Abstract
Background and aims:
Inappropriate medication prescription is highly prevalent in older adults and is associated with adverse health outcomes. The aim of this study was to examine the associations between potentially inappropriate medications (PIMS) and potential prescribing omissions with physical function in older adults situated in diverse environments.
Methods:
A systematic search was completed using the following databases: MEDLINE, CINAHL, PsycINFO, EMBASE and COCHRANE. Results were extracted from the included studies.
Results:
In total, 55 studies reported on 2,767,594 participants with a mean age of 77.1 years (63.5% women). Study designs comprised 26 retrospective cohort studies, 21 prospective cohort studies and 8 cross-sectional studies. Inappropriate medications in community and hospital settings were significantly associated with higher risk of falls (21 out of 30 studies), higher risk of fractures (7 out of 9 studies), impaired activities of daily living (ADL; 8 out of 10 studies) and impaired instrumental ADL (IADL) score (4 out of 6 studies). Five out of seven studies also showed that PIMs were associated with poorer physical performance comprising the Timed Up and Go test, walking speed, grip strength, time to functional recovery, functional independence and scale of functioning. Many medication classes were implicated as PIMs in falls, fractures and impairment in physical performance including antipsychotic, sedative, anti-anxiety, anticholinergic, antidiabetic, opioid and antihypertensive medications. For patients not receiving musculoskeletal medications, such as calcium, vitamin D and bisphosphonates, older adults were found to be at risk of a hospital admission for a fall or fracture.
Conclusion:
Inappropriate medication prescriptions are associated with impaired physical function across longitudinal and cross-sectional studies in older adults situated in diverse settings. It is important to support older people to reduce their use of inappropriate medications and prevent prescribing omissions.
Plain language summary
Inappropriate medications and physical function
Background and aims: The use of inappropriate medications is very common in older adults and is associated with harmful health problems. The aim was to examine associations between potentially inappropriate medications and potential prescribing omissions with physical function in older adults situated in diverse environments. Methods: Library databases were examined for possible studies to include and a systematic search was completed. Relevant information was obtained from the included studies. Results: In total, 55 studies reported on 2,767,594 participants who were an average age of 77.1 years and about 6 out of 10 were women. A variety of different study designs were used. Inappropriate medication prescriptions in community and hospital settings were significantly associated with higher risk of falls (21 out of 30 studies), higher risk of fractures (7 out of 9 studies), problems with activities of daily living (ADL), such as eating, bathing, dressing, grooming, walking and toileting (8 out of 10 studies) and problems with instrumental ADL such as managing medications, house cleaning and shopping (4 out of 6 studies). Five out of seven studies also showed that inappropriate medications were associated with poorer physical performance involving the Timed Up and Go test, walking speed, grip strength, time to functional recovery, functional independence and scale of functioning. Many types of medication classes were shown to be associated with a risk of falls, fractures and problems with physical performance. Omitted medications were also associated with falls and fractures. Conclusion: Inappropriate medication prescriptions are associated with problems relating to physical function. It is important to support older people to reduce their use of inappropriate medications and prevent prescribing omissions.
Keywords: activities of daily living, aged, functional independence, independent living, medication therapy management, physical function
Introduction
Prescription of a medication is defined as inappropriate if the potential harm from it outweighs the benefit. Inappropriate medications comprises two subtypes: potentially inappropriate medications (PIMs), which include the prescribing of medications with an increased risk of side effects or drug-interactions, or over-prescription of medications that lack a therapeutic benefit, and potential prescribing omissions (PPOs), which include the absence of medications being proven to be beneficial. 1
The prevalence of inappropriate medication prescriptions provided to community dwelling older adults is around 20% and between 36% and 51% in institutionalised older adults. 2 The prevalence can be attributed to multi-morbidity, polypharmacy and age-related physiological changes that alter pharmacokinetics and increase sensitivity to pharmacodynamics.3,4 Inappropriate prescriptions are related to poor health outcomes, such as increased hospitalisations, emergency department visits, and increased risk of mortality. 5 Physical function, which is defined as a person’s ability to carry out activities requiring mobility, physical performance, balance, muscle strength or endurance, is critical for maintaining independence. 6 Inappropriate prescriptions have been shown to be associated with a significant decline in physical performance, 7 ADL during hospitalisation, 8 as well as falls and injuries in frail older adults. 9 Previous reviews have examined associations between polypharmacy and physical function in older adults, 10 and between inappropriate medication use and functional decline. 11
The aim of this systematic review is to examine the associations between inappropriate medication prescriptions and physical function in older adults situated in diverse environments.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was used for reporting this review. The search strategy was completed with a senior university librarian and included terms relating to PIMs and physical function (Supplemental file S1). The search timeframe was from inception up to 3 April 2021. Articles were included by searching MEDLINE, CINAHL, PsycINFO EMBASE and COCHRANE (within MEDLINE).
Article titles and abstracts as well as full-texts of all included articles were independently screened by two reviewers (EM, MZK), and any discrepancies were resolved by a third reviewer (ABM). The study selection was undertaken on the Rayyan QCRI Platform. 12
Eligibility criteria
Articles that utilised a validated tool to assess medication appropriateness, along with reporting physical function were included. Physical function was defined as falls, fractures, ADL, instrumental activities of daily living (IADL), physical performance balance, muscle strength and cardiovascular endurance. Articles focussing on a specific medication or a class of medication were included if a validated tool was used to assess its appropriateness of use. For this systematic review, older adults were situated in diverse settings, including hospitals, aged care facilities and the community. Conference abstracts, case reports with fewer than five cases, letters to the Editor, reviews and any non-English articles were excluded from the review.
Data extraction
The data extraction process for each study was conducted independently by two authors (EM, MZK) into a standardised electronic data extraction sheet. Any discrepancies were resolved by a third reviewer (ABM). The following information were extracted: first author/year, country, mean age, sex ratio, sample size, study setting, the PIMs and PPOs examined as predictors, the approach used to identify PIMs and PPOs and the method for measuring the outcome. Attempts were made to contact authors of studies if there appeared to be missing information.
Quality assessment
Two authors (EM, MZK) independently assessed the quality of included studies using a modified Newcastle–Ottawa Scale (Supplemental Files S2 and S3). Points were given to the eligible categories: (a) selection of the study population, (b) comparability and (c) description of the outcome.
Results
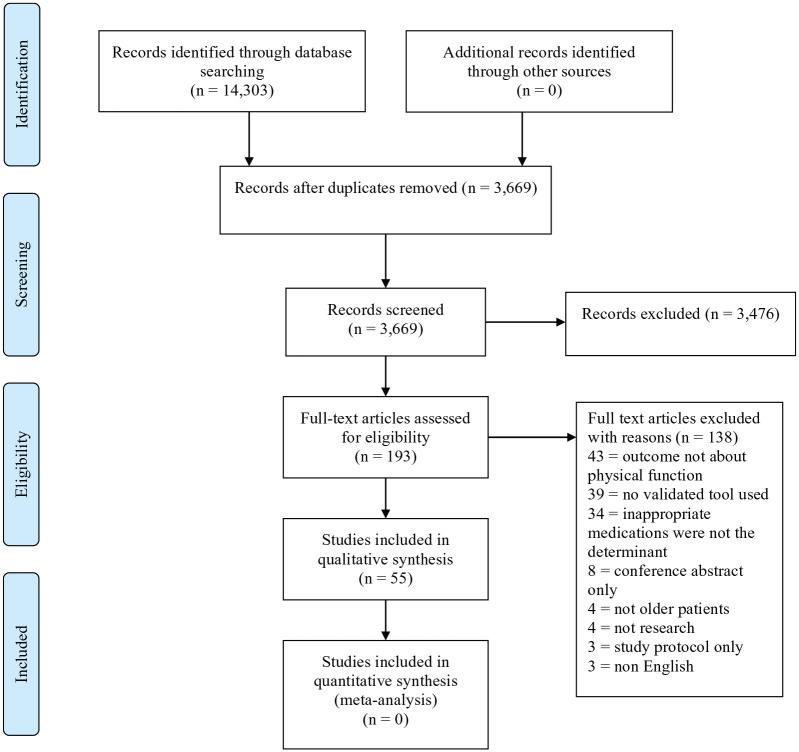
The article selection is outlined in Figure 1. A total of 14,303 studies were initially identified. After title and abstract screening, 193 full-text articles were retrieved and 55 studies met the inclusion criteria reporting on 2,767,594 participants (mean age 77.1 years, 63.5% were female). It was not possible to undertake a quantitative synthesis using meta-analysis due to the heterogeneity in physical function results that were obtained.
Figure 1.
PRISMA flow diagram.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Characteristics of included studies
The characteristics of included studies are summarised in Table 1. A total of 37 studies used various versions of the Beers criteria,8,13–48 19 studies used the Screening Tool of Older Person’s prescriptions (STOPP) criteria,21,23,25,34,36,37,43,48–59 9 studies used the Screening to Alert to Right Treatment (START) criteria,36,47–50,52,55,56,59 2 studies used the Meds 75+ Database,60,61 2 studies used the European Union (EU)(7)-PIM list,28,62 4 studies used the Drug Burden Index,24,43,63,64 and 1 study used the Norwegian General Practice (NORGEP) criteria list to identify inappropriate medications. 8 In three studies, the Anticholinergic Cognitive Burden scale was used,24,34,65 while in one study, the Quantitative Drug Index was used. 66 In some studies, more than one tool was used.8,15,17,21,23–25,28,34,36,37,43,47–50,52,55,56,59 Table 2 shows the associations between inappropriate medication prescriptions and physical function. Table 3 provides an illustrative summary of the associations between inappropriate medication prescriptions and physical function.
Table 1.
Characteristics of included studies (N = 55).
| Author | Country | Study design | Setting | Age, years | Sample size, N | Female, % | |
|---|---|---|---|---|---|---|---|
| Cut-off | Mean (SD) | ||||||
| Longitudinal studies | |||||||
| Ackroyd-Stolarz et al. 13 | Canada | RCS | Tertiary care hospital | ⩾65 | NG | 8976 | NG |
| Agashivala and Wu 14 | US | RCS | Nursing home | ⩾65 | 84.1 (7.97) | 11,940 | 74.7 |
| Beer et al. 15 | Australia | RCS | Community dwelling | 65–83 | 77.0 (3.6) | 4260 | 0 |
| Berdot et al. 16 | France | PCS | Community dwelling | ⩾65 | 73.7 (5.3) | 6343 | 59.0 |
| Borenstein et al. 17 | USA | PCS | Medical and surgical units | ⩾65 | 75.0 (13.4) | 214 | 57.9 |
| Cardwell et al. 63 | New Zealand, UK | PCS | Community dwelling | 80 | Maori: 82.3 (2.6) | 671 | 59.9 |
| Non-Maori: 84.6 (0.5) | |||||||
| Chan et al. 41 | US | PCS | Geriatric psychiatry unit | NG | 81.5 (6.2) | 118 | 78.0 |
| Chin et al. 42 | US | PCS | Emergency department | ⩾65 | 76.3 (7.9) | 898 | 63.0 |
| Chun et al. 20 | US | RCS | Assisted living facilities | ⩾65 | 83.9 [65–99] | 95 | 68.4 |
| De Vincentis et al. 34 | Italy | PCS | Medical units | ⩾65 | Median 79 [IQR 12] | 2631 | 51.4 |
| Delgado et al. 57 | UK | RCS | Community dwelling linked to hospitals | ⩾65 | 84.4 (7.3) | 11,175 with dementia + 43,463 controls | 64.8 |
| Early et al. 21 | US | PCS | Community dwelling | 65–99 | 77 | 1,678,037 | 63.4 case group |
| Fernández et al. 22 | Columbia | PCS | Community dwelling | ⩾65 | 69.3 (2.96) | 273 | 48.0 |
| Fick et al. 31 | US | RCS | Community dwelling | ⩾65 | 72.9 (10.6) | 960 | 41.1 |
| Fick et al. 32 | US | RCS | Community dwelling | ⩾65 | 73.5 (6.5) PIM exposed group | 17,971 | 71.0 PIM exposed group |
| Frankenthal et al. 49 | Israel | PCS | Chronic care geriatric facility | ⩾65 | NG | 542 | 62.5 |
| García-Gollarte et al. 50 | Spain | PCS | Nursing home | >65 | 84.4 (12.7) | 716 | 73.0 |
| Gosch et al. 59 | Austria | PCS | Geriatric evaluation and management unit | >65 | 80.6 (7.1) | 457 | 82.5 |
| Hamilton et al. 23 | US | PCS | Medical and surgical units | ⩾65 | Median 77.0 [IQR 72.0–83.0] | 600 | 59.8 |
| Hill-Taylor et al. 51 | Canada | RCS | Community and hospital | 66 | NG | 1327 | 83.1 |
| Hyttinen et al. 60 | Finland | RCS | Community dwelling | ⩾65 | 80.6 | 47,850 | 63.8 |
| Hyttinen et al. 61 | Finland | RCS | Community dwelling | ⩾65 | 74.6 (5.5) | 20,666 | 62.3 |
| Iaboni et al. 44 | US | PCS | Various hospitals | 60 | 78.5 (8.4) PIM exposed group | 477 | 68.7 PIM exposed group |
| 78.4 (9.1) Non PIM exposed group | 82.0 Non PIM exposed group | ||||||
| Ie et al. 24 | US | RCS | Community dwelling | ⩾65 | 78.3 (6.6) | 343 | 89.4 |
| Kersten et al. 8 | Norway | RCS | Emergency department | 75 | 86.0 (5.7) | 232 | 59.1 |
| Kose et al. 45 | Japan | RCS | Rehabilitation ward | ⩾65 | 79.0 (72–85) | 272 | 62.5 |
| Kose et al. 46 | Japan | RCS | Rehabilitation ward | ⩾65 | Median 79.0 [IQR 73.0–85.0] | 569 | 66.4 |
| Koyama et al. 38 | US | PCS | Community dwelling | >75 | 83.0 (3.1) | 1429 | 100 |
| Lu et al. 33 | Taiwan | RCS | Community and hospitals | ⩾65 | NG | 59,042 | 48.8 |
| Manias et al. 52 | Australia | RCS | Geriatric subacute wards | ⩾65 | 88.0 [IQR 86.0–91.0] | 249 | 61.4 |
| McMahon et al. 25 | Ireland | RCS | Emergency department | >70 | 82.7 (6.1) | 1016 | 69.7 |
| Moriarty et al. 36 | Ireland | PCS | Community dwelling | ⩾65 | Median 76.0 [IQR 72.0–80.0] | 1753 | 54.4 |
| Nagai et al. 53 | Japan | RCS | Surgical units | ⩾65 | 75.6 (8.6) PIM exposed group | 253 | 86.6 PIM exposed group |
| 72.8 (7.7) non PIM exposed group | 191 propensity matched group | 85.3 non PIM exposed group | |||||
| Nagai et al. 54 | Japan | RCS | Rehabilitation units | ⩾65 | 81.3 (8.1) | 170 | 66.5 |
| Naples et al. 39 | US | PCS | Community dwelling | ⩾65 | 74.6 (2.9) | 2402 | 51.3 |
| Narayan and Nishtala 26 | New Zealand | RCS | Community and hospitals | ⩾65 | 74.7 (7.6) | 537,387 | 54.9 |
| Ota et al. 27 | US | RCS | Ambulatory setting | ⩾65 | 71.9 (6.4) | 2704 | 66.5 |
| Pasina et al. 65 | Italy | PCS | Internal medicine and geriatric wards | ⩾65 | 78.5 (7.2) | 1380 | 48.8 |
| Renom-Guiteras et al. 62 | England, Estonia, Finland, France, Germany, The Netherlands, Spain, Sweden | PCS | Long-term care or at risk of long-term care | ⩾65 | 83.0 (6.6) | 2004 | 67.5 |
| Schiek et al. 28 | Germany | PCS | Military hospital | ⩾65 | Median 79 [IQR 69–86] | 174 | 54 |
| Sengul Aycicek et al. 40 | Turkey | PCS | Tertiary care hospital | ⩾65 | 72 (65–86) | 101 | 55.4 |
| Shibasaki et al. 47 | Japan | RCS | Neurology and Rehabilitation Hospital | ⩾65 | 82.9 (6.6) | 217 | 80.6 |
| Stockl et al. 29 | US | RCS | Community and hospitals | ⩾65 | 75.2 (6.4) | 27,084 | 69.0 PIM exposed group |
| Tosato et al. 37 | Italy | PCS | Internal medicine and geriatric wards | ⩾65 | 80.2 (7.0) | 871 | 53.2 |
| Umit et al. 48 | Turkey | RCS | Tertiary hospital | ⩾65 | 69.5 (65–86) | 80 | 57.5 |
| Walker et al. 30 | US | RCS | Trauma centre | ⩾65 | 78.5 (range 65–104) | 2181 | 52.0 |
| Weeks et al. 55 | Spain | RCS | Nursing home | 70–99 | 86.7 (6.5) Antipsychotic exposed group | 1653 | 76.8 |
| Cross-sectional studies | |||||||
| Anson et al. 66 | US | CSS | Community dwelling | >65 | 79 (range 66–92) | 57 | 72 |
| Bonfiglio et al. 58 | Italy | CSS | Outpatient department | ⩾64 | 78.3 (5.8) | 160 | 54.4 |
| Cameron et al. 18 | Canada | CSS | Long term care facility | ⩾65 | Median 85.0 [IQR 77–90] | 395 | 68.1 |
| Carter et al. 19 | US | CSS | Emergency department | ⩾65 | 75.2 (6.4) | 259,775 | 69.0 PIM exposed group |
| Dalleur et al. 56 | Belgium | CSS | Teaching hospital | 75 | Median 84.0 [IQR 81–88] | 302 | 62.6 |
| Gnjidic et al. 64 | Australia | CSS | Community dwelling | 70 | 76.9 (5.5) | 1705 | 0 |
| Hasan et al. 43 | Malaysia | CSS | Tertiary care hospital | 60 | 70.0 (6.77) | 344 | 44.9 |
| Mohamed et al. 35 | US | CSS | Cancer center | ⩾65 | 76.9 (5.4) | 439 | 45 |
Study by Anson et al. 66 involved a secondary analysis of patient results at baseline of an RCT.
CSS, cross-sectional study; IQR, interquartile range; NG, not given; PCS, prospective cohort study; PIM, potentially inappropriate medications; RCS, retrospective cohort study; SD, standard deviation; UK, United Kingdom; US, United States.
Table 2.
Results of included studies (N = 55).
| Author | Criteria used | Type of medications | Outcome measured | Adjustments | Statistical unit | Result (95% CI) | p value |
|---|---|---|---|---|---|---|---|
| Falls | |||||||
| Ackroyd-Stolarz et al. 13 | Beers | Benzodiazepine | Fall | Unadjusted | Prevalence | 4.5% (PIM use)3.8% (no PIM use) | 0.30 |
| Fall-related injuries | 2.6% (PIM use)1.8% (no PIM use) | 0.08 | |||||
| Agashivala and Wu 14 | Beers | PIPM | Falls in past 30 days | Unadjusted | OR | 1.349 (1.333–1.366) | <0.01 |
| OR of other Psychoactive medications with PIPM as reference | 0.83 (0.702–0.980) | 0.028 | |||||
| OR of non-psychoactive medications with PIPM as reference | 0.624 (0.517–0.754) | <0.01 | |||||
| Beer et al. 15 | Beers McLeod |
PIM use | Falls history | Unadjusted | OR | 1.66 (1.42–1.94) | <0.001 |
| Potential under utilisation | Unadjusted | OR | 1.24 (1.06–1.45) | 0.008 | |||
| Any marker for suboptimal medication use | Unadjusted | OR | 1.63 (1.29–2.04) | <0.001 | |||
| PIM use | Adjusted | OR | 1.23 (1.04–1.45) | 0.018 | |||
| Potential under utilisation | Adjusted | OR | 1.10 (0.93–1.31) | 0.278 | |||
| Any marker for suboptimal medication use | Adjusted | OR | 1.17 (0.91–1.49) | 0.227 | |||
| Berdot et al. 16 | Beers | PIM occasional user | Falls | Unadjusted | OR | 1.48 (1.26–1.74) | <0.001 |
| Falls | Adjusted | OR | 1.23 (1.04–1.5) | 0.016 | |||
| PIM regular user | Falls | Unadjusted | OR | 1.45 (1.26–1.66) | <0.001 | ||
| Falls | Adjusted | OR | 1.08 (0.94–1.25) | 0.29 | |||
| Borenstein et al. 17 | McLeod Beers | PIM | Falls | Unadjusted | OR | 2.93 (1.17–7.34) | <0.05 |
| Falls | Adjusted | OR | 3.05 (1.19–7.83) | <0.05 | |||
| Cameron et al. 18 | Beers | PIM | Falls | Adjusted – any PIM | Beta | 0.34 (0.037–0.65) | 0.028 |
| PIM | Falls | Adjusted – benzodiazepine | Beta | NG – reduced falls | 0.009 | ||
| PIM | Falls | Adjusted – Selective serotonin reuptake inhibitor/serotonin noradrenaline reuptake inhibitor use | Beta | NG – increased falls | 0.007 | ||
| Cardwell et al. 63 | Drug burden index | PIM | Falls | Adjusted | Relative risk | Maori: | |
| 12 months: 1.49 (0.76–2.92) | 0.25 | ||||||
| 24 months: 1.32 (0.68–2.57) | 0.41 | ||||||
| 36 months: 1.08 (0.53–2.19) | 0.83 | ||||||
| Non-Maori: | |||||||
| 12 months: 1.09 (0.76–1.56) | 0.65 | ||||||
| 24 months:1.06 (0.75–1.51) | 0.73 | ||||||
| 36 months: 1.13 (0.80–1.62) | 0.49 | ||||||
| Carter et al. 19 | Beers | PIM | Fall related ED visit | Not adjusted | Observed counts | 3442 falls comprising 47.8% of ED visits. 735 (11.7%) of ED visits had at least 1 PIM | NG |
| Chun et al. 20 | Beers | PIM | Falls | NG | Nagelkerke R2 | 0.017 | 0.079 |
| Early et al. 21 | Beers, STOPP | Fall-risk drugs, PIM | Falls | Adjusted | OR | Single PIM: 1.021 (0.998–1.044) | >0.05 |
| Two classes of PIM: 1.128 (1.102–1.154) | <0.05 | ||||||
| Five or more classes of PIM: 1.579 (1.540–1.619) | <0.05 | ||||||
| Fernández et al. 22 | Beers | PIM | Recurring falls | Adjusted | OR | 2.43 (1.08–5.84) | 0.028 |
| Frankenthal et al. 49 | STOPP/START | PIM and PPO | Average number of falls | NG | Difference | −0.5 (−0.9245 to −0.0755) | 0.006 |
| Physical component score | NG | Difference | 1.1 (−0.59 to 2.80) | 0.07 | |||
| García-Gollarte et al. 50 | STOPP/START | PIM and PPO | Falls | NG | Mean Difference | −0.08 | 0.251 |
| Hamilton et al. 23 | STOPP Beers | PIM | Benzodiazepines users (STOPP) + Falls | Proportion (%) | 100 | ||
| Benzodiazepines users (Beers) + Falls | 91.7 | ||||||
| Opiate users (STOPP) + Falls | 100 | ||||||
| Opiate users (Beers) + Falls | 0 | ||||||
| Sedative-Hypnotics users (STOPP) + Falls | 0 | ||||||
| Sedative-Hypnotics users (Beers) + Falls | 0 | ||||||
| Neuroleptics-users (STOPP) + Fall | 100 | ||||||
| Neuroleptics-users (Beers) + Falls | 20 | ||||||
| Hill-Taylor et al. 51 | STOPP | Benzodiazepine and zoplicone | Proportion of fallers taking these PIMs | Proportion | 21.60% | ||
| Ie et al. 24 | Fall risk-increasing drugs | PIM | Fall-months | Adjusted | Rate ratio | ⩾2: 1.67 (1.04–2.68) | <0.05 |
| Beers | PIM | ⩾1: 1.15 (0.72–1.84) | >0.05 | ||||
| Anticholinergic Cognitive Burden | PIM | >0.655 score: (1.24 (0.80–1.92) | >0.05 | ||||
| Drug Burden Index | PIM | >0.15 score: 1.51 (0.88–2.58) | >0.05 | ||||
| Manias et al. 52 | STOPP/START | PIM | Falls | Adjusted | Exp(B) incident count | 1.071 (0.883–1.299) | 0.484 |
| PPO | Falls | Adjusted | 1.096 (1.000–1.202) | 0.051 | |||
| McMahon et al. 25 | STOPP | PIM | % prescribing in fallers (pre-fall) | NG | Prevalence | 42.2% | 0.70 |
| Beers | PIM | % prescribing in fallers (pre-fall) | Prevalence | 44.0% | 0.10 | ||
| Nagai et al. 53 | STOPP-J | PIM | Subsequent falls in patients with distal radius fractures | Adjusted | OR | 1.713 (1.246–2.357) | <0.001 |
| Narayan and Nishtala 26 | Beers | PIM | Fall-related hospitalisation | Adjusted | IRR | 1.45 (1.37–1.52) | <0.05 |
| Ota et al. 27 | Beers | PIM | Fall, or fracture or injury | Adjusted | OR | 0.77 (0.51–1.13) | >0.05 |
| Renom-Guiteras et al. 62 | EU(7) - PIM List | PIM | Falls | Adjusted | OR | 1.54 (1.04–2.30) | <0.05 |
| Schiek et al. 28 | PRISCUS | PIM | FRIARs (fall-risk-increasing adverse reactions) | Unadjusted | OR | 1.966 (1.164–3.320) | <0.05 |
| EU(7)-PIM | PIM | 1.668 (0.900–3.091) | >0.05 | ||||
| Beers | PIM | 1.345 (1.065–1.698) | <0.05 | ||||
| Stockl et al. 29 | Beers | PIM | Fall or Fracture | Adjusted | HR | 1.22 (1.10–1.35) | <0.001 |
| Walker et al. 30 | Beers | PIM | Risk of falling | Adjusted | OR | 1.14 (1.00–1.29) | 0.0492 |
| Weeks et al. 55 | STOPP/START | PIM and PPO | Fall and physical restraints | NG | NG | No difference between exposure and controls | >0.05 |
| Falls and Fractures | |||||||
| Dalleur et al. 56 | STOPP/START | PIM | Fall | Adjusted | OR | 5.2 (2.2–12.3) | <0.001 |
| PPO | Osteoporotic fractures | Adjusted | OR | 5.0 (2.2–11.4) | <0.001 | ||
| PIM | PIM related fall admission in patients with fall-risk-PIM | NG | PPV | 0.68 | |||
| PPO | PPO related fall admission in patients with fall-risk-PPO | PPV | 0.25 | ||||
| Delgado et al. 57 | STOPP | PIM | Fall | Adjusted | HR | 1.37 (1.15–1.63) | <0.01 |
| PIM | Fracture | Adjusted | HR | 0.92 (0.70–1.19) | 0.51 | ||
| Fick et al. 31 | Beers | PIM | Fall | Adjusted | OR | 4.00 (1.76–9.76) | <0.0001 |
| Beers | PIM | Fracture | Adjusted | OR | 1.14 (0.50–2.65) | 0.72 | |
| Fick et al. 32 | Beers | PIM | Fall | Adjusted | OR | 4.05 (1.89–8.69) | <0.01 |
| Beers | PIM | Hip fracture | Adjusted | OR | 3.10 (1.71–5.62) | <0.01 | |
| Beers | PIM | Femur fracture | Adjusted | OR | 6.80 (1.95–23.67) | <0.01 | |
| Fractures | |||||||
| Hyttinen et al. 60 | Meds75+ Database | PIM | Hip fracture rates | Unadjusted but time-varying model | HR | 1.15 (0.94–1.40) | >0.05 |
| Unadjusted but time-varying model for the incident PIM use period | HR | 1.26 (1.02–1.56) | <0.05 | ||||
| Adjusted time varying model | HR | 1.21 (1.00–1.48) | 0.056 | ||||
| Adjusted time varying model for the incident PIM use period | HR | 1.31 (1.06–1.63) | 0.014 | ||||
| Hyttinen et al. 61 | Meds75+ Database | PIM | Fracture related hospitalisations (1 month after exposure) | Adjusted | HR | 1.61 (1.11–2.33) | 0.013 |
| Fracture related hospitalisations (3 months after exposure) | Adjusted | HR | 1.50 (1.22–1.84) | <0.01 | |||
| Fracture related hospitalisations (6 months after exposure) | Adjusted | HR | 1.38 (1.21–1.57) | <0.01 | |||
| Lu et al. 33 | Beers | PIM | Fracture related hospitalisations | Adjusted | OR | 1.55 (1.48–1.62) | <0.001 |
| ADL | |||||||
| Bonfiglio et al. 58 | STOPP-J | PIM | Bartel Index | Not adjusted | Independent t-test | With PIM: mean = 97.8 (SD = 5.5) | 0.541 |
| Without PIM: mean = 98.7 (SD = 3.1) | |||||||
| De Vincentis et al. 34 | Beers | PIM | Barthel Index at 3-month follow up | Adjusted | HR | −2 (−7.03 to 3.31) | 0.454 |
| STOPP | PIM | Barthel Index at 3-month follow up | Adjusted | HR | −1 (−6.59 to 4.92) | 0.734 | |
| Anticholinergic Cognitive Burden | PIM | Barthel Index at 3-month follow up | Adjusted | HR | −7.55 (−12.37 to −2.47) | 0.004 | |
| Gosch et al. 59 | STOPP/START | PIM and PPO | ADLs | NG | NG | Low Functional Status | <0.001 |
| Manias et al. 52 | STOPP/START | PIM | Independence in personal activities of daily living | Adjusted | OR | 1.07 (0.95–1.19) | 0.261 |
| Independence in domestic ADL | Adjusted | OR | 1.17 (1.01–1.34) | 0.036 | |||
| Independence in community ADL | Adjusted | OR | 1.25 (1.06–1.48) | 0.010 | |||
| Mohamed et al. 35 | Beers | PIM | Katz ADLs | Adjusted | OR | 1.42 (0.87–2.32) | >0.05 |
| Moriarty et al. 36 | STOPP | PIM | ADL | Adjusted | OR | ⩾2 PIM 1.22 (0.74– 2.01) | 0.439 |
| Beers | PIM | ⩾2 PIM 2.11 (1.36–3.28) | 0.001 | ||||
| ACOVE PIMs | PIM | ⩾2 PIM 1.10 (0.54–2.24) | 0.792 | ||||
| START | PPO | ⩾2 PPO 1.98 (1.20–3.26) | 0.008 | ||||
| ACOVE PPOs | PPO | ⩾2 PPO 1.82 (1.16–2.86) | 0.009 | ||||
| Nagai et al. 54 | STOPP-J | PIM | Bartel Index gain | Adjusted | Beta | −0.313 (−13.188 to −4.430) | <0.001 |
| Pasina et al. 65 | Anticholinergic Cognitive Burden | With anticholinergic medications | Barthel Index ADL | Adjusted | ANOVA | 83.5 (81.9–85.0) | 0.03 |
| No anticholinergic medications | 86.3 (84.4–88.1) | ||||||
| Renom-Guiteras et al. 62 | EU(7) - PIM List | PIM | Katz-index of 0–2 versus 6 | Adjusted | OR | 2.93 (1.85–4.65) | <0.001 |
| Katz-index of 3–5 versus 6 | Adjusted | OR | 1.848 (1.19–2.86) | 0.006 | |||
| Tosato et al. 37 | STOPP Beers |
STOPP (PIM versus no PIM) | Decline in physical ADL | Adjusted | OR | 2.00 (1.10–3.64) | <0.05 |
| Beers (PIM versus no PIM) | Decline in physical ADL | Adjusted | OR | 1.57 (0.85–2.89) | >0.05 | ||
| STOPP (⩾2 PIMs) | Decline in physical ADL | Adjusted | OR | 3.50 (1.77–6.91) | <0.05 | ||
| Beers (⩾2 PIMs) | Decline in physical ADL | Adjusted | OR | 1.90 (0.95–3.81) | >0.05 | ||
| IADL | |||||||
| Bonfiglio et al. 58 | STOPP-J | PIM | IADL | Not adjusted | Independent t-test | With PIM: mean = 0.8 (SD = 0.1) | 0.203 |
| Without PIM: mean = 0.9 (SD = 0.1) | |||||||
| Cardwell et al. 63 | Drug burden index | PIM | Functional status, change in Nottingham Extended ADL | Adjusted | Difference in mean score | Māori: | |
| 12 months: 0.49 (0.82–1.11) | 0.77 | ||||||
| 24 months: 0.55 (−1.36 to 0.81) | 0.62 | ||||||
| 36 months: 1.01 (−1.99 to 1.98) | 1.00 | ||||||
| Non-Māori: | |||||||
| 12 months: 0.36 (−1.22 to 0.20) | 0.16 | ||||||
| 24 months: 0.41 (−1.20 to 0.39) | 0.31 | ||||||
| 36 months: 0.49 (−1.01 to 0.89) | 0.90 | ||||||
| Koyama et al. 38 | Beers | PIM | IADL impairments | Adjusted | OR | 1.36 (1.05–1.75) | <0.05 |
| Mohamed et al. 35 | Beers | PIM | IADL impairment | Adjusted | OR | 1.72 (1.09–2.73) | <0.05 |
| Physical performance | |||||||
| Anson et al. 66 | Quantitative drug index | Falls-risk medications | Berg Balance Scale | Adjusted | Multiple regression | Standardised beta: −0.26 | 0.02 |
| TUG Test | Adjusted | Multiple regression | Standardised beta: 0.32 | 0.007 | |||
| TUG Test with cognitive dual task | Adjusted | Multiple regression | Standardised beta: 0.27 | 0.02 | |||
| Activities-specific Balance Confidence | Adjusted | Multiple regression | Standardised beta: −0.32 | 0.009 | |||
| Gosch et al. 59 | STOPP/START | PIM and PPO | TUG Test | Adjusted | NG | Low mobility patients have more STOPP items | 0.036 |
| Unadjusted | NG | Low mobility patients have more STOPP items | 0.006 | ||||
| Gnjidic et al. 64 | Drug burden index | Anticholinergic and sedative medications | Chair Stand Test (CST) | NG | Difference in time | CST: 0.58 (−0.11 to 1.27) | >0.05 |
| 6 m Walking Speed (6WS) | Difference in speed | 6WS: −0.03 (−0.05 to 0.00) | <0.05 | ||||
| 20 cm NWS | Difference in speed | NWS: −0.03 (−0.05 to −0.01) | <0.05 | ||||
| Grip Strength (GS) | Difference in kg (GS) | GS: −1.09 (−1.90 to −0.28) | <0.01 | ||||
| Balance | Difference in performance score (Balance) | Balance: −0.11 (−0.18 to −0.03) | <0.01 | ||||
| IADL | Difference in IADL Score | IADL: 0.18 (0.04–0.32) | <0.01 | ||||
| Kersten et al. 8 | NORGEP Beers | PIM | TUG Test | Adjusted | ANOVA F | 0.20 | 0.80 |
| HGS (Left Hand) | ANOVA F | 2.20 | 0.10 | ||||
| HGS (Right Hand) | ANOVA F | 1.10 | 0.30 | ||||
| Naples et al. 39 | Beers | PIM | GSD | Unadjusted | OR | 1.06 (0.92–1.24) | >0.05 |
| GSD | Adjusted (with time- varying age) | OR | 1.08 (0.93–1.26) | >0.05 | |||
| GSD | Adjusted (without time-varying age) | OR | 1.06 (0.90–1.24) | >0.05 | |||
| GSD (slow walkers) | Unadjusted | OR | 1.28 (1.03–1.58) | <0.05 | |||
| GSD (slow walkers) | Adjusted (with time- varying age) | OR | 1.27 (1.02–1.57) | <0.05 | |||
| GSD (slow walkers) | Adjusted (without time-varying age) | OR | 1.23 (0.97–1.55) | >0.05 | |||
| GSD (fast walkers) | Unadjusted | 1.15 (0.92–1.44) | >0.05 | ||||
| GSD (fast walkers) | Adjusted (with time- varying age) | 1.13 (0.90–1.42) | >0.05 | ||||
| GSD (fast walkers) | Adjusted (without time-varying age) | 1.03 (0.81–1.31) | >0.05 | ||||
| Sengul Aycicek et al. 40 | Beers | PIM | BPBS – balance | Adjusted | OR | 11.05 (2.39–51.10) | 0.002 |
| Functional independence score | |||||||
| Bonfiglio et al. 58 | STOPP-J | PIM | Quality of Life VAS | Adjusted | OR | 0.973 (0.939–1.008) | 0.131 |
| STOPP-J | PIM | Fried Criteria for Frailty | Adjusted | OR | 1.171 (0.676–2.028) | 0.573 | |
| Chan et al. 41 | Beers | PIM | SOF Score | NG | Correlation between change in # of PIMs and change in SOF score from admission to discharge | r = −0.44 | <0.001 |
| Chin et al. 42 | Beers | PIM | Health Related Quality of Life | NG | Score change if prescribed prior to admission | −3.5 (−6.9 to −0.1) | <0.05 |
| Score change if prescribed in the emergency department | −10.7 (−17.1 to −4.4) | <0.05 | |||||
| Score change if prescribed upon discharge from emergency department | −12.7 (−20.5 to −4.8) | <0.05 | |||||
| Hasan et al. 43 | Beers | PIM | Groningen Frailty Indicator | NG | Spearman’s correlation r | 0.025 (outpatient) | 0.745 (outpatient) |
| 0.097 (inpatient) | 0.206 (inpatient) | ||||||
| STOPP | Potential inappropriate prescribing | 0.041 (outpatient) | 0.595 (outpatient) | ||||
| −0.065 (inpatient) | 0.399 (inpatient) | ||||||
| Drug burden index | Sedatives and anticholinergics | −0.096 (outpatient) | 0.210 (outpatient) | ||||
| −0.158 (inpatient) | 0.038 (inpatient) | ||||||
| Beers | PIM | Older People’s Quality of Life | NG | Spearman’s correlation r | −0.157 (outpatient) | 0.040 (outpatient) | |
| −0.085 (inpatient) | 0.267 (inpatient) | ||||||
| STOPP | Potential inappropriate prescribing | −0.052 (outpatient) | 0.501 (outpatient) | ||||
| 0.022 (inpatient) | 0.774 (inpatient) | ||||||
| Drug burden index | Sedatives and anticholinergics | −0.069 (outpatient) | 0.369 (outpatient) | ||||
| 0.034 (inpatient) | 0.656 (inpatient) | ||||||
| Iaboni et al. 44 | Beers | PIM | Time to full functional recovery following hip fracture | Adjusted | HR | 0.69 (0.52–0.92) | 0.012 |
| Kose et al. 45 | Beers | PIM | FIM | Adjusted | FIM gain | −1.393 × change in number of PIM + 5.7 | <0.0001 |
| Kose et al. 46 | Beers | PIM | FIM–motor | Adjusted | Linear regression, changes in number of PIMs | Beta = −0.988 (−1.919 to −0.056) | 0.0377 |
| Mohamed et al. 35 | Beers | PIM | OARS PH survey | Adjusted | OR | 1.97 (1.15–3.37) | <0.05 |
| Shibasaki et al. 47 | Beers | PIM | FIM gain: FIM at discharge – | Adjusted | Standardised β | 0.084 | 0.260 |
| START | PPO | FIM at admission | 0.180 | 0.016 | |||
| Umit et al. 48 | Beers START/STOPP |
Prolonged use of benzodiazepines | ECOG Performance status (men) | NG | OR | 2.46 (1.91–3.27) | 0.007 |
ACOVE, assessing care of vulnerable elders indicators; ADL, activities of daily living; BPBS, Biosway Portable Balance System; ECOG, Eastern Cooperative Oncology Group; FIM, functional independence measure; GSD, gait speed decline; HGS, hand grip strength; HR, hazard ratio; IADL, instrumental activities of daily living; IRR, incidence rate ratio; NG, not given; NORGEP, Norwegian General Practice; NWS, narrow walking speed; OARS PH, Older Americans Resources and Services Physical Health; OR, odds ratio; PIM, potentially inappropriate medications; PIPM, potential inappropriate psychoactive medications; PPO, potential prescribing omissions; PPV, positive predictive value; SOF, scale of functioning; START, screening tool to alert to right treatment; STOPP, screening tool of older people’s prescriptions; TUG, timed up and go test.
Table 3.
Effect of inappropriate medication prescriptions on physical function.
| Type of physical function | Outcome | ||
|---|---|---|---|
| Falls | 21 a | 9 b | 0 c |
| Fractures | 7 a | 2 b | 0 c |
| Activities of daily living | 8 a | 2 b | 0 c |
| Instrumental activities of daily living | 4 a | 2 b | 0 c |
| Physical performance | 5 a | 2 b | 0 c |
| Functional independence score | 9 a | 1 b | |
Significantly associated with impediment of physical function.
No significant association with physical function.
Significantly associated with improvement of physical function.
Associations of PIMs and PPOs with falls and fractures
A total of 30 studies examined the association between inappropriate medications and falls (Table 2)13–32,49–53,55–57,62,63; 18 studies used the Beer’s criteria, 5 used the STOPP/START criteria, 5 used the STOPP, 2 used the EU (7)-PIM list, 2 used the Drug Burden Index, 1 used the PRISCUS list, and 1 used the Anticholinergic Drug Burden. Out of 30 studies, 21 showed a significant positive association between PIMs and risk of falls.14–18,22–24,26,28–32,49,51,53,56,57,62 One study showed a positive predictive value of 25% for the proportion of patients with PPO-related admissions for a fall with a fracture. 56 Benzodiazepine, opiate and sedative use were common PIMs associated with falls.14,23,31,51,52
Nine studies examined inappropriate medication use and its association with fractures.27,29,31–33,56,57,60,61 Seven out of nine studies showed a significantly higher number of fractures when exposed to PIMs.29,32,33,56,57,60,61 In the one study that examined the effect of PPOs on fractures, multivariate logistic regression analysis showed PPO-related admission was associated with increased odds of osteoporotic fracture [odds ratio (OR) = 5.0, 95% confidence interval (CI) 2.2–11.4, p < 0.001]. 56 Antidiabetic, psychotropic, opioid and antihypertensive use impacted on older people’s associated risk of experiencing fractures.32,61
Associations of PIMs and PPOs with ADL and IADL
A total of 10 studies examined associations between inappropriate medication use and ADL,34–37,52,54,58,59,62,65 while 6 studies examined associations between inappropriate medication use and IADL (Table 2).35,38,52,58,63,64 Of the 10 studies focusing on ADLs, 8 showed that inappropriate medication use was associated with ADL impairment,34,36,37,52,54,59,62,65 while 4 studies involving IADLs showed that inappropriate medication use was associated with IADL impairment.35,38,52,64 In the one study involving anticholinergic burden and ADLs, patients who were prescribed any anticholinergic medication had a mean Bartel Index of 83.5 (95% CI 81.9–85.0), while those who were not prescribed any anticholinergic medication had a mean Bartel Index of 86.3 (95% CI 84.4–88.1, p = 0.03). 65 In the study by Tosato et al., there were variations in results depending on the type of tool used for inappropriate medication use. 37 They showed PIM use defined with the STOPP criteria was significantly associated with ADL impairment, while PIM use defined with the Beer’s criteria showed no significant association with ADL impairment.
Associations of PIMs and PPOs with physical performance
Seven studies involved examination of associations between inappropriate medication use and physical performance (Table 2).8,39,40,49,59,64,66 Aside from two studies,8,49 the included studies showed significant associations between PIM use and physical performance. In the study by Kersten et al., 8 PIM use had no significant association with the Timed Up and Go test. In the study by Naples et al., 39 while variable results were found in terms of effects of inappropriate medication use on physical performance, the investigators showed that any drug–drug or drug–disease interaction was significantly associated with a meaningful decline in gait speed of ⩾0.1 m/s, for slow versus fast walkers based on a median split at 1.15 m/s (OR 1.27, 95% CI 1.02–1.57, p < 0.05).
Associations of PIMs and PPOs with functional independence scores
Of the 10 studies involved in the examination of inappropriate medication use and measures of functional independence, 9 demonstrated that inappropriate medication use was significantly associated with increased impediment with functional independence.35,41–48 PIM use was associated significantly with a decrease in the Health-Related Quality-of-Life Score. 42 In one study, a lowering in the number of PIMs was associated with a significant increase in the Functional Independence Measure. 45 In one study, PIM use was associated with a longer time to full functional recovery in older patients who had surgery for a hip fracture, especially those patients who were using two or more PIMs at 2–14 days after surgical hip fracture repair. 44
Quality of included studies
Table 4 shows the results of the modified Newcastle-Ottawa Scale (Supplemental Files S2 and S3), which assesses the quality of included studies. The median total NOS score was 6.0 (IQR 5–7).
Table 4.
Quality of included studies (N = 55).
| Author | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | O1 | O2 | O3 | ||
| Longitudinal studies | |||||||||
| Ackroyd-Stolarz et al. 13 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| Agashivala and Wu 14 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Beer et al. 15 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Berdot et al. 16 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Borenstein et al. 17 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Cardwell et al. 63 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Chan et al. 41 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Chin et al. 42 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Chun et al. 20 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| De Vincentis et al. 34 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Delgado et al. 57 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Early et al. 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Fernández et al. 22 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Fick et al. 31 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| Fick et al. 32 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Frankenthal et al. 49 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| García-Gollarte et al. 50 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| Gosch et al. 59 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Hamilton et al. 23 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Hill-Taylor et al. 51 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Hyttinen et al. 60 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Hyttinen et al. 61 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Iaboni et al. 44 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| Ie et al. 24 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Kersten et al. 8 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Kose et al. 45 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Kose et al. 46 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Koyama et al. 38 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Lu et al. 33 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Manias et al. 52 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| McMahon et al. 25 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Moriarty et al. 36 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Nagai et al. 53 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| Nagai et al. 54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Naples et al.39 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Narayan and Narayan 26 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Ota et al. 27 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Pasina et al. 65 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Renom-Guiteras et al. 62 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Schiek et al. 28 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Sengul Aycicek et al. 40 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Shibasaki et al. 47 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Stockl et al. 29 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Tosato et al. 37 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 |
| Umit et al. 48 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Walker et al. 30 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Weeks et al. 55 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Cross-sectional studies | |||||||||
| Anson et al. 66 | 1 | 1 | 1 | 0 | 0 | 1 | NA | NA | 4 |
Discussion
The systematic review showed that PIMs were associated with a higher rate of falls and fractures. There was one study examining the association of PPOs on falls and fractures. PIMs and PPOs were also associated with impairment in ADLs and IADL impairment. PIMs and PPOs were also associated with poor physical performance comprising the Timed Up and Go test, walking speed, grip strength, time to functional recovery, functional independence and scale of functioning. In contrast to extensive work conducted with PIMs, there was a small amount of research related to associations of PPOs and physical function.
A number of medication classes were implicated as PIMs in falls, fractures and impairment in physical performance including antipsychotic, sedative, anti-anxiety, anticholinergic, antidiabetic, opioid and antihypertensive medications.14,23,32,51,52,61,65 Aside from the use of PIMs, the combination of different medications can lead to drug interactions that could have exacerbated the adverse effects experienced by older adults, thereby leading to higher propensity for impaired physical function. 10 Furthermore, adverse drug reactions can occur independently of PIMs, which can contribute to accentuating the impact on physical function. 67 Anticholinergic cognitive burden is also associated with increased susceptibility of delirium, longer hospital stays and increased prescription of more medications. This combination of events may also further impede physical performance experienced by older patients. 68
There has been limited research examining the association of PPOs on physical function. Of studies examining PPOs, their impact has been considered as a large group entity rather than determining which PPO criteria or medication groups may be associated with physical function.49,50,55 Conversely, a study by Dalleur et al. study provided valuable insight into the association of prescribing omissions with physical function. 56 In that study, prescribing omissions were associated with a significant number of hospital admissions in relation to osteoporotic fractures and fall admissions in patients with fall-risk PPOs. For their study, a pharmacist and a geriatrician independently used the STOPP and START criteria to detect PIMs and PPOs and their association with outcomes, which could contribute to reporting bias. Furthermore, for patients not receiving musculoskeletal medications, such as calcium, vitamin D and bisphosphonates, patients were found to be at risk of a hospital admission for a fall with a fracture. Further work is needed on other PPOs, and their associations with physical function. Examples include the lack of use of angiotensin converting enzyme inhibitors for cardiac failure, or the lack of use of regular inhaled beta-2 agonist or anticholinergic medication for chronic obstructive pulmonary disease, or the lack of use of platelet aggregation inhibitors, statins or antithrombotic agents for ischaemic heart disease. Omissions of these medications may lead to symptoms affecting patients’ physical function and mobility.
Methodological limitations of past studies related to their focus on PIMs rather than PPOs. Most studies focussed on older people living in the community and hospitals. The results may therefore not be extended to different clinical situations. There has been an increased focus in recent years on comparing results between screening tools for inappropriate medication prescribing. Further work is needed to determine the sensitivity in the use of various tools in terms of the associations between inappropriate prescribing and physical function. While many studies comprised large sizes, some studies had small samples, which could have impacted results related to physical function. In most studies, the dose effect of how the number of inappropriate medications was associated with physical function related adverse outcomes was not examined. Fewer than half of the studies involved a prospective cohort design. Further research is also needed on how changes in inappropriate prescribing across transitions of care are associated with physical function.
Strengths and limitations
A strength of the systematic review is that studies were included only if they used a validated tool to assess the appropriateness of medications. This approach was undertaken to eliminate sources of bias that could arise from a geriatrician or a pharmacist labelling a medication as inappropriate. All settings were included in the systematic review, which facilitated a comprehensive examination of the topic. A limitation of the systematic review was that only studies published in English were included. Conference papers were excluded from the systematic review because of the limited information contained in these sources. It is possible that additional insights may have been obtained from such sources.
Conclusion
Inappropriate medication prescribing is associated with poor physical function. Health professionals should focus on supporting older people to reduce the use of PIMs and PPOs. More research is required to investigate the associations of PPOs and physical function.
Supplemental Material
Supplemental material, sj-pdf-1-taw-10.1177_20420986211030371 for Inappropriate medications and physical function: a systematic review by Elizabeth Manias, Md Zunayed Kabir and Andrea B. Maier in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-2-taw-10.1177_20420986211030371 for Inappropriate medications and physical function: a systematic review by Elizabeth Manias, Md Zunayed Kabir and Andrea B. Maier in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-3-taw-10.1177_20420986211030371 for Inappropriate medications and physical function: a systematic review by Elizabeth Manias, Md Zunayed Kabir and Andrea B. Maier in Therapeutic Advances in Drug Safety
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Elizabeth Manias  https://orcid.org/0000-0002-3747-0087
https://orcid.org/0000-0002-3747-0087
Contributor Information
Elizabeth Manias, School of Nursing and Midwifery, Centre for Quality and Patient Safety Research, Institute for Health Transformation, 221 Burwood Highway, Burwood, VIC 3125, Australia; Department of Medicine, The Royal Melbourne Hospital, The University of Melbourne, Parkville, VIC, Australia.
Md Zunayed Kabir, Department of Medicine and Aged Care, The Royal Melbourne Hospital, The University of Melbourne, Parkville, VIC, Australia.
Andrea B. Maier, Department of Medicine and Aged Care, @ Age Melbourne, The Royal Melbourne Hospital, The University of Melbourne, VIC, Australia Department of Human Movement Sciences, Faculty of Behavioural and Movement Sciences, @AgeAmsterdam, Vrije Universiteit Amsterdam, Amsterdam Movement Sciences, Amsterdam, The Netherlands; Healthy Longevity Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Centre for Healthy Longevity, @AgeSingapore, National University Health System, Singapore.
References
- 1. Primejdie DP, Bojita MT, Popa A. Potentially inappropriate medications in elderly ambulatory and institutionalized patients: an observational study. BMC Pharmacol Toxicol 2016; 17: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morin L, Fastbom J, Laroche ML, et al. Potentially inappropriate drug use in older people: a nationwide comparison of different explicit criteria for population-based estimates. Br J Clin Pharmacol 2015; 80: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Junius-Walker U, Theile G, Hummers-Pradier E. Prevalence and predictors of polypharmacy among older primary care patients in Germany. Fam Pract 2007; 24: 14–19. [DOI] [PubMed] [Google Scholar]
- 4. Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007; 370: 185–191. [DOI] [PubMed] [Google Scholar]
- 5. Chen CC, Cheng SH. Potentially inappropriate medication and health care outcomes: an instrumental variable approach. Health Serv Res 2016; 51: 1670–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beswick AD, Rees K, Dieppe P, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008; 371: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landi F, Russo A, Liperoti R, et al. Impact of inappropriate drug use on physical performance among a frail elderly population living in the community. Eur J Clin Pharmacol 2007; 63: 791–799. [DOI] [PubMed] [Google Scholar]
- 8. Kersten H, Hvidsten LT, Gløersen G, et al. Clinical impact of potentially inappropriate medications during hospitalization of acutely ill older patients with multimorbidity. Scand J Prim Health Care 2015; 33: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maerz AH, Walker BS, Collier BR, et al. The Beers criteria: not just for geriatrics anymore? Analysis of Beers criteria medications in nongeriatric trauma patients and their association with falls. J Trauma Acute Care Surg 2019; 87: 147–152. [DOI] [PubMed] [Google Scholar]
- 10. Katsimpris A, Linseisen J, Meisinger C, et al. The association between polypharmacy and physical function in older adults: a systematic review. J Gen Internal Med 2019; 34: 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peron EP, Gray SL, Hanlon JT. Medication use and functional status decline in older adults: a narrative review. Am J Geriatr Pharmacotherap 2011; 9: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan — a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ackroyd-Stolarz S, Mackinnon NJ, Sketris I, et al. Potentially inappropriate prescribing of benzodiazepines for older adults and risk of falls during a hospital stay: a descriptive study. Can J Hosp Pharm 2009; 62: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agashivala N, Wu WK. Effects of potentially inappropriate psychoactive medications on falls in US nursing home residents: analysis of the 2004 National Nursing Home Survey database. Drugs Aging 2009; 26: 853–860. [DOI] [PubMed] [Google Scholar]
- 15. Beer C, Hyde Z, Almeida OP, et al. Quality use of medicines and health outcomes among a cohort of community dwelling older men: an observational study. Br J Clin Pharmacol 2011; 71: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berdot S, Bertrand M, Dartigues JF, et al. Inappropriate medication use and risk of falls – a prospective study in a large community-dwelling elderly cohort. BMC Geriatr 2009; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borenstein J, Aronow HU, Bolton LB, et al. Early recognition of risk factors for adverse outcomes during hospitalization among Medicare patients: a prospective cohort study. BMC Geriatr 2013; 13: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cameron EJ, Bowles SK, Marshall EG, et al. Falls and long-term care: a report from the care by design observational cohort study. BMC Fam Pract 2018; 19: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carter MW, Gupta S. Characteristics and outcomes of injury-related ED visits among older adults. Am J Emerg Med 2008; 26: 296–303. [DOI] [PubMed] [Google Scholar]
- 20. Chun JC, Appel SJ, Simmons S. 2015 Beers criteria medication review in assisted living facilities. J Am Assoc Nurse Pract 2018; 30: 648–654. [DOI] [PubMed] [Google Scholar]
- 21. Early NK, Fairman KA, Hagarty JM, et al. Joint effects of advancing age and number of potentially inappropriate medication classes on risk of falls in Medicare enrollees. BMC Geriatr 2019; 19: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernández A, Gómez F, Curcio C-L, et al. Prevalence and impact of potentially inappropriate medication on community-dwelling older adults. Biomedica 2021; 41: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 2011; 171: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 24. Ie K, Chou E, Boyce RD, et al. Fall risk-increasing drugs, polypharmacy, and falls among low-income community-dwelling older adults. Innov Aging 2021; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahon CG, Cahir CA, Kenny RA, et al. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing 2014; 43: 44–50. [DOI] [PubMed] [Google Scholar]
- 26. Narayan SW, Nishtala PS. Associations of potentially inappropriate medicine use with fall-related hospitalisations and primary care visits in older New Zealanders: a population-level study using the updated 2012 Beers criteria. Drugs Real World Outcomes 2015; 2: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ota T, Patel RJ, Delate T. Effectiveness of best practice alerts for potentially inappropriate medication orders in older adults in the ambulatory setting. Perm J. Epub ahead of print 22 November 2020. DOI: 10.7812/TPP/7819.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiek S, Hildebrandt K, Zube O, et al. Fall-risk-increasing adverse reactions-is there value in easily accessible drug information? A case-control study. Eur J Clin Pharmacol 2019; 75: 849–857. [DOI] [PubMed] [Google Scholar]
- 29. Stockl KM, Le L, Zhang S, et al. Clinical and economic outcomes associated with potentially inappropriate prescribing in the elderly. Am J Manag Care 2010; 16: e1–e10. [PubMed] [Google Scholar]
- 30. Walker BS, Collier BR, Bower KL, et al. The prevalence of Beers criteria medication use and associations with falls in geriatric patients at a level 1 trauma center. Am Surg 2019; 85: 877–882. [PubMed] [Google Scholar]
- 31. Fick D, Kolanowski A, Waller J. High prevalence of central nervous system medications in community-dwelling older adults with dementia over a three-year period. Aging Ment Health 2007; 11: 588–595. [DOI] [PubMed] [Google Scholar]
- 32. Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health 2008; 31: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu WH, Wen YW, Chen LK, et al. Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: a retrospective cohort study. CMAJ 2015; 187: e130–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Vincentis A, Gallo P, Finamore P, et al. Potentially inappropriate medications, drug-drug interactions, and anticholinergic burden in elderly hospitalized patients: does an association exist with post-discharge health outcomes? Drugs Aging 2020; 37: 585–593. [DOI] [PubMed] [Google Scholar]
- 35. Mohamed MR, Ramsdale E, Loh KP, et al. Association of polypharmacy and potentially inappropriate medications with physical functional impairments in older adults with cancer. J Natl Compr Canc Netw. Epub ahead of print 22 January 2021. DOI: 10.6004/jnccn.2020.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moriarty F, Bennett K, Kenny RA, et al. Comparing potentially inappropriate prescribing tools and their association with patient outcomes. J Am Geriatr Soc 2020; 68: 526–534. [DOI] [PubMed] [Google Scholar]
- 37. Tosato M, Landi F, Martone AM, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing 2014; 43: 767–773. [DOI] [PubMed] [Google Scholar]
- 38. Koyama A, Steinman M, Ensrud K, et al. Long-term cognitive and functional effects of potentially inappropriate medications in older women. J Gerontol A Biol Sci Med Sci 2014; 69: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naples JG, Marcum ZA, Perera S, et al. Impact of drug-drug and drug-disease interactions on gait speed in community-dwelling older adults. Drugs Aging 2016; 33: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sengul Aycicek G, Arik G, Kizilarslanoglu MC, et al. Association of polypharmacy with postural instability and impaired balance in community-dwelling older adults in Turkey. Marmara Med J 2021; 34: 12–17. [Google Scholar]
- 41. Chan VT, Woo BKP, Sewell DD, et al. Reduction of suboptimal prescribing and clinical outcome for dementia patients in a senior behavioral health inpatient unit. Int Psychogeriatr 2009; 21: 195–199. [DOI] [PubMed] [Google Scholar]
- 42. Chin MH, Wang LC, Jin L, et al. Appropriateness of medication selection for older persons in an urban academic emergency department. Acad Emerg Med 1999; 6: 1232–1242. [DOI] [PubMed] [Google Scholar]
- 43. Hasan SS, Burud IAS, Kow CS, et al. Use of potentially inappropriate medications among older outpatients and inpatients in a tertiary care hospital in Malaysia. Int J Clin Pract 2021; 75: e13714. [DOI] [PubMed] [Google Scholar]
- 44. Iaboni A, Rawson K, Burkett C, et al. Potentially inappropriate medications and the time to full functional recovery after hip fracture. Drugs Aging 2017; 34: 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kose E, Hirai T, Seki T, et al. Role of potentially inappropriate medication use in rehabilitation outcomes for geriatric patients after strokes. Geriatr Gerontol Int 2018; 18: 321–328. [DOI] [PubMed] [Google Scholar]
- 46. Kose E, Hirai T, Seki T, et al. The impact of decreasing potentially inappropriate medications on activities of daily living in a convalescent rehabilitation setting. Int J Clin Pharm. Epub ahead of print 2 November 2020. DOI: 10.1007/s11096-11020-01165-11093. [DOI] [PubMed] [Google Scholar]
- 47. Shibasaki K, Asahi T, Kuribayashi M, et al. Potential prescribing omissions of anti-osteoporosis drugs is associated with rehabilitation outcomes after fragility fracture: retrospective cohort study. Geriatr Gerontol Int 2021; 21: 386–391. [DOI] [PubMed] [Google Scholar]
- 48. Umıt EG, Baysal M, Bas V, et al. Polypharmacy and potentially inappropriate medication use in older patients with multiple myeloma, related to fall risk and autonomous neuropathy. J Oncol Pharm Pract 2020; 26: 43–50. [DOI] [PubMed] [Google Scholar]
- 49. Frankenthal D, Lerman Y, Kalendaryev E, et al. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 50. García-Gollarte F, Baleriola-Júlvez J, Ferrero-López I, et al. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc 2014; 15: 885–891. [DOI] [PubMed] [Google Scholar]
- 51. Hill-Taylor B, Sketris IS, Gardner DM, et al. Concordance with a STOPP (Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions) criterion in Nova Scotia, Canada: benzodiazepine and zoplicone prescription claims by older adults with fall-related hospitalizations. J Popul Ther Clin Pharmacol 2016; 23: e1–e12. [PubMed] [Google Scholar]
- 52. Manias E, Maier A, Krishnamurthy G. Inappropriate medication use in hospitalised oldest old patients across transitions of care. Aging Clin Exp Res 2019; 31: 1661–1673. [DOI] [PubMed] [Google Scholar]
- 53. Nagai T, Nagaoka M, Tanimoto K, et al. Relationship between potentially inappropriate medications and functional prognosis in elderly patients with distal radius fracture: a retrospective cohort study. J Orthop Surg Res 2020; 15: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nagai T, Wakabayashi H, Maeda K, et al. Influence of potentially inappropriate medications on activities of daily living for patients with osteoporotic vertebral compression fractures: a retrospective cohort study. J Orthop Sci 2021; 26: 448–452. [DOI] [PubMed] [Google Scholar]
- 55. Weeks WB, Mishra MK, Curto D, et al. Comparing three methods for reducing psychotropic use in older demented Spanish care home residents. J Am Geriatr Soc 2019; 67: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 56. Dalleur O, Spinewine A, Henrard S, et al. Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging 2012; 29: 829–837. [DOI] [PubMed] [Google Scholar]
- 57. Delgado J, Jones L, Bradley MC, et al. Potentially inappropriate prescribing in dementia, multi-morbidity and incidence of adverse health outcomes. Age Ageing 2021; 50: 457–464. [DOI] [PubMed] [Google Scholar]
- 58. Bonfiglio V, Umegaki H, Kuzuya M, et al. Potentially inappropriate medications and polypharmacy: a study of older people with mild cognitive impairment and mild dementia. J Alzheimers Dis 2019; 71: 889–897. [DOI] [PubMed] [Google Scholar]
- 59. Gosch M, Wörtz M, Nicholas JA, et al. Inappropriate prescribing as a predictor for long-term mortality after hip fracture. Gerontol 2014; 60: 114–122. [DOI] [PubMed] [Google Scholar]
- 60. Hyttinen V, Taipale H, Tolppanen AM, et al. Incident use of a potentially inappropriate medication and hip fracture in community-dwelling older persons with Alzheimer’s disease. Ann Pharmacother 2017; 51: 725–734. [DOI] [PubMed] [Google Scholar]
- 61. Hyttinen V, Jyrkkä J, Saastamoinen LK, et al. The association of potentially inappropriate medication use on health outcomes and hospital costs in community-dwelling older persons: a longitudinal 12-year study. Eur J Health Econ 2019; 20: 233–243. [DOI] [PubMed] [Google Scholar]
- 62. Renom-Guiteras A, Thürmann PA, Miralles R, et al. Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing 2018; 47: 68–74. [DOI] [PubMed] [Google Scholar]
- 63. Cardwell K, Kerse N, Ryan C, et al. The association between Drug Burden Index (DBI) and health-related outcomes: a longitudinal study of the ‘oldest old’ (LiLACS NZ). Drugs Aging 2020; 37: 205–213. [DOI] [PubMed] [Google Scholar]
- 64. Gnjidic D, Cumming RG, Le Couteur DG, et al. Drug burden index and physical function in older Australian men. Br J Clin Pharmacol 2009; 68: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pasina L, Djade CD, Lucca U, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging 2013; 30: 103–112. [DOI] [PubMed] [Google Scholar]
- 66. Anson E, Thompson E, Odle BL, et al. Influences of age, obesity, and adverse drug effects on balance and mobility testing scores in ambulatory older adults. J Geriatr Phys Ther 2018; 41: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saka SA, Nlooto M, Oosthuizen F. American Geriatrics Society-Beers criteria and adverse drug reactions: a comparative cross-sectional study of Nigerian and South African older inpatients. Clin Interv Aging 2018; 13: 2375–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rigor J, Rueff Rato I, Ferreira PM, et al. Prehospital anticholinergic burden is associated with delirium but not with mortality in a population of acutely ill medical patients. J Am Med Dir Assoc 2020; 21: 481–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taw-10.1177_20420986211030371 for Inappropriate medications and physical function: a systematic review by Elizabeth Manias, Md Zunayed Kabir and Andrea B. Maier in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-2-taw-10.1177_20420986211030371 for Inappropriate medications and physical function: a systematic review by Elizabeth Manias, Md Zunayed Kabir and Andrea B. Maier in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-3-taw-10.1177_20420986211030371 for Inappropriate medications and physical function: a systematic review by Elizabeth Manias, Md Zunayed Kabir and Andrea B. Maier in Therapeutic Advances in Drug Safety