Abstract
Background:
Most patients with advanced pancreatic adenocarcinoma (PA) treated with FOLFIRINOX experience adverse events requiring dose reduction. We aimed to assess the association between relative dose intensity (RDI) and disease control in a European setting.
Methods:
We retrospectively included patients with advanced PA treated with three or more cycles of FOLFIRINOX between 2011 and 2018 in six French centers. We computed the cumulative single-agent RDI (csRDI) before the first reassessment for each FOLFIRINOX agent (oxaliplatin, irinotecan, 5FU bolus, and 5FU intravenous infusion) and the cumulative multi-drug RDI (cmRDI) of their combination. The association between RDI and disease control or objective response at first reassessment was evaluated using multivariable logistic regression models controlling for performance status, liver metastases, and center.
Results:
We included 243 patients. Median csRDIs were 81%, 79%, 75%, and 85% for oxaliplatin, irinotecan, 5FU bolus, and 5FU intravenous infusion, respectively. Median cmRDI was 80%. None of the RDIs was significantly associated with disease control or objective response. Including RDI in a clinical model did not improve its ability to predict disease control; the area under the curve was 0.79 (95% CI: 0.73–0.85) with RDI versus 0.78 (95% CI: 0.72–0.85) without. Similar results were observed for the objective response.
Conclusion:
Pragmatic dose adjustments of FOLFIRINOX should be made by oncologists without considering a loss of effect.
Keywords: advanced pancreatic cancer, disease control rate, FOLFIRINOX, relative dose intensity, response rate
Introduction
Pancreatic adenocarcinoma (PA) remains one of the most common causes of cancer death in developed countries, and its incidence is steadily increasing.1–5 Since publication of the phase III trial PRODIGE-4/ACCORD-11 results in 2011, the triple chemotherapy regimen FOLFIRINOX (oxaliplatin 85 mg/m², irinotecan 180 mg/m², and 5FU 400 mg/m² bolus followed by 5FU 2400 mg/m² continuous infusion over 46 h) has become the standard first-line treatment for locally advanced and metastatic PA. 6 However, this regimen causes significant toxicities such as hematological, gastrointestinal, or cumulative peripheral neuropathy, which is induced by oxaliplatin, and generally limits treatment continuation after a median of seven cycles. 7 These significant toxicities require frequent dose adjustments, and thus determining the minimum dose intensity required to obtain a therapeutic response with FOLFIRINOX remains a major issue.
The concept of relative dose intensity (RDI) was first studied in 1984 by Hryniuk and Bush 8 in advanced breast cancer followed by other types of cancer, such as lung cancer, ovarian cancer, and lymphoma.9–11 In a more recent retrospective study, Lee et al. 12 established a modified Hryniuk model and defined a cumulative multi-drug RDI (cmDRI) for FOLFIRINOX in Korean patients treated for advanced PA (http://www.rdicalc.com). The authors recommended different thresholds of the resulting RDI to preserve the optimal objective response and disease control. To our knowledge, no similar study has been performed for a Western European population with locally advanced or metastatic PA.
Our primary objective was to evaluate the association between disease control at first reassessment and the RDI of FOLFIRINOX according to the method described by Lee et al. 12 using the cmRDI as well as the cumulative dose for each agent (cumulative single-agent relative dose intensity, csRDI). Our secondary objective was to evaluate the association between objective response and RDI for all agents of the FOLFIRINOX protocol using the same methodology. We also evaluated the association of cmRDI with depth of tumor response at first reassessment, survival outcomes, and severe toxicity.
Methods
Study design and population
This was a retrospective, multicenter study that included patients over 18 years of age with histologically confirmed locally advanced or metastatic PA who were treated with at least three cycles of first-line FOLFIRINOX between January 2011 and December 2018 and whose tumor response was evaluable. Patients with known dihydropyrimidine dehydrogenase or UDP-glycosyltransferase 1 polypeptide A1 (UGT1A1) deficiency or who objected to the use of their medical data were excluded from the study. Six French centers (three university hospitals, two cancer centers, one general hospital) participated in the study, and the data were retrospectively collected from the electronic medical records.
Evaluation criteria
The primary endpoint was disease control rate (DCR), which was the sum of complete response (CR), partial response (PR), and stable disease (SD) rates according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, 13 with a central review of baseline and first reassessment imaging when available. The secondary endpoints were response rate (sum of CR and PR), depth of tumor response (relative variation in the sum of the diameters of the target lesions between baseline and first reassessment), progression-free survival (PFS), and overall survival (OS) from the start of FOLFIRINOX treatment, as well as grade III/IV toxicities between treatment initiation and first reassessment according to the US National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Statistical considerations
Calculation of sample size
We initially planned to divide the study sample, placing two-thirds in a training sample to fit the model and one-third in a validation sample to obtain an unbiased estimate of the model fit. Based on the results of the French randomized trial evaluating FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer, 6 we assumed that disease control would be achieved in 70% of patients overall. As we had no data regarding the distribution of RDI in our European population setting, we calculated the sample size for studying the association between RDI and disease control by considering a binary variable for RDI (RDI <median value versus RDI ⩾median value), leading to two balanced groups. We assumed a 20% difference in disease control [60% in patients with low RDI versus 80% in patients with high RDI, equivalent to an odds ratio (OR) of 2.67]. Accordingly, a total of 160 patients were required for the training sample to ensure 80% power for performing the planned comparison with a significance level of 0.05 and a two-sided test. Therefore, we planned to include 240 patients. As we did not find any association between the RDI of FOLFIRINOX and disease control, the analysis ultimately included the entire population, leading to a power of 93% for a 20% difference (60% versus 80%) and 78% for a 16% difference (62% versus 78%).
Statistical analysis
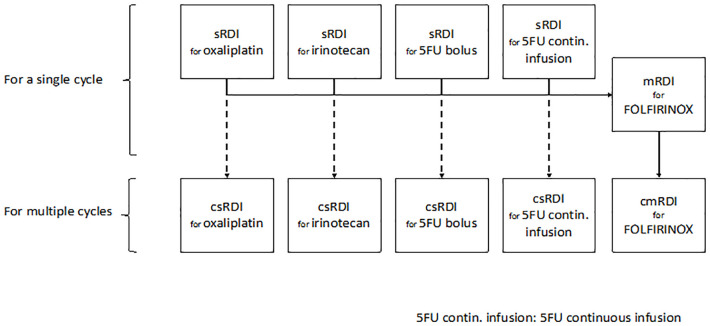
As illustrated in Figure 1 and detailed in Supplemental Figure S1, we determined the cmRDI of FOLFIRINOX as described by Lee et al. 12 by first calculating the RDI of each component per cycle (sRDI), the RDI of all components per cycle (mRDI), and finally the cumulative RDI of the combination over all cycles up to the first reassessment imaging. We also calculated the csRDI of each component, defined as the cumulative sRDI over all cycles for each component.
Figure 1.
Scheme for determining the cumulative relative dose intensity of each FOLFIRINOX agent and their combination.
cmRDI, cumulative multi-drug relative dose intensity; csRDI, cumulative single-agent relative dose intensity.
We estimated cumulative incidence curves for dose reductions per agent before the first reassessment using Kalbleisch and Prentice’s method, where the time interval between the start of treatment and the first dose reduction was calculated, considering treatment discontinuation unrelated to toxicity as a competing event.
Logistic regression models were used to evaluate factors associated with disease control and objective response following a multistep process. First, we developed a “clinical” model that included all clinical variables deemed relevant a priori and sufficiently documented to be included in the model. The candidate variables were Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), the presence of liver metastases, and initial CA19-9 levels. Second, we incorporated RDI into this clinical model as a continuous variable. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to assess the discrimination ability of the models with or without RDI. Based on the ROC curve, thresholds were defined by maximizing Youden statistics (sensitivity + specificity − 1). The same approach was used considering the csRDI of the four treatment components first, followed by the cmRDI. To illustrate the results for each agent, we estimated the OR for four categories of RDI distribution: ⩽70%, 70–80%, 80–90%, and >90%, with the last category used as a reference.
We also adjusted all models for the treatment center and number of cycles before reassessment to control for possible confounding bias. A linear regression model was used to evaluate the association between cmRDI and the depth of tumor response after adjusting for patient clinical characteristics, the center, and number of cycles before the first reassessment. The main analysis focused on all patients, with other analyses performed using two homogeneous subgroups: (1) metastatic patients and (2) patients assessed after three or four cycles.
We estimated the cumulative probability of any kind of grade III or IV toxicity using the Kaplan–Meier method from the start of treatment (1–Kaplan–Meier curve). Patients who did not develop toxicity were censored on the date of reassessment. The association between cmRDI and the risk of toxicity was assessed using the Cox model, adjusting for PS and center. For patients who experienced toxicity, the cmRDI was recalculated up to the date of toxicity onset.
All estimates are presented with a 95% confidence interval (CI), and the tests were performed at a two-tailed significance level of 5%. All statistical analyses were performed using Stata® software (version 15.0; StataCorp LLC, College Station, TX).
Ethical requirements
Ethical approval was not required for this study. The French Data Protection Authority waived the requirement for informed consent for this retrospective study (agreement no. 918110). None of the patients objected to the use of their clinical data for research purposes.
Results
Description of population and treatment
A total of 243 patients from the six participating centers were included (Supplemental Figure S2). Locally advanced PA was diagnosed in 65 patients (26.7%) and metastatic PA in 178 patients (73.3%). The number of cycles received before the first reassessment was usually four (39.5%) or six (32.9%). Patient characteristics, treatments, and outcomes are described in Table 1.
Table 1.
Patient characteristics and treatment at inclusion (N = 243).
| Characteristic | Total |
|---|---|
| Gender | |
| Men | 155 (63.8%) |
| Women | 88 (36.2%) |
| Age at start of treatment (years) | |
| Median (min–max) | 60.0 (30.0–86.0) |
| ECOG PS | |
| 0 | 95 (39.1%) |
| 1 | 148 (60.9%) |
| Body mass index (kg/m2) | |
| Median (min–max) | 23.6 (14.5–45.2) |
| Indication for FOLFIRINOX | |
| From the outset (locally advanced or metastatic disease) | 192 (79.0%) |
| After recurrence | 51 (21.0%) |
| Previous adjuvant chemotherapy with gemcitabine (n = 50, MD = 1) | |
| No | 9 (18.0%) |
| Yes | 41 (82.0%) |
| Location of the primary pancreatic tumor (MD = 1) | |
| Head | 128 (52.9%) |
| Isthmus | 21 (8.7%) |
| Body/tail | 93 (38.4%) |
| Number of metastatic sites at inclusion | |
| 0 | 65 (26.7%) |
| 1–2 | 169 (69.5%) |
| 3 or more | 9 (3.7%) |
| Metastatic sites | |
| Hepatic | 138 (56.8%) |
| Pulmonary | 38 (15.6%) |
| Peritoneal and/or carcinoma cells in ascites | 36 (14.8%) |
| Other | 22 (9.1%) |
| CA19-9 at inclusion >normal (MD = 35) | |
| No | 32 (13.2%) |
| Yes | 176 (72.4%) |
| Number of cycles of FOLFIRINOX before first reassessment | |
| 3 | 28 (11.5%) |
| 4 | 96 (39.5%) |
| 5 | 38 (15.6%) |
| ⩾6 | 81 (33.3%) |
| Continuation of treatment beyond first reassessment | |
| Yes | 177 (72.8%) |
| No | 66 (27.2%) |
| Reason for treatment discontinuation (n = 62, MD = 4) | |
| Progression | 51 (77.3%) |
| Deterioration in overall health | 7 (10.6%) |
| Toxicity | 1 (1.5%) |
| Other | 3 (4.5%) |
| Duration of treatment with FOLFIRINOX up to reassessment (days) | |
| Median (min–max) | 70 (38–134) |
| G-CSF prophylaxis from cycle 1 of FOLFIRINOX | |
| No | 177 (72.8%) |
| Yes | 66 (27.2%) |
| Cumulative relative dose intensity (%) | |
| csRDI oxaliplatin, median (min–max) | 80.8 (23.8–102.5) |
| csRDI irinotecan, median (min–max) | 79.3 (8.5–102.1) |
| csRDI 5FU bolus, median (min–max) | 74.9 (0–102.5) |
| csRDI 5FU continuous infusion, median (min–max) | 84.6 (23.8–102.5) |
| cmRDI FOLFIRINOX, median (min–max) | 80.3 (22.8–102.2) |
| Tumor response at first evaluation (RECIST v 1.1) | |
| Complete response | 5 (2.1%) |
| Partial response | 44 (18.1%) |
| Stable disease | 131 (53.9%) |
| Disease progression | 63 (25.9%) |
| Progression-free survival (PFS) | |
| Median PFS duration, months | 9.2 (7.9–10.2) |
| Overall survival (OS) | |
| Median OS duration, months | 13.2 (11.5–15.7) |
CA19-9, carbohydrate antigen 19-9; cmRDI, cumulative multi-drug relative dose intensity; csRDI, cumulative single-agent relative dose intensity; ECOG, Eastern Cooperative Oncology Group; G-CSF, granulocyte colony-stimulating factor; MD, missing data; PS, performance status; RECIST, response evaluation criteria in solid tumors.
Description of dose adjustments
Initial dose
The initial dose for 159 patients (65.4%) was 100% for all four FOLFIRINOX agents. Overall, the oxaliplatin, irinotecan, 5FU bolus, and 5FU continuous infusion doses were immediately reduced during cycle 1 in 57 (23.5%), 78 (32.1%), 66 (27.2%), and 56 (23%) patients, respectively.
Adjustment of subsequent doses
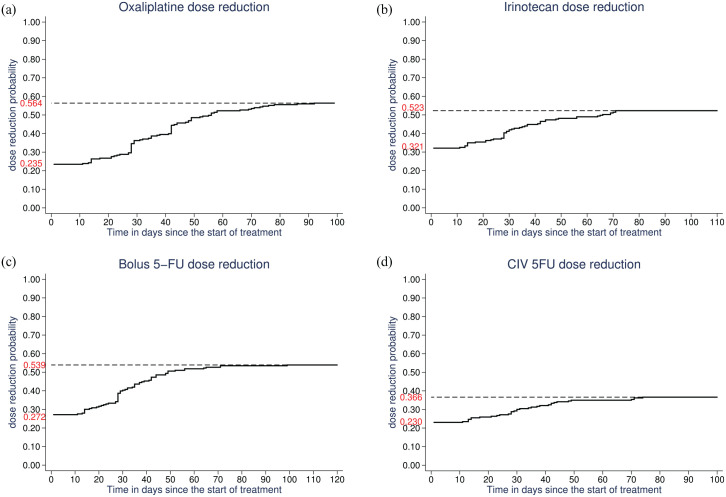
For patients with a cycle 1 dose reduction, there was a subsequent re-escalation of oxaliplatin in 47 of 57 (82.5%), irinotecan in 69 of 78 (88.5%), 5FU bolus in 52 of 66 (78.8%), and 5FU continuous infusion in 48 of 56 (85.7%) patients. Conversely, for patients without a cycle 1 dose reduction, a reduction was implemented during subsequent cycles in 80 of 186 patients (43.0%) for oxaliplatin, 49 of 165 (29.7%) for irinotecan, 65 of 177 (36.7%) for 5FU bolus, and 33 of 187 (17.6%) for 5FU continuous infusion, resulting in a cumulative dose reduction probability of 56.4%, 52.3%, 53.9%, and 36.6% for oxaliplatin, irinotecan, 5FU bolus, and 5FU continuous infusion, respectively (Figure 2).
Figure 2.
Probability of dose reduction as a function of time (in days) between the first treatment cycle and first reassessment for oxaliplatin (a), irinotecan (b), 5FU bolus (c), and 5FU continuous infusion (d).
RDI of FOLFIRINOX
The median csRDIs of oxaliplatin, irinotecan, 5FU bolus, and 5FU continuous infusion were 80.8%, 79.3%, 74.9%, and 84.6%, respectively (Supplemental Table S1). The median cmRDI of FOLFIRINOX for the entire study population was 80.3%. Only 13 patients (5.3%) had a cmRDI <50%. Fifteen patients (6.2%) did not receive a 5FU bolus.
Response to treatment
Description of response to treatment
An OR was achieved at the first tumor reassessment in 49 of 243 patients (20.2%), including 5 CRs and 44 PRs, while 130 had SD, leading to a DCR of 179 of 243 (73.7%) (Supplemental Figure S3).
Clinical model of disease control
As detailed in Supplemental Table S2, clinical factors associated with disease control at first reassessment were PS 0 (p = 0.002) and no liver metastases (p = 0.004). We did not include CA19-9 levels due to the absence of any significant association in the multivariable model (p = 0.23). In multivariable analysis, the probability of disease control varied significantly from one center to another (p < 0.02) and increased with the number of cycles received before first reassessment (p < 0.0001). The AUC of this clinical model was 0.78 (95% CI: 0.72–0.85; Supplemental Figure S4).
Association between RDI and disease control
In multivariable analysis after adjustment for clinical characteristics, center, and number of cycles received before first reassessment, the cmRDI of FOLFIRINOX was not found significantly associated with disease control (OR = 1.06; 95% CI: 0.86–1.31; p = 0.58). Similar results were obtained for the csRDIs of each of the four FOLFIRINOX agents (Table 2).
Table 2.
Factors associated with disease control in the entire population (N = 243).
| Characteristic | Multivariable model with csRDIs * | Multivariable model with cmRDI † | ||||
|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI adjusted OR | Adjusted p-value | Adjusted OR | 95% CI adjusted OR | Adjusted p-value | |
| Relative dose intensity | ||||||
| OR/10% | ||||||
| csRDI oxaliplatin | 1.01 | (0.71–1.43) | 0.97 | |||
| csRDI irinotecan | 1.09 | (0.83–1.43) | 0.55 | |||
| csRDI 5FU bolus | 0.98 | (0.83–1.15) | 0.80 | |||
| csRDI 5FU continuous infusion | 0.99 | (0.59–1.65) | 0.96 | |||
| cmRDI FOLFIRINOX | 1.06 | (0.86–1.31) | 0.58 | |||
| ECOG PS | 0.003 | 0.004 | ||||
| 1 | 0.29 | (0.13–0.65) | 0.29 | (0.13–0.65) | ||
| 0 | 1 (ref) | 1 (ref) | ||||
| Liver metastasis | 0.004 | 0.004 | ||||
| Yes | 0.35 | (0.16–0.72) | 0.35 | (0.17–0.72) | ||
| No | 1 (ref) | 1 (ref) | ||||
| Number of cycles before first reassessment ‡ | <0.0001 | <0.0001 | ||||
| OR/1 cycle | 2.23 | (1.46–3.39) | 2.27 | (1.50–3.42) | ||
| Center | 0.043 | 0.027 | ||||
| Center 1 | 1 (ref) | 1 (ref) | ||||
| Center 2 | 0.51 | (0.2–1.57) | 0.50 | (0.2–1.44) | ||
| Center 3 | 0.41 | (0.1–1.73) | 0.40 | (0.1–1.64) | ||
| Center 4 | 0.20 | (0.1–0.76) | 0.18 | (0.1–0.67) | ||
| Center 5 | 1.44 | (0.4–4.66) | 1.38 | (0.4–4.31) | ||
| Center 6 | 2.25 | (0.6–8.88) | 2.23 | (0.6–7.66) | ||
The multivariable model evaluating the effect of csRDI included the csRDI of the four component agents (oxaliplatin, irinotecan, 5FU bolus, and 5FU continuous infusion) as well as ECOG PS, liver metastasis, number of cycles before first assessment, and center.
The multivariable model evaluating the effect of cmRDI included cmRDI, ECOG PS, liver metastasis, and center.
The variable “number of cycles before first reassessment” was incorporated as a continuous variable into the model after checking the monotonicity of the association. Taking three cycles before reassessment as a reference, the ORs for 4, 5, and 6 cycles were 2.76, 2.98, and 10.33, respectively.
CI, confidence interval; cmRDI, cumulative multi-drug relative dose intensity; csRDI, cumulative single-agent relative dose intensity; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; PS, performance status; ref, reference.
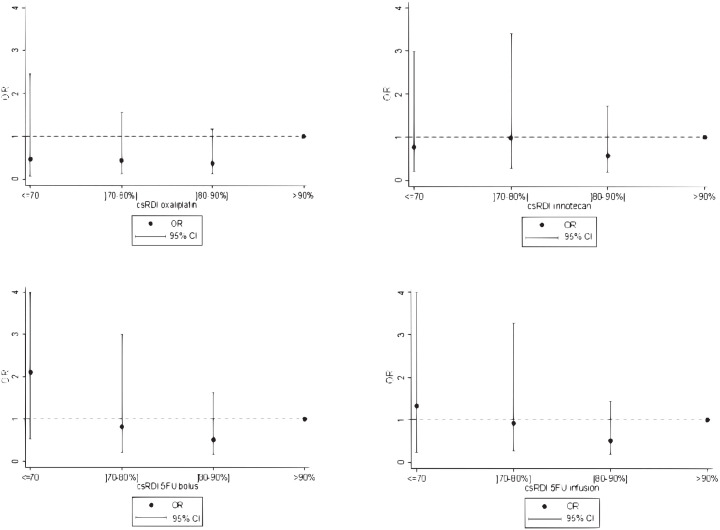
The AUC of the model that included the cmRDI of FOLFIRINOX was 0.79 (95% CI: 0.72–0.85), while the AUC of the one that included the csRDIs of the four FOLFIRINOX agents was 0.79 (95% CI: 0.73–0.85). Hence, as illustrated by the superimposed ROC curves (Supplemental Figure S4), including csRDI or cmRDI did not result in any significant gain in AUC compared with the initial clinical model. Moreover, there was a lack of any dose–response relationship between the csRDI of each FOLFIRINOX agent and disease control (Figure 3).
Figure 3.
Assessment of the dose–response relationship between the cumulative relative dose intensity of each FOLFIRINOX agent (csRDI) and disease control.
95%CI, 95% confidence interval estimated in a multivariable model including the csRDI of the four FOLFIRINOX agents as well as ECOG, liver metastasis, number of cycles before first reassessment and center; OR, odds ratio.
Association between RDI and objective response
No significant association was observed between RDI and objective response (Supplemental Figure S5). Multivariable analysis adjusted for clinical variables in the entire study sample estimated a cmRDI OR of 1.11 (95% CI: 0.87–1.42; p = 0.40).
Association between cmRDI and depth of tumor response
Multivariable analysis was performed after adjusting for patient clinical characteristics, center, and number of cycles before first reassessment; when the depth of tumor response was analyzed as a continuous variable, it was not found significantly associated with the cmRDI of FOLFIRINOX (regression coefficient = −2.88 for 10% of cmRDI; 95% CI: −9.2 to 3.40; p = 0.367) (Supplemental Table S3).
Progression-free and overall survival
As detailed in Supplementary Tables S4 and S5, we did not observe any significant association between cmRDI and PFS [hazard ratio (HR) = 1.02; 95% CI, 0.93–1.13; p = 0.67] or OS (HR = 0.99; 95% CI, 0.90–1.11; p = 0.99) via multivariable analysis.
Grade III/IV toxicities
Grade III or IV toxicities occurred in 48.6% of patients, with the majority involving neutropenia (febrile or not; 25.1%), deterioration in overall health (18.5%), and gastrointestinal disorders (16.5%) (Supplemental Table S6). Grade III/IV neutropenia occurred in 57 of 177 patients (32.2%) who did not receive primary prophylaxis versus 4 of 62 (6.1%) patients who did (p < 0.001). A significant difference was also observed for grade III/IV febrile neutropenia between the same two populations, where it occurred in 15 of 177 patients who did not receive primary prophylaxis (8.5%) versus 0 of 66 patients who did (p = 0.013).
The median time to onset of grade III/IV toxicity was 74 days (95% CI: 28 days to “not reached”). The probability of grade III/IV toxicity was 30.1% (95% CI: 25.2–36.8%) and 59.6% (95% CI: 48.3–71.1%) at 30 and 120 days, respectively, after the start of treatment with FOLFIRINOX (Supplemental Figure S6). Using multivariable analysis, the occurrence of grade III/IV toxicity was significantly associated with the cmRDI of FOLFIRINOX (p < 0.001). After adjusting for PS and center, the risk of occurrence of grade III/IV toxicity increased by 44% (HR = 1.44; 95% CI: 1.41–1.72) for every 10% increase in the cmRDI of FOLFIRINOX (Table 3).
Table 3.
Factors associated with the occurrence of grade III/IV toxicity.
| Characteristic | Model with cmRDI | ||
|---|---|---|---|
| Adjusted HR | 95% CI adjusted HR | p-Value | |
| Relative dose intensity | |||
| HR/10% | |||
| cmRDI of FOLFIRINOX | 1.44 | (1.21–1.72) | <0.001 |
| ECOG PS | 0.14 | ||
| 1 | 1.34 | (0.91–1.97) | |
| 0 | 1 (ref) | ||
| Center | 0.23 | ||
| Center 1 | 1 (ref) | ||
| Center 2 | 0.68 | (0.40–1.16) | |
| Center 3 | 0.71 | (0.32–1.53) | |
| Center 4 | 1.38 | (0.64–2.95) | |
| Center 5 | 1.26 | (0.75–2.11) | |
| Center 6 | 0.75 | (0.39–1.42) | |
CI, confidence interval; cmRDI, cumulative multi-drug relative dose intensity; ECOG, Eastern Cooperative Oncology Group; PS, performance status; HR, hazard ratio.
Discussion
This is the first multicenter study to evaluate the impact of FOLFIRINOX RDI on disease control and objective response in advanced PA in Western Europe. Our study did not ultimately demonstrate any significant association between FOLFIRINOX cmRDI or the csRDI of each agent and the disease control or objective response. Consequently, no relevant RDI threshold could be defined. Our results contrast with the study published by Lee et al., 12 who concluded that a threshold of 55.3% cmRDI was associated with a sensitivity of 93.6% and a specificity of 62.5% for disease control. We also did not find a significant association between cmRDI and the depth of tumor response, PFS, or OS. Conversely, there was a significant association between FOLFIRINOX cmRDI and the occurrence of grade III/IV toxicity.
We chose to evaluate the csRDI of each FOLFIRINOX agent and the cmRDI of the entire protocol, whereas Lee et al. 12 only found a significant association between cmRDI and tumor response in an Asian population, without exploring the csRDI of each FOLFIRINOX agent. Since disease control is the primary goal of treatment in patients with inoperable PA, whether locally advanced or metastatic, our objective was to evaluate whether individual agents of the FOLFIRINOX protocol may have RDIs associated with better or poorer disease control. Another difference between our studies is that the FOLFIRINOX cmRDI at first reassessment appears higher in our European cohort than in the Asian cohort: the median cmRDI was 80.3% in our study and 71.8% in the Lee et al. 12 study. The proportion of patients with a cmRDI >90% was also significantly higher in our study population [74/243 (30.5%) versus 22/133 (16.5%); p < 0.001]. Only 13 of the 243 (5.3%) patients in our study population had a cmRDI <50%. Consequently, we cannot exclude the possibility that large dose reduction may be associated with poor oncologic outcomes. However, the proportion of patients with a cmRDI <50% in our study population was not significantly lower than that reported by Lee et al. 12 (10/133, 7.5%; p = 0.40). Moreover, objective response was achieved at first tumor reassessment in 20.2% of patients in our study versus 36% in the Lee et al. 12 study and 31% in the Conroy et al. 6 study (but after a median of 10 cycles of FOLFIRINOX, which may correspond to the second reassessment in this case). These findings support the hypothesis of differences in tumor response and toxicity of chemotherapy between Asian and European/Caucasian populations. An example is the possible variation in the metabolism of irinotecan mediated by pharmacogenetic specificities.14,15
Our study has several limitations. First, we excluded patients who received less than three cycles of FOLFIRINOX. This was initially intended to obtain RDI data that we considered more comprehensive, and thus potentially more informative for our primary objective regarding an association between RDI and disease control. This choice limits the external validity of our study because some patients progress or die early on FOLFIRINOX. In the original study by Conroy et al., 6 14.6% of patients could not be evaluated owing to death, early progression, or deterioration in overall health. Additionally, our central review of radiological images gave rise to a measurement bias because some images were unavailable, as did the original radiological reassessment because of local tumor changes. In fact, locoregional therapeutic response is difficult to assess in pancreatic cancer because of residual fibrosis.16–18 Lastly, our study was designed to ensure sufficient power for large differences in terms of oncologic outcomes between patients with low versus high RDI, as reported by Lee et al., 12 which may be underpowered for detecting smaller differences.
Nevertheless, we highlighted a center effect that may have resulted from a possible recruitment bias due to the long recruitment window from January 2011 to December 2018. In addition, the different number of cycles before first reassessment between the centers (most often four or six) led to a confounding bias that was taken into account in our multivariable analysis. In addition, the results were very stable when focusing on the 124 patients who had an early response assessment (28 patients after three cycles and 96 after four cycles). Therefore, current recommendations do not favor any specific time interval between the first treatment cycle and tumor reassessment. 19
The results of our study raise several questions; in particular, the probability of disease control at first reassessment was significantly associated with the number of cycles administered; in other words, it can be interpreted that more cycles of FOLFIRINOX (i.e., six versus four) lead to better disease control. However, patients progressing clinically, whose overall health was deteriorating or whose laboratory results (in particular, hepatic tests) were abnormal, had the date of the first radiological reassessment brought forward, and had thus received fewer treatment cycles.
Pharmacodynamic and/or pharmacokinetic parameters are not factored into dose reduction or modified protocols of FOLFIRINOX. Limited data exist on the pharmacological profile of each agent, and even less data exist on the FOLFIRINOX combination. 20 We followed the study design established by Lee et al. 12 in our weighing of the RDI of each agent in the final calculation of the cmRDI. This method does not take into account the highly probable pharmacodynamic and pharmacokinetic differences between a bolus and continuous infusion of 5FU or interindividual variability; for example, there are variations in the elimination of 5FU via dihydropyrimidine dehydrogenase21,22 and irinotecan via UGT1A1, 23 both of which result in differing plasma concentrations. However, the lack of any association in our study between the RDI of FOLFIRINOX and tumor response was perhaps due to sufficient plasma concentrations despite the dose reductions factored into the RDI.
We also explored the clinical and laboratory characteristics of the patients and found that the neutrophil/lymphocyte ratio and CA19-9 levels are promising candidates for establishing prognostic models.24,25 It may be worth evaluating the nutritional status of patients, namely their body mass index and albumin levels, or the impact of body surface area exceeding 2 m2, although this last parameter is of limited value because in most centers the 2 m2 threshold is not exceeded when calculating the final dose of FOLFIRINOX to be administered to the patient. Overall, these findings indicate that more consideration should be given to clinical, laboratory, and pharmacological variables in the design of future prospective studies.
Furthermore, our results and those of the available literature on the various “modified” FOLFIRINOX protocols led us to consider the utility of alternative protocols in which doses are reduced, or the 5FU bolus is sometimes withdrawn, but efficacy is maintained.25–31 In routine clinical practice, our findings may also lead to a preference for pragmatic dose reductions as soon as they appear warranted, with the aim of continuing the protocol while maintaining an acceptable quality of life during treatment. The significantly low incidence (p = 0.013) of febrile neutropenia in patients taking prophylactic G-CSF starting from the first cycle of FOLFIRINOX should also lead to a wider discussion of this treatment option. 32
Conclusion
We did not observe any significant association between the reduced RDI of FOLFIRINOX and disease control in the first-line treatment of advanced PA. However, considering the high-grade toxicities associated with FOLFIRINOX and the fragility of patients with PA, pragmatic dose adjustments must be made by oncologists based on arising toxicities to preserve quality of life. Future dose-reduction studies supported by pharmacological data are necessary.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_17588359211029825 for FOLFIRINOX relative dose intensity and disease control in advanced pancreatic adenocarcinoma by Antonin Vary, Loïc Lebellec, Frédéric Di Fiore, Nicolas Penel, Claire Cheymol, Emilia Rad, Farid El Hajbi, Astrid Lièvre, Julien Edeline, André Michel Bimbai, Marie-Cécile Le Deley and Anthony Turpin in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Stéphanie Bacquaert for data capture, Severine Marchant (Center Oscar Lambret), Lille University Faculty of Medicine, and Editage (www.editage.com) for English language editing.
Footnotes
Author contributions: Conceptualization, A.T.; Supervision, A.T., M.-C.L.D., A.M.B., L.L., N.P.; Data collection, A.V.; Methodology, M.-C.L.D., A.M.B., L.L., N.P.; Formal analysis, M.-C.L.D., A.M.B., L.L., N.P.; Visualization, C.C., E.R., F.E.H., A.L., J.E., F.D.F.; Investigation, C.C., E.R., F.E.H., A.L., J.E., F.D.F.; Writing–original draft, A.T., A.V.; Writing–review and editing, A.T., A.V., M.-C.L.D., A.M.B., L.L., N.P.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article
ORCID iDs: Julien Edeline  https://orcid.org/0000-0002-8289-7741
https://orcid.org/0000-0002-8289-7741
Anthony Turpin  https://orcid.org/0000-0002-2282-0101
https://orcid.org/0000-0002-2282-0101
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Antonin Vary, Lille University, Lille, France.
Loïc Lebellec, Lille University, Lille, France Medical Oncology; Department, Oscar Lambret Center, Lille, France.
Frédéric Di Fiore, Medical Oncology Department, Henri Becquerel Center, Rouen, France; Digestive Oncology Department, Rouen University Hospital, Rouen, France.
Nicolas Penel, Lille University, Lille, France; Medical Oncology Department, Oscar Lambret Center, Lille, France; Biostatistics and Methodology Unit, Oscar Lambret Center, Lille, France.
Claire Cheymol, Onco-Hematology Department, Saint Vincent de Paul Hospital, Lille, France.
Emilia Rad, Medical Oncology Department, Victor Provo Hospital, Roubaix, France.
Farid El Hajbi, Medical Oncology Department, Oscar Lambret Center, Lille, France.
Astrid Lièvre, Department of Gastroenterology, CHU Pontchaillou, Rennes, France; University of Rennes 1, Rennes, France; INSERM U1242, Oncogenesis, Stress & Signaling, Rennes, France.
Julien Edeline, Medical Oncology Department, Eugène Marquis Center, Rennes, France.
André Michel Bimbai, Biostatistics and Methodology Unit, Oscar Lambret Center, Lille, France.
Marie-Cécile Le Deley, Biostatistics and Methodology Unit, Oscar Lambret Center, Lille, France.
Anthony Turpin, University of Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, UMR9020 - UMR-S 1277 - Canther-Cancer Heterogeneity, Plasticity and Resistance to Therapies, Lille, France; Department of Medical Oncology, Lille University Hospital, 2 Avenue Oscar Lambret, Lille, 59000 France.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 2015; 12: 319–334. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 4. Hidalgo M, Cascinu S, Kleeff J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 2015; 15: 8–18. [DOI] [PubMed] [Google Scholar]
- 5. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016; 22: 9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. Epub ahead of print 12 May 2011. DOI: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7. Artru P, Bennouna J, Lievre A, et al. Cancer colorectal métastatique : place du traitement d’entretien et de la pause thérapeutique. Bull Cancer (Paris) 2018; 105: 408–414. [DOI] [PubMed] [Google Scholar]
- 8. Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol 1984; 2: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 9. Brunetto AT, Carden CP, Myerson J, et al. Modest reductions in dose intensity and drug-induced neutropenia have no major impact on survival of patients with non-small cell lung cancer treated with platinum-doublet chemotherapy. J Thorac Oncol 2010; 5: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 10. Hanna RK, Poniewierski MS, Laskey RA, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol 2013; 129: 74–80. [DOI] [PubMed] [Google Scholar]
- 11. Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol 2008; 87: 277–283. [DOI] [PubMed] [Google Scholar]
- 12. Lee J, Kim JW, Ahn S, et al. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer 2017; 76: 125–133. [DOI] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14. Phan VH, Moore MM, McLachlan AJ, et al. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol 2009; 5: 243–257. [DOI] [PubMed] [Google Scholar]
- 15. Chen S, Sutiman N, Zhang CZ, et al. Pharmacogenetics of irinotecan, doxorubicin and docetaxel transporters in Asian and Caucasian cancer patients: a comparative review. Drug Metab Rev 2016; 48: 502–540. [DOI] [PubMed] [Google Scholar]
- 16. Cassinotto C, Cortade J, Belleannée G, et al. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol 2013; 82: 589–593. [DOI] [PubMed] [Google Scholar]
- 17. Elbanna KY, Jang H-J, Kim TK. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging 2020; 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee ES. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014; 20: 7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neuzillet C, Gaujoux S, Williet N, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis 2018; 50: 1257–1271. [DOI] [PubMed] [Google Scholar]
- 20. Deyme L, Barbolosi D, Gattacceca F. Population pharmacokinetics of FOLFIRINOX: a review of studies and parameters Cancer Chemother Pharmacol 2019; 83: 27–42. [DOI] [PubMed] [Google Scholar]
- 21. Henricks LM, Lunenburg CATC, de Man FM, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 2018; 19: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 22. Wigle TJ, Tsvetkova EV, Welch SA, et al. DPYD and fluorouracil-based chemotherapy: mini review and case report. Pharmaceutics 2019; 11: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujii H, Yamada Y, Watanabe D, et al. Dose adjustment of irinotecan based on UGT1A1 polymorphisms in patients with colorectal cancer. Cancer Chemother Pharmacol 2019; 83: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fornaro L, Leone F, Vienot A, et al. Validated nomogram predicting 6-month survival in pancreatic cancer patients receiving first-line 5-fluorouracil, oxaliplatin, and irinotecan. Clin Colorectal Cancer 2019; 18: e394–e401. [DOI] [PubMed] [Google Scholar]
- 25. Vivaldi C, Caparello C, Musettini G, et al. First-line treatment with FOLFOXIRI for advanced pancreatic cancer in clinical practice: patients’ outcome and analysis of prognostic factors: first-line treatment with FOLFOXIRI for advanced pancreatic cancer. Int J Cancer 2016; 139: 938–945. [DOI] [PubMed] [Google Scholar]
- 26. Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma: Pancreas 2013; 42: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 27. Ghorani E, Wong HH, Hewitt C, et al. Safety and efficacy of modified FOLFIRINOX for advanced pancreatic adenocarcinoma: a UK single-centre experience. Oncology 2015; 89: 281–287. [DOI] [PubMed] [Google Scholar]
- 28. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018; 379: 2395–2406. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Ma T, Zhang Q, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: a prospective study in Chinese population. Cancer Lett 2017; 406: 22–26. [DOI] [PubMed] [Google Scholar]
- 30. Ozaka M, Ishii H, Sato T, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 2018; 81: 1017–1023. [DOI] [PubMed] [Google Scholar]
- 31. Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016; 114: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macaire P, Paris J, Vincent J, et al. Impact of granulocyte colony-stimulating factor on FOLFIRINOX-induced neutropenia prevention: a population pharmacokinetic/pharmacodynamic approach. Br J Clin Pharmacol. Epub ahead of print 9 May 2020. DOI: 10.1111/bcp.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_17588359211029825 for FOLFIRINOX relative dose intensity and disease control in advanced pancreatic adenocarcinoma by Antonin Vary, Loïc Lebellec, Frédéric Di Fiore, Nicolas Penel, Claire Cheymol, Emilia Rad, Farid El Hajbi, Astrid Lièvre, Julien Edeline, André Michel Bimbai, Marie-Cécile Le Deley and Anthony Turpin in Therapeutic Advances in Medical Oncology