Abstract
Background
The benefit of adjuvant cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors with endocrine therapy (ET) in hormone receptor-positive, human epidermal growth factor 2 receptor-negative (HR+/HER2-) early breast cancer (EBC) is uncertain. Hence, we performed a meta-analysis to determine the efficacy and safety of adjuvant CDK4/6 inhibitors plus ET and to identify potential preferred subpopulations for this regimen.
Methods
A literature search was conducted in PubMed, Embase, Cochrane databases up to Jan 15, 2021. Hazard ratios (HRs) for invasive disease-free survival (IDFS) and risk ratios (RRs) for grade 3/4 adverse events (AEs) and treatment discontinuation were extracted. Analysis with predefined subgroup variables was done. Trial sequential analysis (TSA) was performed to assess the conclusiveness of survival outcomes.
Results
Three trials were eligible (N = 12647). Compared with ET, adjuvant CDK4/6 inhibitors with ET prolonged IDFS in patients with HR+/HER2- EBC (HR 0.87, 95% CI 0.76–0.98, p = 0.03, I2 = 19%), with positive therapeutic responses observed in patients with N2/N3 nodal status (HR 0.83, 95% CI 0.71–0.97, p = 0.02, I2 = 0%). None of the cumulative z-curves crossed the trial monitoring boundaries in TSA, and no reliable conclusion could be drawn. The combination treatment carried a higher risk of grade 3/4 AEs (RR 4.14, 95% CI 3.33–5.15, p < 0.00001) and an increase in treatment discontinuation due to AEs (RR 19.16, 95% CI 9.27–39.61, p < 0.00001).
Conclusions
Adjuvant CDK4/6 inhibitors with ET might provide survival benefit in HR+/HER2- EBC. A statistically significantly improved IDFS was only observed in N2/N3 subgroup. However, overall evidence favoring the use of this combination regimen was inadequate.
Keywords: CDK4/6 inhibitors, Endocrine therapy, Adjuvant, Early breast cancer, Meta-analysis
Highlights
-
•
Primary results from clinical trials investigating adjuvant CDK4/6 inhibitors were inconsistent.
-
•
The meta-analysis demonstrated potential IDFS benefit of adjuvant CDK4/6 inhibitors with endocrine therapy in HR+/HER2-early breast cancer patients.
-
•
A statistically significantly improved IDFS was observed in patients with N2/N3 nodal status.
-
•
Overall evidence favoring the use of adjuvant CDK4/6 inhibitors remained inadequate.
Introduction
Approximately 70% of early breast cancer (EBC) are diagnosed as hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) [[1], [2], [3], [4]]. Adjuvant endocrine therapy (ET) (aromatase inhibitors and/or antiestrogens with or without ovarian suppression) is a fundamental component of systemic therapy for standard treatment of HR+/HER2- EBC and has contributed to a significant decrease in risk of recurrence and death [5]. Still, up to 20% of patients may experience recurrence with/without distant metastases in the first 10 years [6]. Risk of recurrence is even higher for patients with high-risk clinicopathologic features, especially during the first several years of adjuvant ET [7]. Therefore, it is critical to optimize adjuvant therapy for these patients.
Recent studies have revealed an important role of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors in endocrine-resistant breast cancer [[8], [9], [10], [11], [12], [13]]. Cell cycle progression is strictly regulated by a wide range of pathways including the cyclin-dependent kinases (CDK)-retinoblastoma (RB)-E2F pathway [9,14]. The dysregulated CDK-RB-E2F pathways are associated with endocrine-resistance in HR + breast cancer [10,15]. The most relevant therapeutic improvement in this subset is represented by the introduction of CDK4/6 inhibitors (palbociclib, ribociclib and abemaciclib) to standard ET. These drugs bind to CDK4/6 and inhibit their aberrant functioning, causing cell-cycle arrest and apoptosis [16]. Pivotal trials led to the approval of CDK4/6-inhibitors plus ET combinations after showing almost indistinguishable statistically significant and clinically meaningful improvements in progression-free survival (PFS) and/or overall survival in first/second-line setting of patients with HR+/HER2-metastatic breast cancer (MBC) [[17], [18], [19], [20], [21], [22], [23]]. Median PFS and overall response rates of all the intervention arms were roughly doubled compared to the comparison arms of standard ET [[17], [18], [19], [20], [21], [22], [23]].
Given the success of CDK4/6 inhibitors for HR+/HER2- MBC, there is great interest in determining whether the survival benefit translates into an adjuvant breast cancer setting [24]. All three CDK4/6 inhibitors are being studied in the adjuvant setting in phase III trial [[25], [26], [27], [28]]. Three trials have completed enrollment. The monarchE trial (NCT03155997) explored the adjuvant use of abemaciclib. The PALLAS trial (NCT02513394) studied the addition of palbociclib to standard of care ET in stage II-III HR+/HER2-breast cancer. The PENELOPE-B trial (NCT01864746) investigated the combination of palbociclib with standard ET for patients with HR + residual disease after neoadjuvant chemotherapy. In contrast to the similar benefit observed in MBC, the three trials demonstrated inconsistent primary outcomes regarding the effectiveness of adjuvant CDK4/6 inhibitors in EBC. Thus, we performed this meta-analysis to evaluate the efficacy and safety of CDK4/6 inhibitors in combination with standard endocrine agents as compared to standard ET for the adjuvant treatment of HR+/HER2- EBC, and attempted to identify the potential candidates that may benefit most from this novel therapeutic regimen. Trial sequential analysis (TSA) was also applied to compute the required information size and evaluate the quality of information obtained from the conventional cumulative meta-analysis.
Materials and methods
This was a meta-analysis of randomized controlled trials investigating the efficacy and safety of adjuvant CDK4/6 inhibitors with ET in HR+/HER2- EBC patients. The study was registered on PROSPERO with registration number CRD42021231421.
Data sources and search strategy
A literature search in PubMed, Embase, and Cochrane Register of Controlled Trials with key words related to “palbociclib”, “ribociclib”, “abemaciclib”, “CDK4/6 inhibitors”, “adjuvant”, “endocrine therapy”, “early breast cancer”, and “randomized controlled trial” was conducted up to January 15, 2021. Conference proceedings of American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and San Antonio Breast Cancer Symposium (SABCS) were also consulted up to January 2021 to identify unpublished studies. No language restriction was applied. The citation lists of the relevant literature were reviewed for potentially eligible articles.
Study selection
Inclusion criteria were: (1) randomized-controlled trials of CDK4/6 inhibitors with ET for the adjuvant treatment of HR+/HER2-early breast cancer; (2) trials reporting invasive disease-free survival (IDFS) as the primary outcome and hazard ratios (HRs) with 95% confidence intervals (CIs) for IDFS of the overall patients and the subgroups. Studies not fulfilling the inclusion criteria were excluded. Other exclusion criteria were as followed: (1) on-going trials with no results published or presented; (2) non-randomized, single-arm study; (3) other types of publication including review, trial protocol and report on patient characteristics.
Data extraction and risk of bias assessment
Two investigators (YYL and TZ) independently extracted the data and discrepancies were resolved by consensus. The following data from eligible studies were collected: trial name, design, treatment regimen, dose, population characteristics, the number of participants, median follow-up period, IDFS, number of grade 3/4 adverse events (AEs) and number of treatment discontinuation. The Cochrane Collaboration's tool [29] was used to assess the risk of bias of individual studies.
Statistical analysis
We used HR and 95% CI to evaluate IDFS, and risk ratios (RRs) to evaluate AEs and treatment discontinuation. The Cochran Q test and Higgins I2 statistic were applied to assess statistical heterogeneity [30]. The pooled HR was calculated by inverse-variance-weighted method; the pooled RR was synthesized by Mantel-Haenszel method. When moderate heterogeneity was observed (p-value <0.10 or I2 >30%), a random-effects model was employed; otherwise, a fixed-effects model was used. A p-value <0.05 was considered statistically significant. We conducted meta-analysis of IDFS, AEs and treatment discontinuation; and explored the effect of predefined subgroup variables (TNM stage, tumor stage, nodal stage, Ki-67, histologic grade, prior neoadjuvant chemotherapy [NAC], age, ethnicity, menopausal status, and type of CDK4/6 inhibitor) on IDFS. To resolve different subgroup categorizations in individual study, some subgroups were combined into a single group before the final analysis was done. Sensitivity analysis was applied to identify the source of heterogeneity when I2 >75% in the analysis containing more than two studies. Publication bias was not assessed due to inadequate trials included in the analysis. Statistical analyses were carried out using Review Manager version 5.4 software (Cochrane Tech, London, UK).
Positive results from meta-analysis of IDFS were examined with TSA. We set a two-sided 5% risk of type I error (α = 5%) and 20% risk of type II error (β = 20%). Analyses were conducted with both a priori information size (APIS) and low-bias heterogeneity-adjusted information size (LBHIS) to estimate a realistic sample size [31,32]. APIS was computed for an a priori prespecified relative risk reduction (RRR) of 15%, the intervention effect seems relevant in most therapeutic area [31]. LBHIS was based on I2 from all included studies [30] and the information size from trials with adequate allocation concealment [33]. Trial sequential monitoring boundaries (TSMB) using APIS (TSMBAPIS) and LBHIS (TSMBLBHIS) were determined by O'Brien–Fleming α-spending function [34]. When I2 ≤ 30%, a fixed-effects model was employed to calculate cumulative z-scores; otherwise, a random-effects model was used. We constructed a cumulative z-curve and assessed its crossing of TSMBAPIS and TSMBLBHIS to ascertain the conclusiveness of the effectiveness of CDK4/6 inhibitor-based adjuvant regimen. TSA was performed using the metacumbounds command of Stata (version 16.0) [35].
Results
Characteristics of eligible studies
The literature search returned 115 records and three eligible phase III trials were identified (Fig. 1). Some data were retrieved from a later-published full article [36] and two latest conference proceedings [37,38]. A total of 12647 HR+/HER2- EBC patients were randomized to receive adjuvant CDK4/6 inhibitors in combination with ET (N = 6322; abemaciclib 44.4%, palbociclib 55.6%) versus adjuvant ET (N = 6325). The risk of bias was low for PENELOPE-B [27] and unclear for monarchE [25] and PALLAS [26] (Supplementary Figure 1). Main characteristics of the included studies are summarized in Table 1. Combination of subgroups was done for monarchE and PALLAS due to different subgroup categorizations (Supplementary Table 2).
Fig. 1.
Flow diagram of identifying eligible studies.
Table 1.
Characteristics of included studies.
| Trial | Design | Population characteristics | Regimen | Dose | ITT N | Median age, year | Median follow-up, mo | Primary endpoint | IDFS HR (95% CI) of overall patients |
|---|---|---|---|---|---|---|---|---|---|
| monarchE | Open label, randomized (1:1), phase III | HR + HER2-, pre- or post-menopausal, high riska, stage II or III, node positive, with or without NACT | Abemaciclib + ET vs ET alone | Abemaciclib 150 mg bid × 2 years; standard adjuvant ETd | 5637 (2808/2829) | 51; 51 | 15.5; 15.5 | IDFS | 0.75 (0.60–0.93) |
| PALLAS | Open label, randomized (1:1), phase III | HR + HER2-, pre- or post-menopausal, low risk or high riskb, stage II or III, node positive or negative, with or without NACT | Palbociclib + ET vs ET alone | Palbociclib 125 mg once daily, d1-21 in a 28-day cycle × 2 years; standard adjuvant ETd | 5760 (2883/2877) | 52; 52 | 23.7; 23.7 | IDFS | 0.93 (0.76–1.15) |
| PENELOPE-B | Double blind, randomized (1:1), phase III | HR + HER2-, pre- or post-menopausal, high riskc, early BC, node positive or negative, no pCR after NACT | Palbociclib + ET vs placebo + ET | Palbociclib 125 mg once daily, p.o., d1-21, q28d for 13 cycles; Placebo d1-21, q28d for 13 cycles; ET according to local standard | 1250 (631/619) | 49; 48 | 42.8; 42.8 | IDFS | 0.93 (0.74–1.17) |
ITT intention-to-treat; IDFS invasive disease-free survival; HR hazard ratio; 95% CI 95% confidence interval; TNM tumor, node, metastasis; ET endocrine therapy (aromatase inhibitors and/or antiestrogens with or without ovarian suppression); NACT neoadjuvant chemotherapy; BC breast cancer; pCR pathologic complete remission.
Defined as patients with four or more positive pathologic axillary lymph nodes or one to three positive axillary lymph nodes and at least one of the following: tumor size≥5 cm, histologic grade 3, or centrally assessed Ki-67 ≥ 20%.
Defined as patients with≥4 nodes involved (≥N2), or 1–3 nodes with either T3/T4 and/or G3 disease.
Defined as patients with CPS-EG (Clinical-Pathologic Scoring System incorporating estrogen receptor-negative disease and nuclear grade 3 tumor pathology) score≥3, or 2 with ypN+.
Consist of tamoxifen or an aromatase inhibitor (letrozole, anastrozole exemestane), with or without an LHRH agonist.
Invasive disease-free survival
An overall pooled IDFS benefit was observed for adjuvant CDK4/6 inhibitors in combination with ET compared to standard ET (HR 0.87, 95% CI 0.76–0.98, p = 0.03, I2 = 19%; Fig. 2).
Fig. 2.
Forest plot of pooled hazard ratio for invasive disease-free survival of CDK4/6 inhibitors plus endocrine therapy versus ET in the overall patients. ET endocrine therapy.
Predefined subgroup analysis
Moderate heterogeneity was detected in six subgroups (stage IIB/III, stage T0/T1/Tis/TX, stage T3/T4, non-Asian, prior NAC, and premenopausal status) and a random-effects model was applied. Only one study reported the findings for Ki-67, and thus analysis was not conducted for this subgroup.
TNM stage
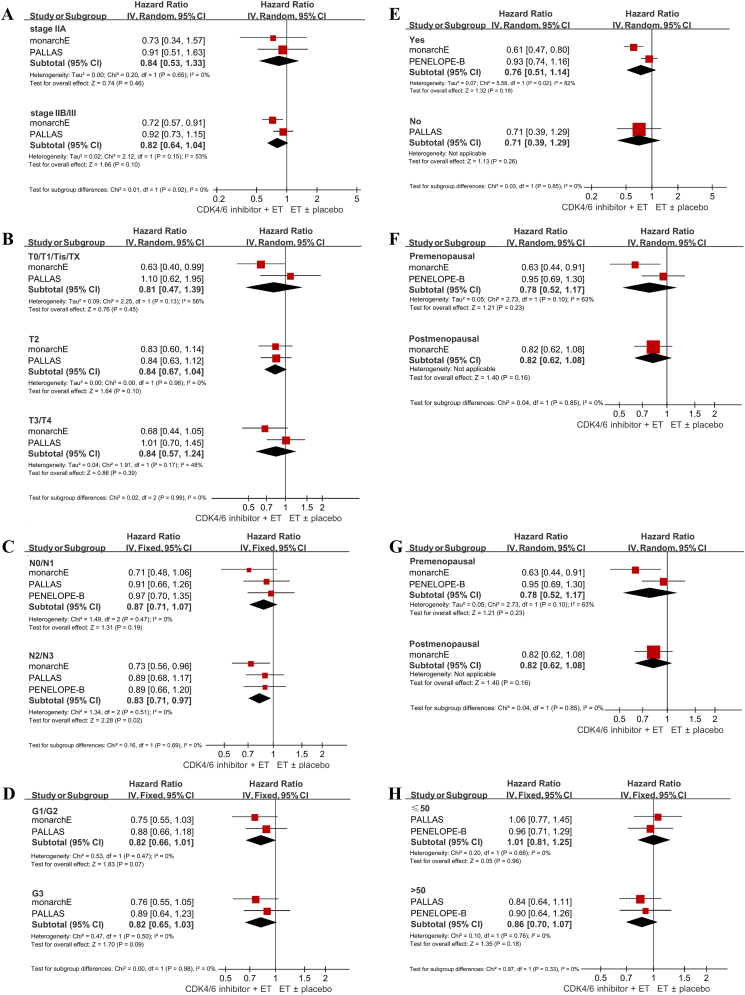
Two studies reported IDFS HR for patients with stage IIA (N = 1689). The pooled effect of adjuvant CDK4/6 inhibitors plus ET versus ET was not significant (HR 0.84, 95% CI 0.53–1.33, p = 0.46). Two studies reported the results for patient with stage IIB/III (N = 9468) and the cumulative effect was also statistically insignificant (HR 0.82, 95% CI 0.64–1.04, p = 0.10) (Fig. 3A). No significant subgroup differences were detected (p = 0.92).
Fig. 3.
Forest plot of pooled hazard ratios comparing invasive disease-free survival in stage IIA and stage IIB/III subgroups (A); in stage T0/T1/Tis/TX, stage T2 and stage T3/T4 subgroups (B); in N0/N1 and N2/N3 subgroups (C); in G1/G2 and G3 subgroups (D); in patients with or without prior neoadjuvant chemotherapy (E); in non-Asians and Asians (F); in patients ≤50 years old and patients >50 years old (G); in premenopausal and postmenopausal patients (H). ET endocrine therapy.
Tumor stage
Two studies provided the findings for patients in stage T0/T1/Tis/TX (N = 2602), and the intervention effect of adjuvant CDK4/6 inhibitors plus ET in this subgroup was statistically insignificant (HR 0.81, 95% CI 0.47–1.39, p = 0.45). Two studies provided the findings for patients in stage T2 (N = 6027). The cumulative effect for the combination therapy was not significant (HR 0.84, 95% CI 0.67–1.04, p = 0.10). Results for patients in stage T3/T4 were also provided in two studies (N = 2685). The pooled result remained insignificant (HR 0.84, 95% CI 0.57–1.24, p = 0.39) (Fig. 3B). No significant subgroup differences were observed (p = 0.99).
Nodal stage
All the included studies reported IDFS for patients in stage N0/N1 (N = 6474) and N2/N3 (N = 6157). A statistically significant effect adjuvant CDK4/6 inhibitors plus ET was observed in stage N2/N3 (HR 0.83, 95% CI 0.71–0.97, p = 0.02), but not in stage N0/N1 (HR 0.87, 95% CI 0.71–1.07, p = 0.19) (Fig. 3C). Subgroup differences between these groups were insignificant (p = 0.69).
Histologic grade
Two studies reported the results for patients with G1/G2 histologic grade (N = 7085), and patients with G3 histologic grade (N = 3759). The cumulative estimates of adjuvant CDK4/6 inhibitors plus ET versus ET in both subgroups were statistically insignificant (HR 0.82, 95% CI 0.66–1.01, p = 0.07; and HR 0.82, 95% CI 0.65–1.03, p = 0.09, respectively) (Fig. 3D). There were no significant subgroup differences (p = 0.48).
Prior NAC
Survival outcomes for patients with prior NAC (N = 3306) were reported in two studies. The cumulative result of adjuvant CDK4/6 inhibitors plus ET was statistically insignificant (HR 0.76, 95% CI 0.51–1.14, p = 0.19). One study reported the outcome for patients without prior NAC or adjuvant chemotherapy (N = 1005) and the effect was also insignificant (HR 0.71, 95% CI 0.39–1.29, p = 0.26) (Fig. 3E). Differences between the two subpopulations were insignificant (p = 0.85).
Ethnicity
Two studies reported IDFS HR for non-Asians (N = 5366). The pooled estimate of adjuvant CDK4/6 inhibitors plus ET was not remarkable (HR 0.83, 95% CI 0.64–1.07, p = 0.15). Two studies reported the results for Asians (N = 1439), and the intervention effect was also unremarkable (HR 0.82, 95% CI 0.54–1.26, p = 0.37) (Fig. 3F). No significant subgroup differences were detected (p = 0.99).
Age
Results for patients ≤50 (N = 3314) and >50 years old (N = 3759) were provided in two studies. No statistically significant effect of adjuvant CDK4/6 inhibitors plus ET was observed in both subgroups (HR 1.01, 95% CI 0.81–1.25, p = 0.96, and HR 0.86, 95% CI 0.70–1.07, p = 0.18, respectively) (Fig. 2G). There were no significant differences between the two groups (p = 0.33).
Menopausal status
Two studies reported the subgroup findings for premenopausal patients (N = 3069), and one study reported the finding for postmenopausal patients (N = 3184). The treatment effects of adjuvant CDK4/6 inhibitors combined with ET in both subgroups were statistically insignificant (HR 0.78, 95% CI 0.52–1.17, p = 0.23; and HR 0.82, 95% CI 0.62–1.08, p = 0.16, respectively) (Fig. 3H).
Type of CDK4/6 inhibitor
The pooled HR for abemaciclib and palbociclib was 0.75 (95% CI 0.60–0.93, p = 0.01) and 0.93 (95% CI 0.80–1.08, p = 0.35), respectively. There were no statistically significant discrepancies between the two types of CDK4/6 inhibitor regarding clinical efficacy (p = 0.12).
Trial sequential analysis
TSA of two meta-analyses of IDFS was conducted using fixed-effects models. We found lack of evidence for an overall favorable effect of adjuvant CDK4/6 inhibitors with ET over standard endocrine agents in HR+/HER2- EBC. The cumulative z-curve only touched the TSMBAPIS and TSMBLBHIS, with the accumulated information size exceeding APIS (Fig. 4A), but not LBHIS (Fig. 4B), implying a potential weaker intervention effect than anticipated from a prespecified 15% RRR and more trials should be included in the analysis to provide substantial evidence for an intervention effect of 13% RRR. Likewise, evidence for the superiority of the combination therapy over standard ET in patients with stage N2/N3 was inconclusive. The cumulative z-curve just crossed TSMBAPIS before APIS and did not cross TSMBLBHIS despite accruing adequate information size (Fig. 4C and D), suggesting this meta-analysis was underpowered to detect a 17% RRR suggested by studies with low-bias risk and future studies were warranted to confirm the prespecified 15% RRR.
Fig. 4.
Trial sequential analyses of meta-analysis of adjuvant CDK4/6 inhibitor-endocrine therapy in overall patients (A, B) and patients with stage N2/N3 (C, D) HR+/HER2-early breast cancer. The blue full line represents the cumulative z-curve; the green full line represents the conventional boundary for statistical significance (p = 0.05); the red dotted line represents the trial monitoring boundary; and the red full line represents the required information size determined by APIS (A, C) and LBHIS (B, D). APIS a priori information size; LBHIS low-bias heterogeneity-adjusted information size; RRR relative risk reduction; alpha risk of type I error; power statistical power to reject type II error.
Grade 3/4 adverse events
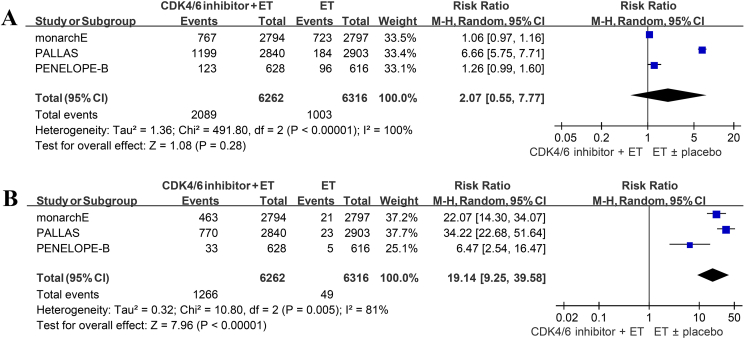
All the studies provided the incidence of total grade 3/4 AEs. The pooled RR was 4.14 with significant heterogeneity emerged (95% CI 3.33–5.15, p < 0.00001, I2 = 91%; Fig. 5A). After excluding PALLAS, I2 for heterogeneity dramatically reduced from 91% to 0% (Supplementary Table 3). Subgroup analysis with type of CDK4/6 inhibitor demonstrated no statistically significant differences between abemaciclib and palbociclib in total grade 3/4 AEs (p = 0.08; Supplementary Fig. 2A).
Fig. 5.
Forest plot of pooled risk ratio for grade 3/4 adverse events in the overall patients (A); forest plot of pooled risk ratio for grade 3/4 hematologic and non-hematologic adverse events (B). ET endocrine therapy.
Grade 3/4 hematologic AEs were more commonly observed in CDK4/6 intervention arm in the three studies (RR 45.96, 95% 13.57–155.70, p < 0.00001). The RRs were 67.39 (95% CI 19.56–232.17, p < 0.0001) for neutropenia, 86.69 (95% CI 20.52–366.23, p = 0.0005) for leukopenia, 4.17 (95% CI 2.37–7.32, p < 0.00001) for anemia, 11.12 (95% CI 7.20–17.18, p < 0.00001) for lymphopenia, and 8.26 (95% CI 2.33–29.34, p = 0.001) for thrombocytopenia. The pooled estimate of grade 3/4 non-hematologic AEs in the three studies was statistically insignificant (RR 2.23, 95% CI 0.77–6.44, p = 0.14). The RRs were 5.86 for fatigue (95% CI 1.64–20.93, p = 0.006), 2.73 for nausea (95% CI 0.70–10.68, p = 0.15), 5.24 for diarrhea (95% CI 0.30–92.27, p = 0.26), and 0.62 for arthralgia (95% CI 0.31–1.25, p = 0.18) (Fig. 5B, Supplementary Fig. 2B and C). The results of sensitivity analysis are presented in Supplementary Table 4–9.
Treatment discontinuation
Pooled analysis of the three included studies revealed an insignificant increase in patients discontinuing treatment in the combination arm (RR 2.07, 95% CI 0.55–7.77, p = 0.28, I2 = 100%; Fig. 6A). Sensitivity analysis identified PALLAS as the major source of heterogeneity (Supplementary Table 10).
Fig. 6.
Forest plot of pooled risk ratio for treatment discontinuation (A); forest plot of pooled risk ratio for treatment discontinuation due to adverse events (B). ET endocrine therapy.
The incidence of treatment interruption due to AEs was markedly higher in the intervention group than the control group. The pooled RR was 19.14 (95% CI 9.25–39.58, p < 0.00001, I2 = 81%; Fig. 6B), and 27.64 (95% CI 17.95–42.55, p < 0.00001, I2 = 52%; Supplementary Table 11) after excluding PENELOPE-B. Two studies provided the number of patients discontinuing treatment due to development of recurrent disease or secondary malignancy, and the cumulative result was statistically insignificant (RR 0.91, 95% CI 0.45–1.83, p = 0.78, I2 = 85%; Supplementary Fig. 3A). The pooled estimate of treatment interruption owing to death was also insignificant (RR 1.36, 95% CI 0.77–2.43, p = 0.29, I2 = 0%; Supplementary Fig. 3B).
Discussion
Optimizing adjuvant therapy for HR+/HER2- EBC is imperative to preventing early recurrence and metastases. Three large-scale, randomized, phase III trials reported inconsistent primary outcomes regarding the efficacy of adjuvant CDK4/6 inhibitors with ET in EBC. The present study is the first meta-analysis with TSA that summarizes the association between CDK4/6 inhibitor-based adjuvant therapy and survival outcomes in HR+/HER2- EBC. Results showed a potential favorable effect of adjuvant CDK4/6 inhibitors combined with ET over standard ET in HR+/HER2- EBC, and patients in stage N2/N3 were the only subpopulation that could derive statistically significant survival benefit from the combination treatment. However, overall evidence favoring the use of this novel regimen was inadequate. Future trials are needed to establish firm evidence for these findings.
Our meta-analysis demonstrated survival benefit of adjuvant CDK4/6 inhibitors with ET in EBC (IDFS HR 0.87, 95% CI 0.76–0.98, p = 0.03). The cumulative z-curve did not cross the TSMB in TSA, indicating that the current meta-analysis is of insufficient evidence to substantiate the result, and the overall intervention effect of the combination therapy may be around 13% RRR, which awaits validation by including more trials in the analysis to accrue ample information size. We further focused our meta-analysis on predefined subgroups of clinical relevance. The combinatorial regimen did not significantly prolong IDFS in most subgroups irrespective of TNM stage (stage IIA vs stage IIB/III), tumor stage (T0/T1/Tis/TX, T2 vs T3/T4), histologic grade (G1/G2 vs G3), prior NAC (yes vs no), ethnicity (non-Asian vs Asian), age (≤50 years old vs > 50 years old), or menopausal status (premenopausal vs postmenopausal). A statistically significant treatment effect was only observed in patients with stage N2/N3 (HR 0.83, 95% CI 0.71–0.97, p = 0.02). Results from TSA suggested a possible therapeutic effect of 15% RRR without reaching the required information size, which also calls for an update analysis involving more studies. Lymph node involvement is one of the most important risk factors for disease recurrence in HR + EBC. Unlike positive hormonal status, no statistical correlation between node infiltration and strong cyclin D1 protein expression and gene amplification was heretofore established [[39], [40], [41]]. Therefore, the mechanisms underlying the prognostic value of advanced nodal status in HR+/HER2- EBC patients receiving adjuvant CDK4/6 inhibitors plus standard ET is still unclear. Recent updates of subgroup analyses in PENELOPE-B revealed that numerically significant benefit from palbociclib was seen in a small group of patients (N = 64) with a luminal-B tumor assessed by Absolute Intrinsic Molecular Subtyping (IDFS HR 0.50, 95% CI 0.24–1.05), and a small subgroups of premenopausal patients (N = 119) receiving tamoxifen and gonadotropin-releasing hormone analogue as adjuvant ET (HR 0.52, 95% CI 0.27–1.02) [38,42]. Given the limited number of patient cohort, these results are mainly hypothesis generating and merit confirmation by examining the corresponding subgroup outcomes in other parallel clinical trials. Overall, our findings suggest the lack of definitive clinicopathologic features indicative of preferred therapeutic responses to adjuvant CDK4/6 inhibitors treatment. Combined with the fact that there are no available genomic signatures or validated predictive biomarkers to adequately select patients for CDK4/6 inhibitors [43,44], introduction of adjuvant CDK4/6 inhibitors to ET in HR+/HER2- EBC warrants deliberation.
The efficacy of the three CDK4/6 inhibitors abemaciclib, palbociclib, and ribociclib in combination with ET for HR+/HER2- MBC is almost parallel across different clinical trials. In the adjuvant setting, the effectiveness of abemaciclib and palbociclib contradicts one another (HR 0.75 [95% CI 0.60–0.93] vs. 0.93 [95% CI 0.80–1.08]), despite a statistically insignificant test for subgroup differences (p = 0.12). Possible explanations for the conflicting findings can be encapsulated in three aspects. One consideration is the differences in study population. Both monarchE and PENELOPE-B specifically enrolled EBC patients with high-risk of recurrence. In contrast, only 58.7% of patients in PALLAS were of high clinical risk disease. Enrollment of low-risk patients may have contributed to the negative results of PALLAS. A substantial proportion of patients in the intervention group in PALLAS discontinued treatment prematurely (42.2% versus 16.6% in monarchE and 19.5% in PENELOPE-B), which may also precipitate the observed lack of benefit from palbociclib. Additionally, even though both monarchE and PENELOPE-B exclusively included high-risk patients, the two trials applied different eligibility criteria, possibly making the positive results of monarchE irreproducible in PENELOPE-B. There are also concerns with the discrepancies in trial design. The course of treatment varied across studies. Both monarchE and PALLAS adopted a two-year treatment schedule, whereas PENELOPE-B only adopted a one-year treatment schedule. It is not impossible that positive results could be obtained in PENELOPE-B with continuation of therapy beyond one year. The duration of follow-up also raises attention. The median follow-up was 15.5 months for monarchE, 23.7 months for PALLAS, and 42.8 months for PENELOPE-B. Some researchers suggested that the follow-up in PALLAS was inadequate and might obscure potential significant delayed effect of palbociclib; and for monarchE, HR interpretation might be confounded by temporal fluctuations given the shortest period of follow-up period among the three trials [45]. The latest updates in monarchE partly addressed such concern, and showed that with an extended period of follow-up to over 24 months, the IDFS improvement for HR+/HER2- EBC who received prior NAC remained statistically significant (HR 0.61, 95% CI 0.47–0.80) [37], suggesting a lasting treatment benefit from adjuvant abemaciclib.
Results of grade 3/4 toxicity profiles in the present study mirrored those observed in the metastatic setting (RR 4.14, 95% CI 3.33–5.15, p < 0.0001) [17,18,20,23]. Administration of CDK4/6 inhibitors was associated with a significantly higher risk of grade 3/4 hematologic AEs (RR 45.96, 95% 13.57–155.70, p < 0.00001) compared to non-hematologic AEs (RR 2.23, 95% CI 0.77–6.44, p = 0.14), and an increase in early treatment discontinuation caused by AEs (RR 19.14, 95% CI 9.25–39.58, p < 0.00001). No significant differences between the two types of CDK4/6 inhibitor regarding the incidence of total grade 3/4 AEs was found (p = 0.08). PALLAS was the primary source of heterogeneity in the pooled analysis for grade 3/4 AEs and early treatment discontinuation. It is possible that such findings are attributed to the relatively high proportion of low-risk patients included in the study.
Our study has several limitations. First, the aggregated data were from published articles instead of individual patient data. Second, subgroup characterizations differed across the included studies, making it unfeasible to extract all the primary results for each predefined subgroup. Third, the inclusion of two different CDK4/6 inhibitors (abemaciclib and palbociclib) might introduce heterogeneity to the analysis.
A recent meta-analysis has also evaluated CDK4/6 inhibitors as adjuvant treatment for HR+/HER2- EBC. Similarly to our results, it has showed that administration of adjuvant CDK4/6 inhibitors was associated with a trend toward an IDFS benefit and an increase in the risk of toxicities and treatment discontinuation [46]. However, in this meta-analysis, two out of the three included trials had unpublished data, possibly undermining the accuracy of results. Comparatively, our study collected the latest data from published articles and conference proceedings of the three trials. In addition, we further ascertained the quality of information obtained from the conventional meta-analysis by performing TSA. We found that patients with N2/N3 disease had a tendency to benefit from adding CDK4/6 inhibitors to standard adjuvant treatment, and future analysis renewed with data from the currently ongoing studies (NCT03701334 and NCT03820830) is essential to help determine the ultimate role of CDK4/6 inhibitors in the adjuvant setting. Translation analyses from the included studies are also eagerly awaited to elucidate the relationship between high-risk clinicopathologic features, such as receiving prior NAC, advanced nodal status and luminal-B tumors, and therapeutic responses to CDK4/6 inhibitors. In short, adjuvant use of CDK4/6 inhibitors is not suitable for all HR+/HER2- EBC patients. Careful decision making is required in better tailoring patients’ treatments.
Conclusions
Adjuvant CDK4/6 inhibitors with ET may provide survival benefit in HR+/HER2- EBC. A statistically significant IDFS benefit was only observed in patients with N2/N3 disease. However, overall evidence favoring the use of this combination regimen was inadequate, and future trials are warranted to substantiate the results. Compared with ET, the addition of CDK4/6 inhibitors to ET carries a higher risk of grade 3/4 AEs and early treatment discontinuation. These results highlight the imperative to identify predictive biomarkers to select patients for whom adjuvant CDK4/6 inhibitors constitute effective treatment, and to investigate the correlation between tumor biology and the pharmacodynamics of different CDK4/6 inhibitors to guide adjuvant therapeutic strategy for HR+/HER2- EBC.
Funding acknowledgment
This work was supported by grants from the Science and Technology Planning Project of Guangzhou City (202002030236); the Beijing Medical Award Foundation (YXJL-2020-0941-0758); the Science and Technology Special Fund of Guangdong Provincial People's Hospital (No.Y012018218); the CSCO-Hengrui Cancer Research Fund (Y-HR2016-067); the Guangdong Provincial Department of Education Characteristic Innovation Project (2015KTSCX080); the Guangdong Basic and Applied Basic Research Foundation (2020A1515010346, 2021A1515011570); the Guangzhou Science and Technology Project (202102021055); and the Fundamental Research Funds for the Central Universities (No. 2020ZYGXZR017). Funding sources were not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Data availability statement
The data underlying this study will be shared on reasonable request to the corresponding author.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.07.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Howlader N., Altekruse S.F., Li C.I., Chen V.W., Clarke C.A., Ries L.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju055. Epub 2014/04/30. PubMed PMID: 24777111; PubMed Central PMCID: PMC4580552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso F., Spence D., Mertz S., Corneliussen-James D., Sabelko K., Gralow J. Global analysis of advanced/metastatic breast cancer: decade report (2005-2015) Breast. 2018;39:131–138. doi: 10.1016/j.breast.2018.03.002. Epub 2018/04/22. PubMed PMID: 29679849. [DOI] [PubMed] [Google Scholar]
- 3.Kwan M.L., Kushi L.H., Weltzien E., Maring B., Kutner S.E., Fulton R.S. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11(3):R31. doi: 10.1186/bcr2261. Epub 2009/05/26. PubMed PMID: 19463150; PubMed Central PMCID: PMC2716499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onitilo A.A., Engel J.M., Greenlee R.T., Mukesh B.N. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. doi: 10.3121/cmr.2009.825. Epub 2009/07/04. PubMed PMID: 19574486; PubMed Central PMCID: PMC2705275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . 2021. Breast cancer (version 1.2021)https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Available from: [Google Scholar]
- 6.Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 7.Mamounas E.P., Tang G., Paik S., Baehner F.L., Liu Q., Jeong J.H. 21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: results from NSABP B-28/NRG Oncology. Breast Canc Res Treat. 2018;168(1):69–77. doi: 10.1007/s10549-017-4550-8. Epub 2017/11/13. PubMed PMID: 29128898; PubMed Central PMCID: PMC5996978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy C.G., Dickler M.N. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Canc. 2016;23(8):R337–R352. doi: 10.1530/ERC-16-0121. Epub 2016/07/14. PubMed PMID: 27406875. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton E., Infante J.R. Targeting CDK4/6 in patients with cancer. Canc Treat Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. Epub 2016/03/28. PubMed PMID: 27017286. [DOI] [PubMed] [Google Scholar]
- 10.Finn R.S., Aleshin A., Slamon D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18(1):17. doi: 10.1186/s13058-015-0661-5. Epub 2016/02/10. PubMed PMID: 26857361; PubMed Central PMCID: PMC4746893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro G.I. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24(11):1770–1783. doi: 10.1200/JCO.2005.03.7689. Epub 2006/04/11. PubMed PMID: 16603719. [DOI] [PubMed] [Google Scholar]
- 12.Fry D.W., Harvey P.J., Keller P.R., Elliott W.L., Meade M., Trachet E. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Canc Therapeut. 2004;3(11):1427–1438. Epub 2004/11/16. PubMed PMID: 15542782. [PubMed] [Google Scholar]
- 13.Gelbert L.M., Cai S., Lin X., Sanchez-Martinez C., Del Prado M., Lallena M.J. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest N Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7. Epub 2014/06/13. PubMed PMID: 24919854; PubMed Central PMCID: PMC4169866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J., Thijssen B., McDermott U., Garnett M., Wessels L.F., Bernards R. Targeting the RB-E2F pathway in breast cancer. Oncogene. 2016;35(37):4829–4835. doi: 10.1038/onc.2016.32. Epub 2016/03/01. PubMed PMID: 26923330; PubMed Central PMCID: PMC4950965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker S.J., Reddy E.P. CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer. 2012;3(11–12):658–669. doi: 10.1177/1947601913478972. Epub 2013/05/02. PubMed PMID: 23634254; PubMed Central PMCID: PMC3636745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schettini F., De Santo I., Rea C.G., De Placido P., Formisano L., Giuliano M. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol. 2018;8:608. doi: 10.3389/fonc.2018.00608. Epub 2019/01/12. PubMed PMID: 30631751; PubMed Central PMCID: PMC6315195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sledge G.W., Jr., Toi M., Neven P., Sohn J., Inoue K., Pivot X. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585. Epub 2017/06/06. PubMed PMID: 28580882. [DOI] [PubMed] [Google Scholar]
- 18.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.A., Masuda N. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. Epub 2016/03/08. PubMed PMID: 26947331. [DOI] [PubMed] [Google Scholar]
- 19.Tripathy D., Im S.A., Colleoni M., Franke F., Bardia A., Harbeck N. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi: 10.1016/S1470-2045(18)30292-4. Epub 2018/05/29. PubMed PMID: 29804902. [DOI] [PubMed] [Google Scholar]
- 20.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. Epub 2016/12/14. PubMed PMID: 27959613. [DOI] [PubMed] [Google Scholar]
- 21.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155. Epub 2018/05/03. PubMed PMID: 29718092. [DOI] [PubMed] [Google Scholar]
- 22.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.A. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909. Epub 2018/06/05. PubMed PMID: 29860922. [DOI] [PubMed] [Google Scholar]
- 23.Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. Epub 2017/10/03. PubMed PMID: 28968163. [DOI] [PubMed] [Google Scholar]
- 24.Mayer E.L., DeMichele A., Rugo H.S., Miller K., Waks A.G., Come S.E. A phase II feasibility study of palbociclib in combination with adjuvant endocrine therapy for hormone receptor-positive invasive breast carcinoma. Ann Oncol : official journal of the European Society for Medical Oncology. 2019;30(9):1514–1520. doi: 10.1093/annonc/mdz198. [DOI] [PubMed] [Google Scholar]
- 25.Johnston S.R.D., Harbeck N., Hegg R., Toi M., Martin M., Shao Z.M. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR1, HER22, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38(34):3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer E.L., Dueck A.C., Martin M., Rubovszky G., Burstein H.J., Bellet-Ezquerra M. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–222. doi: 10.1016/S1470-2045(20)30642-2. [DOI] [PubMed] [Google Scholar]
- 27.Sibylle Loibl F.M., Martin Miguel, Untch Michael, Bonnefoi Hervé, Kim Sung-Bae, Bear Harry, Mc Carthy Nicole, Olivé Mireia Melé, Gelmon Karen, editors. Phase III study of palbociclib continued with endocrine therapy in patients with hormone-receptor-positive, HER2-negative primary breast cancer and high relapse risk after neoadjuvant chemotherapy: first results from the PENELOPE-B. San Antonio Breast Cancer Symposium Virtual Meeting; December 8-11. 2020. [Google Scholar]
- 28.Slamon D.J., Fasching P.A., Patel R., Verma S., Hurvitz S.A., Chia S.K.L. NATALEE: phase III study of ribociclib (RIBO) + endocrine therapy (ET) as adjuvant treatment in hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) early breast cancer (EBC) J Clin Oncol. 2019;37 doi: 10.1200/JCO.2019.37.15-suppl.TPS597. [DOI] [Google Scholar]
- 29.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. Epub 2011/10/20. PubMed PMID: 22008217; PubMed Central PMCID: PMC3196245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. Epub 2002/07/12. PubMed PMID: 12111919. [DOI] [PubMed] [Google Scholar]
- 31.Wetterslev J., Thorlund K., Brok J., Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. doi: 10.1016/j.jclinepi.2007.03.013. Epub 2007/12/18. PubMed PMID: 18083463. [DOI] [PubMed] [Google Scholar]
- 32.Brok J., Thorlund K., Gluud C., Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–769. doi: 10.1016/j.jclinepi.2007.10.007. Epub 2008/04/16. PubMed PMID: 18411040. [DOI] [PubMed] [Google Scholar]
- 33.Schulz K.F., Chalmers I., Hayes R.J., Altman D.G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. J Am Med Assoc. 1995;273(5):408–412. doi: 10.1001/jama.273.5.408. Epub 1995/02/01. PubMed PMID: 7823387. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien P.C., Fleming T.R. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. Epub 1979/09/01. PubMed PMID: 497341. [PubMed] [Google Scholar]
- 35.Miladinovic B., Hozo I., Djulbegovic B. Trial sequential boundaries for cumulative meta-analyses. STATA J. 2013;13(1):77–91. http://ageconsearch.umn.edu/record/241329/files/sjart_st0284.pdf 2013. Available from: [Google Scholar]
- 36.Loibl S., Marmé F., Martin M., Untch M., Bonnefoi H., Kim S.-B. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—the penelope-B trial. J Clin Oncol. 2021;20 doi: 10.1200/JCO.20.03639. JCO. [DOI] [PubMed] [Google Scholar]
- 37.Martin M., Hegg R., Kim S.-B., Schenker M., Grecea D., García-Sáenz J.A. Abemaciclib combined with adjuvant endocrine therapy in patients with high risk early breast cancer who received neoadjuvant chemotherapy (NAC) J Clin Oncol. 2021;39(15_suppl):517. doi: 10.1200/JCO.2021.39.15_suppl.517. [DOI] [Google Scholar]
- 38.Marmé F., Martin M., Untch M., Bonnefoi H.R., Kim S.-B., Bear H.D. Palbociclib combined with endocrine treatment in breast cancer patients with high relapse risk after neoadjuvant chemotherapy: subgroup analyses of premenopausal patients in PENELOPE-B. J Clin Oncol. 2021;39(15_suppl):518. doi: 10.1200/JCO.2021.39.15_suppl.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz A.B., Garcia D., Vicente Y., Palka M., Bellas C., Martin P. Prognostic significance of cyclin D1 protein expression and gene amplification in invasive breast carcinoma. PloS One. 2017;12(11) doi: 10.1371/journal.pone.0188068. Epub 2017/11/16. PubMed PMID: 29140993; PubMed Central PMCID: PMC5687747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsheikh S., Green A.R., Aleskandarany M.A., Grainge M., Paish C.E., Lambros M.B.K. CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Canc Res Treat. 2008;109(2):325–335. doi: 10.1007/s10549-007-9659-8. [DOI] [PubMed] [Google Scholar]
- 41.Ahlin C., Lundgren C., Embretsen-Varro E., Jirstrom K., Blomqvist C., Fjallskog M. High expression of cyclin D1 is associated to high proliferation rate and increased risk of mortality in women with ER-positive but not in ER-negative breast cancers. Breast Canc Res Treat. 2017;164(3):667–678. doi: 10.1007/s10549-017-4294-5. Epub 2017/05/22. PubMed PMID: 28528450; PubMed Central PMCID: PMC5495873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denkert C., Marmé F., Martin M., Untch M., Bonnefoi H.R., Witkiewicz A.K. Subgroup of post-neoadjuvant luminal-B tumors assessed by HTG in PENELOPE-B investigating palbociclib in high risk HER2-/HR+ breast cancer with residual disease. J Clin Oncol. 2021;39(15_suppl):519. doi: 10.1200/JCO.2021.39.15_suppl.519. [DOI] [Google Scholar]
- 43.Schoninger S.F., Blain S.W. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in breast cancer. Mol Canc Therapeut. 2020;19(1):3. doi: 10.1158/1535-7163.MCT-19-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwa M., Makris A., Esteva F.J. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol. 2017;14(10):595–610. doi: 10.1038/nrclinonc.2017.74. [DOI] [PubMed] [Google Scholar]
- 45.Di Cosimo S., Porcu L., Cardoso F. CDK 4/6 inhibitors mired in uncertainty in HR positive and HER2 negative early breast cancer. Breast. 2020;55:75–78. doi: 10.1016/j.breast.2020.12.006. Epub 2020/12/23. PubMed PMID: 33352521; PubMed Central PMCID: PMC7758367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agostinetto E., Vian L., Caparica R., Bruzzone M., Ceppi M., Lambertini M. CDK4/6 inhibitors as adjuvant treatment for hormone receptor-positive, HER2-negative early breast cancer: a systematic review and meta-analysis. ESMO Open. 2021;6(2) doi: 10.1016/j.esmoop.2021.100091. Epub 2021/03/21. PubMed PMID: 33743330; PubMed Central PMCID: PMC8010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study will be shared on reasonable request to the corresponding author.