.2) | 0.043 | 71.0 ± 18.1 | −7.9 (−15.8–0.0) | 0.051 | −4.3 (−8.4–0.3) | 0.037 |
| Body image | 61.5 ± 27.2 | 61.8 ± 26.7 | 0.3 (−6.1–6.7) | 0.919 | 3.0 (−3.5–9.4) | 0.366 | 58.0 ± 28.5 | −3.5 (−14.7–7.7) | 0.537 | −2.2 (−7.5–3.2) | 0.419 |
Independent variables computed in this model: age, BMI, smoking, reconstruction type, follow-up duration after reconstruction.
DIEP Deep Inferior Epigastric Perforator flap.
LTP Lateral Thigh Perforator flap.
4. Discussion
This study compared the long-term breast-related and body-related QoL of women with autologous breast reconstruction (ABR) to women with implant-based breast reconstruction (IBR), reported two to five years after the reconstruction procedure.
We found that ABR patients reported better mean long-term outcomes for breast-related QoL when compared with IBR patients. Following ABR, women scored significantly higher satisfaction with breasts, satisfaction with outcome, physical well-being, psychosocial well-being, and sexual well-being than following IBR, after adjusted for potential confounders. These results are consistent with previous findings in the literature and further support the hypothesis that ABR results in a better breast-related QoL, both in the short and long term [14,15,25].
Literature suggests that the difference between IBR and ABR outcomes increases in the longer term in favor of ABR, partly because of the development of ptosis in the ABR, resulting in a more natural appearance of the breast [14,26]. There are certain drawbacks to IBR, such as the risk of capsular contracture and implant rupture, which will eventually lead to the implants having to be replaced [27,28]. In addition, the negative media attention that breast implants have received in recent years can negatively influence patient-reported outcomes. Worrying statements in journalism, such as in ‘The implant files', contribute to unrest among women with breast implants [[29], [30], [31]]. In very few cases, the use of breast implants can lead to breast implant illness [[32], [33], [34]]. Removal of the implants improves health complaints in half of the patients [35]. A tertiary ABR could offer a solution for these women [36].
The second purpose of this study was to measure the influence of donor sites on body-related QoL. Body image concerns are common among breast cancer survivors, as breast cancer treatments can profoundly affect physical appearance temporarily (e.g. hair loss, weight fluctuation) or permanently (e.g. loss of a breast, lymphedema) [37,38]. Protective factors such as a strong romantic relationship or postmenopausal age may explain why some women suffer less from a distorted self-perception than others [39,40]. Different, but not all types of psychosocial interventions on body image outcomes were shown to be effective with varying effect sizes [[41], [42], [43]]. Breast reconstruction aims to mitigate body image distress by restoring the appearance of the breast. Research showed that body image improved significantly after breast reconstruction, regardless of the type of reconstruction [44,45]. However, women who underwent delayed reconstruction after mastectomy showed higher levels of body dissatisfaction [46]. We considered the use of free flaps for breast reconstruction as a potential risk factor for body image distortion due to visible scarring and changes in body shape. Therefore, we used the BODY-Q to measure satisfaction with the appearance of specific body parts that function as donor sites for ABR. Our results show that women who underwent ABR report worse outcomes with regard to Hips & outer thighs, Body, and Scars, compared to women who underwent IBR. Nevertheless, no significant difference in body image was reported between the two groups. Hence, it might be concluded that donor site appearance does not materially affect body image.
A remarkable outcome of this study is the higher satisfaction with the abdomen reported by women who underwent DIEP flap ABR compared to women who underwent IBR. One hypothesis is that DIEP flap harvest in women with a higher BMI on average results in a flatter stomach, as this procedure has close similarities to abdominoplasty [47,48]. Previous research demonstrated equal satisfaction with the aesthetic outcome after these two surgeries [49].
Contrarily, previous studies showed a deterioration in abdominal well-being among women undergoing ABR as assessed with the BREAST-Q [14,50]. However, while the BREAST-Q mainly concentrates on the functional donor site morbidity, the BODY-Q focuses more on the appearance of the abdomen [51]. Apparently those are two different outcomes.
Whenever the abdomen is not a suitable donor site, the LTP flap can be harvested from the lateral thigh [52,53]. Compared to women who underwent IBR, women who underwent an LTP reconstruction scored moderately to significantly lower on all body-related scales. We found a striking mean score difference on Hips & outer thighs, something not seen in DIEP patients or ABR patients in general. While DIEP flap harvest appears to have a positive effect on donor site appearance and body image, this effect does not appear to apply to thigh flap harvest. Surgical refinements have been implemented over time to reduce donor site deformations, including liposuction and lipofilling. This allows better results, even longer after reconstruction [52]. Nevertheless, Body and Body Image scores after LTP reconstruction suggest that, by the aesthetically satisfying outcomes of the breast, women are overall satisfied with the outcome of the surgery.
This study has certain strengths and limitations. We believe this was the first study to compare body-related patient-reported outcomes of women with different reconstruction types. However, usage of the BODY-Q was originally validated for patients after massive weight loss and post-bariatric surgery. Nevertheless, the scales of this questionnaire concern the appearance of body parts that could serve as donor sites for microsurgical free flaps [54]. The BODY-Q items are easily answered by all women, while the Abdomen scale of the BREAST-Q is only intended for abdominal flaps. The BODY-Q enabled the assessment of other donor sites and included a body image scale, which could be considered a comprehensive outcome.
Using an online questionnaire to collect results may involve participation bias. It cannot be ruled out that women who were more satisfied with their outcome were more likely to participate. Furthermore, women may be excluded because their reconstruction failed. This may have resulted in the omission of the worst outcomes from the analysis. Moreover, we explicitly adjusted for radiotherapy in the multivariable models as we acknowledge this may be a very important confounding factor. Furthermore, the sample size of specific flaps, e.g. the LTP flap, was small, leading to a low statistical power for this subgroup analysis. Body-related outcomes of rarely used flaps could not be determined from this study because of the small sample size. Finally, the cross-sectional study design prevented us from following up the outcomes over time. Baseline QoL is a potential confounder for long-term outcomes, which could not be adjusted for in this study. This should be taken into account when interpreting the results. Furthermore, the effectiveness of breast reconstruction on improving body image after mastectomy cannot be demonstrated with this design. We hypothesize that breast reconstruction reduces body image distress and that, in combination with personalized psychological interventions earlier in breast cancer treatment, it can reduce psychological symptoms in the adaptation to breast cancer.
Our study was conducted in a hospital that provides specialist care in autologous breast reconstruction. Clinical and aesthetic outcomes are closely related to the surgical experience of the plastic surgeon. We are aware that ABR in general or certain flaps, such as the LTP flap, cannot be offered to all patients. Although technical expertise in flap surgery is constantly improving, breast reconstruction remains patient-specific: some types of reconstruction may be better suited to one patient than another. When counseling a patient considering breast reconstruction, all reconstructive options and their pros and cons should be discussed. In addition to surgery time, complication risks, and recovery time, this also includes long-term breast-related outcomes and body-related outcomes.
5. Conclusions
Long-term breast-related and body-related results of ABR are superior to IBR. Aesthetic results of the donor site do not adversely affect body image in women undergoing ABR. Contrarily, women who underwent ABR are significantly more satisfied with the abdomen than women who underwent IBR. While LTP flap harvest affects the appearance of the hips and outer thighs, it does not negatively affect body image. The results of this study contribute to the tailor-made approach to breast reconstruction.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study was approved by the Medical Ethics Committees of Maastricht University Medical Center (METC2020-2232) and Zuyderland MC (METCZ20200113).
Declaration of competing interest
None.
Contributor Information
Renée ML. Miseré, Email: renee.misere@mumc.nl.
Sander MJ. van Kuijk, Email: sander.van.kuijk@mumc.nl.
Eva L. Claassens, Email: eva.claassens@mumc.nl.
Esther M. Heuts, Email: e.heuts@mumc.nl.
Andrzej A. Piatkowski, Email: andrzej.piatkowskidegrzymala@mumc.nl.
René RWJ. van der Hulst, Email: r.vander.hulst@mumc.nl.
References
- 1.Integraal Kankercentrum Nederland NKR cijfers. IKNL. http://www.inkl.nl/nkr-cijfers
- 2.van der Waal D., Verbeek A.L., den Heeten G.J., Ripping T.M., Tjan-Heijnen V.C., Broeders M.J. Breast cancer diagnosis and death in The Netherlands: a changing burden. Eur J Publ Health. 2015;25(2):320–324. doi: 10.1093/eurpub/cku088. [DOI] [PubMed] [Google Scholar]
- 3.Jagsi R., Li Y., Morrow M. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction: results of a survey of breast cancer survivors. Ann Surg. 2015;261(6):1198–1206. doi: 10.1097/SLA.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isern A.E., Tengrup I., Loman N., Olsson H., Ringberg A. Aesthetic outcome, patient satisfaction, and health-related quality of life in women at high risk undergoing prophylactic mastectomy and immediate breast reconstruction. J Plast Reconstr Aesthetic Surg. 2008;61(10):1177–1187. doi: 10.1016/j.bjps.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Klapdor R., Weiß C., Kuehnle E. Quality of life after bilateral and contralateral prophylactic mastectomy with implant reconstruction. Breast Care. 2020;15(5):519–526. doi: 10.1159/000505449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordeiro P.G. Breast reconstruction after surgery for breast cancer. N Engl J Med. 2008;359(15):1590–1601. doi: 10.1056/NEJMct0802899. [DOI] [PubMed] [Google Scholar]
- 7.Kouwenberg C.A.E., de Ligt K.M., Kranenburg L.W. Long-term health-related quality of life after four common surgical treatment options for breast cancer and the effect of complications: a retrospective patient-reported survey among 1871 patients. Plast Reconstr Surg. 2020;146(1):1–13. doi: 10.1097/PRS.0000000000006887. [DOI] [PubMed] [Google Scholar]
- 8.Beugels J., Cornelissen A.J.M., van Kuijk S.M.J. Sensory recovery of the breast following innervated and noninnervated DIEP flap breast reconstruction. Plast Reconstr Surg. 2019;144(2):178e–188e. doi: 10.1097/PRS.0000000000005802. [DOI] [PubMed] [Google Scholar]
- 9.Beugels J., van Kuijk S.M.J., Lataster A., van der Hulst R., Tuinder S.M.H. Sensory recovery of the breast following innervated and noninnervated lateral thigh perforator flap breast reconstruction. Plast Reconstr Surg. 2021;147(2):281–292. doi: 10.1097/PRS.0000000000007547. [DOI] [PubMed] [Google Scholar]
- 10.Gopie J.P., Hilhorst M.T., Kleijne A. Women's motives to opt for either implant or DIEP-flap breast reconstruction. J Plast Reconstr Aesthetic Surg. 2011;64(8):1062–1067. doi: 10.1016/j.bjps.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Damen T.H., Wei W., Mureau M.A. Medium-term cost analysis of breast reconstructions in a single Dutch centre: a comparison of implants, implants preceded by tissue expansion, LD transpositions and DIEP flaps. J Plast Reconstr Aesthetic Surg. 2011;64(8):1043–1053. doi: 10.1016/j.bjps.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Matros E., Albornoz C.R., Razdan S.N. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plast Reconstr Surg. 2015;135(4):937–946. doi: 10.1097/PRS.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 13.Razdan S.N., Cordeiro P.G., Albornoz C.R. Cost-effectiveness analysis of breast reconstruction options in the setting of postmastectomy radiotherapy using the BREAST-Q. Plast Reconstr Surg. 2016;137(3):510e–517e. doi: 10.1097/01.prs.0000479935.92904.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santosa K.B., Qi J., Kim H.M., Hamill J.B., Wilkins E.G., Pusic A.L. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153(10):891–899. doi: 10.1001/jamasurg.2018.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson J.A., Allen R.J., Jr., Polanco T. Long-term patient-reported outcomes following postmastectomy breast reconstruction: an 8-year examination of 3268 patients. Ann Surg. 2019;270(3):473–483. doi: 10.1097/SLA.0000000000003467. [DOI] [PubMed] [Google Scholar]
- 16.Toyserkani N.M., Jørgensen M.G., Tabatabaeifar S., Damsgaard T., Sørensen J.A. Autologous versus implant-based breast reconstruction: a systematic review and meta-analysis of Breast-Q patient-reported outcomes. J Plast Reconstr Aesthetic Surg. 2020;73(2):278–285. doi: 10.1016/j.bjps.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Lindenblatt N., Gruenherz L., Farhadi J. A systematic review of donor site aesthetic and complications after deep inferior epigastric perforator flap breast reconstruction. Gland Surg. 2019;8(4):389–398. doi: 10.21037/gs.2019.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grünherz L., Keijzer W., Uyulmaz S. Donor site aesthetics and morbidity after DIEP flap breast reconstruction-A retrospective multicenter study. Breast J. 2020;26(10):1980–1986. doi: 10.1111/tbj.14003. [DOI] [PubMed] [Google Scholar]
- 19.Vyas R.M., Dickinson B.P., Fastekjian J.H., Watson J.P., DaLio A.L., Crisera C.A. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121(5) doi: 10.1097/PRS.0b013e31816b1458. [DOI] [PubMed] [Google Scholar]
- 20.Hopwood P., Hopwood N. New challenges in psycho-oncology: an embodied approach to body image. Psycho Oncol. 2019;28(2):211–218. doi: 10.1002/pon.4936. [DOI] [PubMed] [Google Scholar]
- 21.Begovic-Juhant A., Chmielewski A., Iwuagwu S., Chapman L.A. Impact of body image on depression and quality of life among women with breast cancer. J Psychosoc Oncol. 2012;30(4):446–460. doi: 10.1080/07347332.2012.684856. [DOI] [PubMed] [Google Scholar]
- 22.Lam W.W., Li W.W., Bonanno G.A. Trajectories of body image and sexuality during the first year following diagnosis of breast cancer and their relationship to 6 years psychosocial outcomes. Breast Canc Res Treat. 2012;131(3):957–967. doi: 10.1007/s10549-011-1798-2. [DOI] [PubMed] [Google Scholar]
- 23.Cohen W.A., Mundy L.R., Ballard T.N.S. The BREAST-Q in surgical research: a review of the literature 2009–2015. J Plast Reconstr Aesthetic Surg. 2016;69(2):149–162. doi: 10.1016/j.bjps.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pusic A.L., Klassen A.F., Scott A.M., Klok J.A., Cordeiro P.G., Cano S.J. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 25.Pusic A.L., Matros E., Fine N. Patient-reported outcomes 1 year after immediate breast reconstruction: results of the mastectomy reconstruction outcomes consortium study. J Clin Oncol. 2017;35(22):2499. doi: 10.1200/JCO.2016.69.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atisha D.M., Rushing C.N., Samsa G.P. A national snapshot of satisfaction with breast cancer procedures. Ann Surg Oncol. 2015;22(2):361–369. doi: 10.1245/s10434-014-4246-9. [DOI] [PubMed] [Google Scholar]
- 27.Pool S.M.W., Wolthuizen R., Mouës-Vink C.M. Silicone breast prostheses: a cohort study of complaints, complications, and explantations between 2003 and 2015. J Plast Reconstr Aesthetic Surg. 2018;71(11):1563–1569. doi: 10.1016/j.bjps.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Van Slyke A.C., Carr M., Carr N.J. Not all breast implants are equal: a 13-year review of implant longevity and reasons for explantation. Plast Reconstr Surg. 2018;142(3):281e–289e. doi: 10.1097/PRS.0000000000004678. [DOI] [PubMed] [Google Scholar]
- 29.Adidharma W., Latack K.R., Colohan S.M., Morrison S.D., Cederna P.S. Breast implant illness: are social media and the internet worrying patients sick? Plast Reconstr Surg. 2020;145(1):225e–227e. doi: 10.1097/PRS.0000000000006361. [DOI] [PubMed] [Google Scholar]
- 30.Jewell M.L., Jewell H.L. Breast implant-associated illness: medicine by belief, so says dr. Google. Aesthet Surg J. 2019;39(4):Np87–Np89. doi: 10.1093/asj/sjz007. [DOI] [PubMed] [Google Scholar]
- 31.International Consortium of Investigative Journalists . 2018. Medical devices harm patients worldwide as governments fail on safety.https://www.icij.org/investigations/implant-files/medical-devices-harm-patients-worldwide-as-governments-fail-on-safety/ Published. [Google Scholar]
- 32.Miseré R.M.L., Colaris M.J.L., Tervaert J.W.C., van der Hulst R.R.W.J. The prevalence of self-reported health complaints and health-related quality of life in women with breast implants. Aesthetic Surg J. 2021;41(6):661–668. doi: 10.1093/asj/sjaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colaris M.J.L., de Boer M., van der Hulst R.R., Cohen Tervaert J.W. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017;65(1):120–128. doi: 10.1007/s12026-016-8821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnusson M.R., Cooter R.D., Rakhorst H., McGuire P.A., Adams W.P., Jr., Deva A.K. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;143 doi: 10.1097/PRS.0000000000005573. (3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):74s-81s. [DOI] [PubMed] [Google Scholar]
- 35.de Boer M., Colaris M., van der Hulst R., Cohen Tervaert J.W. Is explantation of silicone breast implants useful in patients with complaints? Immunol Res. 2017;65(1):25–36. doi: 10.1007/s12026-016-8813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miseré R.M.L., van der Hulst R.R.W.J. Self-reported health complaints in women undergoing explantation of breast implants. Aesthetic Surg J. 2020:sjaa337. doi: 10.1093/asj/sjaa337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fobair P., Stewart S.L., Chang S., D'Onofrio C., Banks P.J., Bloom J.R. Body image and sexual problems in young women with breast cancer. Psycho Oncol. 2006;15(7):579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg S.M., Tamimi R.M., Gelber S. Body image in recently diagnosed young women with early breast cancer. Psycho Oncol. 2013;22(8):1849–1855. doi: 10.1002/pon.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cairo Notari S., Notari L., Favez N., Delaloye J.F., Ghisletta P. The protective effect of a satisfying romantic relationship on women's body image after breast cancer: a longitudinal study. Psycho Oncol. 2017;26(6):836–842. doi: 10.1002/pon.4238. [DOI] [PubMed] [Google Scholar]
- 40.Davis C., Tami P., Ramsay D. Body image in older breast cancer survivors: a systematic review. Psycho Oncol. 2020;29(5):823–832. doi: 10.1002/pon.5359. [DOI] [PubMed] [Google Scholar]
- 41.Lewis-Smith H., Diedrichs P.C., Rumsey N., Harcourt D. Efficacy of psychosocial and physical activity-based interventions to improve body image among women treated for breast cancer: a systematic review. Psycho Oncol. 2018;27(12):2687–2699. doi: 10.1002/pon.4870. [DOI] [PubMed] [Google Scholar]
- 42.Sebri V., Durosini I., Triberti S., Pravettoni G. The efficacy of psychological intervention on body image in breast cancer patients and survivors: a systematic-review and meta-analysis. Front Psychol. 2021;12:611954. doi: 10.3389/fpsyg.2021.611954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Sánchez L., Luque-Ribelles V., Gil-Olarte P., Ruiz-González P., Guil R. Enhancing self-esteem and body image of breast cancer women through interventions: a systematic review. Int J Environ Res Publ Health. 2021;18(4) doi: 10.3390/ijerph18041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopie J.P., ter Kuile M.M., Timman R., Mureau M.A., Tibben A. Impact of delayed implant and DIEP flap breast reconstruction on body image and sexual satisfaction: a prospective follow-up study. Psycho Oncol. 2014;23(1):100–107. doi: 10.1002/pon.3377. [DOI] [PubMed] [Google Scholar]
- 45.Fang S.Y., Shu B.C., Chang Y.J. The effect of breast reconstruction surgery on body image among women after mastectomy: a meta-analysis. Breast Canc Res Treat. 2013;137(1):13–21. doi: 10.1007/s10549-012-2349-1. [DOI] [PubMed] [Google Scholar]
- 46.Teo I., Reece G.P., Christie I.C. Body image and quality of life of breast cancer patients: influence of timing and stage of breast reconstruction. Psycho Oncol. 2016;25(9):1106–1112. doi: 10.1002/pon.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salgarello M., Tambasco D., Farallo E. DIEP flap donor site versus elective abdominoplasty short-term complication rates: a meta-analysis. Aesthetic Plast Surg. 2012;36(2):363–369. doi: 10.1007/s00266-011-9804-y. [DOI] [PubMed] [Google Scholar]
- 48.Wechselberger G., Haug M., Schoeller T., Nehoda H., Piza-Katzer H. Breast reconstruction facilitated by vertical banded gastroplasty. Obes Surg. 2000;10(5):460–464. doi: 10.1381/096089200321593968. [DOI] [PubMed] [Google Scholar]
- 49.Ingvaldsen C.A., Tindholdt T.T., Tønseth K.A. DIEAP flap patients equally as satisfied with the abdomen as abdominoplasty patients. Plast Reconstr Surg Glob Open. 2018;6(8) doi: 10.1097/GOX.0000000000001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone J.P., Bello R.J., Siotos C. Patient-related risk factors for worsened abdominal well-being after autologous breast reconstruction. Plast Reconstr Surg. 2020;145(3):475e–480e. doi: 10.1097/PRS.0000000000006536. [DOI] [PubMed] [Google Scholar]
- 51.de Vries C.E.E., Klassen A.F., Hoogbergen M.M., Alderman A.K., Pusic A.L. Measuring outcomes in cosmetic abdominoplasty: the BODY-Q. Clin Plast Surg. 2020;47(3):429–436. doi: 10.1016/j.cps.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Tuinder S.M.H., Beugels J., Lataster A. The lateral thigh perforator flap for autologous breast reconstruction: a prospective analysis of 138 flaps. Plast Reconstr Surg. 2018;141(2):257–268. doi: 10.1097/PRS.0000000000004072. [DOI] [PubMed] [Google Scholar]
- 53.Tessler O., Guste J., Bartow M.J. Stacked lateral thigh perforator flap as a novel option for autologous breast reconstruction. Plast Reconstr Surg. 2019;143(6):1601–1604. doi: 10.1097/PRS.0000000000005670. [DOI] [PubMed] [Google Scholar]
- 54.Poulsen L., McEvenue G., Klassen A., Hoogbergen M., Sorensen J.A., Pusic A. Patient-reported outcome measures: body-Q. Clin Plast Surg. 2019;46(1):15–24. doi: 10.1016/j.cps.2018.08.003. [DOI] [PubMed] [Google Scholar]