Abstract
Background:
Unhealthy food environments may be associated with higher risks of developing diet-related cancers, such as, colorectal cancer. We conducted an ecological analysis to evaluate the relationship between the local food environment and colorectal cancer incidence overall and separately for males and females.
Methods:
Data from the Texas Cancer Registry was utilized to geocode individuals aged 40 years and older diagnosed with colorectal cancer from 2005 to 2015 to their residential 2010 census tract. Total number of establishments classified as Limited Service Restaurants for each census tract was retrieved from the 2005 Business Patterns Survey by using a crosswalk to map zip codes to census tract. Census tract unhealthy food availability was calculated by dividing the estimated number of Limited Service Restaurant establishments in each census tract by the census tract population and divided into quartiles. Generalized estimating equations were used to assess the association between unhealthy food availability quartiles and colorectal cancer incidence.
Results:
Adjusting for the census tract level sociodemographic characteristics, the incidence of colorectal cancer was slightly higher in unhealthy food availability quartile 2 (Incidence Rate Ratio (IRR) = 1.03, 95% CI: 1.00–1.05), but not quartile 3 (IRR = 1.02, 95% CI: 1.00–1.05), and quartile 4 (highest availability, IRR = 1.02, 95% CI: 0.99–1.05) compared to census tracts with lowest unhealthy food availability.
Conclusion:
Colorectal cancer incidence was not strongly associated with census tracts with higher unhealthy food availability. Future observational studies should be conducted to examine the influence of the built environment on colorectal cancer risk.
Keywords: Colorectal Neoplasms, Incidence, Food, Environment
1. Introduction
Colorectal cancer is the third most commonly diagnosed cancer in the U.S. and the second leading cause of cancer deaths in both men and women[1]. Dietary factors like increased intake of red and processed meats instead of fruits and vegetables may increase the risk of developing colorectal cancer[[2], [3], [4], [5]]. An individual’s diet and health outcomes, however, may be a function of the foods available in their food environment[[6], [7], [8], [9]]. For instance, greater exposure to unhealthy food options may encourage an individual to have a less healthy diet [6]. More specifically, less healthy food environments are positively associated with poorer diet-related health outcomes such as obesity [[10], [11]], diabetes[[12], [13], [14]], and high blood pressure[15].
There has been an extensive number of ecological studies examining specific food items and nutrients, such as red meat or fiber, and colorectal cancer incidence in diverse settings[[16], [17], [18], [19], [20], [21], [22]]. However, there is a lack of ecological studies that investigate the link between food environments (e.g., access to unhealthy food stores) and colorectal cancer incidence. One prospective cohort study examined the impact of local food environments, using the ratio of number of convenience stores, liquor stores, and fast-food restaurants to supermarkets and farmers’ markets, on the risk of developing colorectal cancer[23]. Overall, no relationship was observed between food environment and risk of colorectal cancer. Yet, less healthy retail food environments were found to be associated with an increased risk of developing colorectal cancer in white males[23]. No other large-scale observational studies have been conducted to examine the relationship between the food environment and colorectal cancer incidence using a diverse sample. To address this, we assessed the ecological relationship between census tract unhealthy food availability and colorectal cancer incidence using statewide cancer registry data in Texas. Conceptually, we expect to see a relationship between the higher availability to unhealthy food stores and colorectal cancer incidence because the physical environment influences behaviors (e.g. dietary patterns) which impacts health[24].
2. Methods
2.1. Sample of Colorectal Cancer Cases
Cancer data were provided by the Texas Cancer Registry (TCR, https://www.dshs.texas.gov/tcr/). TCR is a gold certified cancer registry with at least 95% complete case ascertainment. This study was approved both by the University of Texas Medical Branch’s Institutional Review Board, and TCR’s Institutional Review Board. In 2010, Texas was divided into 5,265 census tracts. Fifty (0.9%) census tracts were excluded from the overall analysis because of missing information on median age, percent white, percent male, or percentage of individuals living below poverty. Census tracts were also excluded from analysis if they had a population 40 years of age and older that was either missing or zero. Final overall and sex stratified analyses utilized information from 5,215 census tracts.
Individuals aged 40 years and older residing in Texas at the time of diagnosis with a primary malignant and/or invasive cancer of the colon or rectum (ICD-03 codes: C180-C189, C209, C260) were eligible for inclusion (N=84,226). We included only persons diagnosed with colorectal cancer at ages ≥40 years because colorectal cancer incidence younger than 40 years is low (<5 cases per 100,000 people) and increases to ~13 per 100,000 persons in those aged 40–49 years old [25]. Individuals were geolocated to their 2010 census tract boundary based on their residential address at the time of diagnosis using Centrus by TCR. Individuals (N=2,821, 3.3%) who could not be geolocated to census tract 2010 boundaries because of missing census tract data or were assigned a census tract based on their residential zip code were excluded from analysis. Our final sample consisted of 81,419 individuals diagnosed with colorectal cancer aggregated to their residential 2010 census tract. Persons diagnosed with colorectal cancer excluded from the analysis were older (mean age, years: 66 vs 67, p<0.0001), more likely to be male (53.4% vs 56.5%, p=0.001) and white (61.2% vs 64.3%, p<0.0001), and had a smaller number of limited service restaurants per zip code (median establishments: 15 vs 2, p<0.0001).
2.2. Outcome
The outcome was the aggregate number of individuals diagnosed with colorectal cancer, overall and by sex, geolocated to a 2010 census tract based on their residential address for each study year (2005–2015). Census tracts which did not have any reported cases for a specified year were assumed to have 0 cases for that specific year because TCR is a population-based registry.
2.3. Exposure and Cross-Walking Exposure to Census Tracts
Unhealthy food availability was operationalized by retrieving the number of all limited-service restaurants (North American Industry Classification System Code 722211) of any employment size per zip code from the 2005 Business Patterns Survey. The annual zip code Business Patterns Survey reports the aggregate number of businesses and employment within each zip code in the United States based on a North American Industry Classification System Code. Limited-service restaurants are establishments where customers select desired food or drink items and pay before eating. Different sets of administrative boundaries, such as census tracts and zip codes, do not correspond to a 1 to 1 match. Instead, a zip code may intersect multiple census tracts and census tracts may incorporate many zip codes. Therefore, it is necessary to crosswalk, or map, values at the zip code level to the census tract level.
The crosswalk was performed by merging the 2005 Business Patterns Survey based on matching zip codes with the U.S. Department of Housing and Urban Development zip code to census tract crosswalk file from the year 2012 quarter 1 [26]. Then, a file containing aggregate demographic information for all 2010 Texas census tracts was merged to the crosswalk file based on matching census tracts. Zip codes which did not have any reported limited service restaurants were assumed to have zero limited service establishments. The median number of limited service establishments per census tract was calculated by identifying the median number of establishments from all zip codes that overlapped a census tract. There was a median number of 2.0 zip codes per census tract (Q1, Q3: 1.0, 2.0; range: 0–18). Estimates of the median number of establishments per census tract was rounded to the nearest whole number.
Unhealthy food availability density (per 100,000 individuals) in 2005 was calculated by dividing the estimated and rounded number of limited-service restaurants in each census tract by the total population of each census tract and multiplying by 100,000. Then, the unhealthy food availability density was divided into quartiles. Census tracts in quartile 1 had the lowest unhealthy food availability and census tracts in quartile 4 had the highest unhealthy food availability. A sensitivity analysis was also performed in which the unhealthy food availability was categorized into above or below the median.
2.4. Census Tract-Level Covariates
Census tract covariate information was obtained from American Fact Finder (now data.census.gov). Data collected from the 2010 Census Summary File 1 included total population, number of persons 40 years of age or older overall and by males and females, median age, percent individuals who identified their race as white, and percentage of males for every census tract in Texas. Information on the percentage of individuals living below poverty was retrieved from the 2010 American Community Survey 5-year survey.
Census tract level demographic and socioeconomic covariates included median age, percent white, percent male, and percent individuals living below poverty. Covariates were categorized based on distribution of continuous values. Median age was categorized as ≤35 years of age or >35 years of age, percent white was categorized as ≤50%, >50% to ≤75%, and >75%, percent individuals below poverty was categorized as ≤10%, >10% to ≤20%, and >20%, percent male was categorized as ≤50% or >50%.
2.5. Statistical analyses
Descriptive analysis of census tract demographic and socioeconomic characteristics was performed by calculating means, standard deviations, medians, and percentages. ANOVA and chi-square tests were utilized to examine differences in census tract characteristics across different quartiles of unhealthy-food availability. Estimated annual incidence rates and 95% confidence intervals for Texas were calculated by summing the total number of persons diagnosed with colorectal cancer per year and dividing by the estimated number of Texas residents ≥40 years of age from 2005–2015. Sex-specific rates were calculated with sex-specific number of males or females diagnosed with cancer and estimated male or female population counts. Population estimates of persons ≥40 years of age, overall and by sex, were obtained from the American Community Surveys 2005–2015. Crude annual incidence rates were age-adjusted to the 2010 U.S. population aged 40 years and older using PROC STDRATE in SAS 9.4.
Generalized estimating equations (GEEs) specified with a Poisson distribution, log link function, and exchangeable correlation structure were used to model the relationship between number of cases per census tract and census tract unhealthy food availability quartiles adjusting for census tract demographic and socioeconomic covariates. All models had a specified offset of the log of the population 40 years of age and older residing in the census tract in 2010. For sex specific analyses the offset statement specified was the population 40 years of age and older for males and females residing within each census tract. By specifying an offset statement, we model cases as annual incidence rates by dividing the number of cases per year by the census tract population in 2010 who were 40 years and older.
When modelling a Poisson distribution for the observed data, overdispersion may occur. Overdispersion is when the variance of an outcome is greater than what is expected by a standard Poisson distribution and can result in smaller standard errors and more narrow confidence intervals. To account for potential overdispersion, a set of sensitivity analyses utilizing GEEs with a negative binomial distribution, log link function, and an exchangeable correlation structure was also conducted. SAS 9.4 was utilized for data management and analysis with PROC GENMOD for GEE models.
3. Results
Figure 1 shows the estimated annual age-adjusted incidence rate (per 100,000 persons) with 95% confidence intervals for individuals aged 40 years or older, overall and stratified by sex. For every year of observation, males had a higher age-adjusted incidence rate than females. In general, colorectal cancer incidence declined sharply from 2005 to 2015 for both males and females. For males, the age adjusted rate declined from 105.1 per 100,000 persons (2005) to 82.4 per 100,000 persons (2015). For females, the age adjusted colorectal cancer incidence rate declined from 80.4 (2005) to 58.4 (2015) per 100,000 persons. For both genders, there was a small increase in the incidence of colorectal cancer incidence from 2014 to 2015 (males: 78.9 to 82.4, females: 57.3 to 58.4 per 100,000 persons).
Figure 1. Estimated Age-Adjusted Colorectal Cancer Incidence in Individuals Aged 40 Years and Older in Texas from 2005 to 2015.
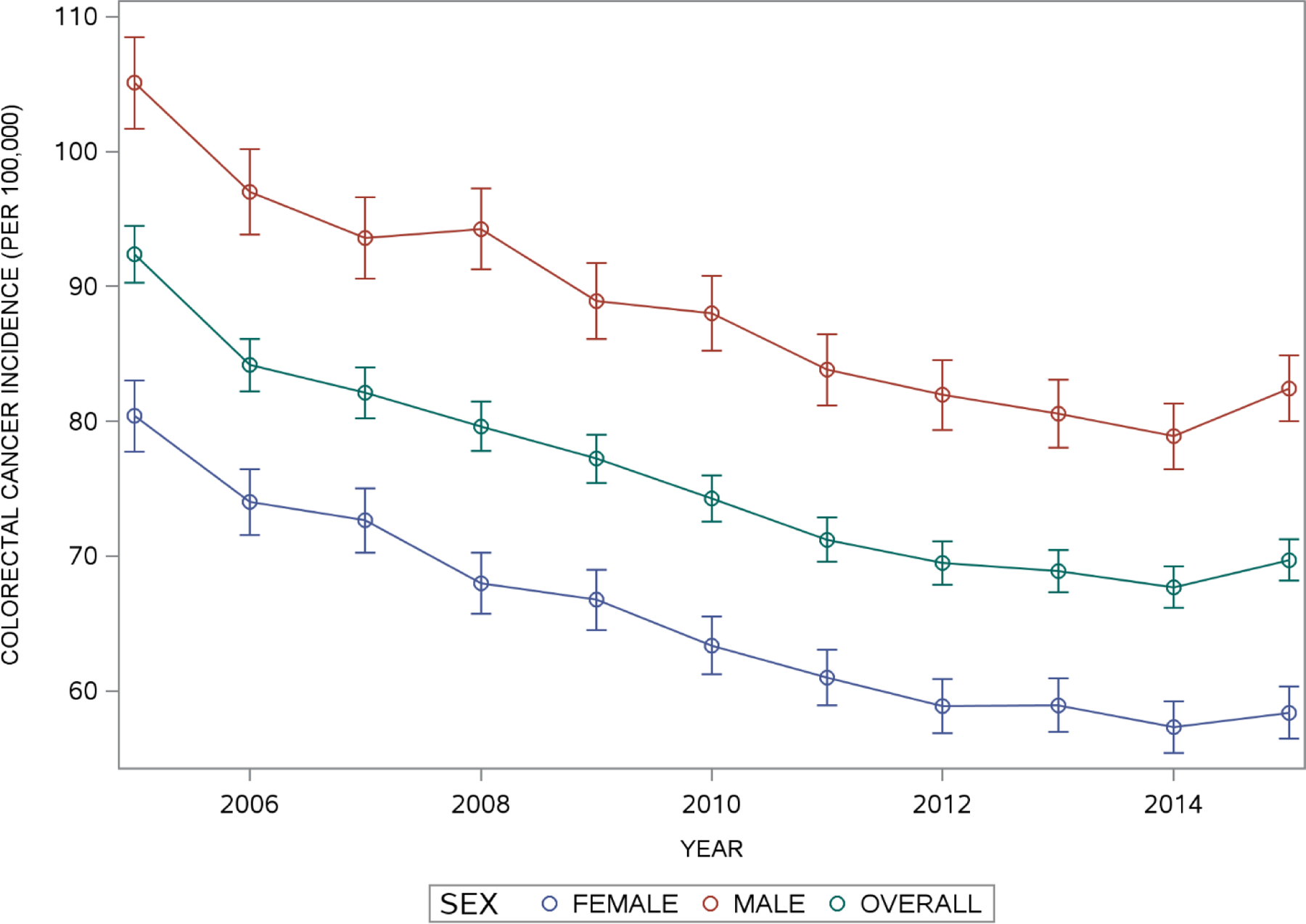
Overall and Sex specific estimates of age-adjusted colorectal cancer incidence rates and 95% confidence intervals were obtained by standardizing annual crude incidence rates for individuals aged 40 years and older to the 2010 U.S. population 40 years of age and older.
Table 1 provides information on census tract demographics, unhealthy food availability, and colorectal cancer incidence in those aged 40 years or older by quartile of unhealthy food availability. There were significant differences between unhealthy food availability quartiles with respect to census tract sociodemographic characteristics. Census tracts with low unhealthy food availability were more likely to have a higher percentage of white residents and higher percentage of male residents, were less likely to have >20% of residents living below the poverty threshold, and more likely to have a median age greater than 35 years old.
Table 1.
Sociodemographic Characteristics and Unhealthy Food Availability of Texas Census Tracts (n=5,215)
| Quartile of Unhealthy Food Availability | |||||
|---|---|---|---|---|---|
| Census tract Characteristics | Low (n = 1303) | Quartile 2 (n = 1304) | Quartile 3 (n = 1304) | High (n = 1304) | p-value |
| Unhealthy Food Availability Score Median (Q1, Q3) | 53 (19, 93) | 231 (183, 282) | 465 (392, 543) | 928 (757, 1254) | -- |
| Population aged 40+ years mean(SD) | 2425.5 (1218.9) | 2247 (903.3) | 1861.9 (716.8) | 1384.4 (657.5) | <0.0001 |
| Percent White n (%) | <0.0001 | ||||
| ≤50 | 100 (7.8) | 25 2(19.3) | 254 (19.5) | 177 (13.6) | |
| >50 to ≤75 | 292 (22.4) | 427 (32.8) | 503 (38.6) | 514 (39.4) | |
| >75 | 911 (69.9) | 625 (47.9) | 547 (42.0) | 613 (47.0) | |
| Percent Persons Living Below Poverty n(%) | <0.0001 | ||||
| ≤10 | 431 (33.1) | 438 (33.6) | 435 (33.4) | 489 (37.5) | |
| >10 to ≤20 | 504 (38.7) | 388 (29.8) | 385 (29.5) | 322 (24.7) | |
| >20 | 368 (28.2) | 478 (36.7) | 484 (37.1) | 493 (37.8) | |
| Percent Male n(%) | <0.0001 | ||||
| ≤50 | 792 (60.8) | 958 (73.5) | 967 (74.2) | 938 (71.9) | |
| >50 | 511 (39.2) | 346 (26.5) | 337 (25.8) | 366 (28.1) | |
| Median Age n(%) | <0.0001 | ||||
| ≤35 years | 502 (38.5) | 754 (57.8) | 804 (61.7) | 745 (57.1) | |
| >35 years | 801 (61.5) | 550 (42.2) | 500 (28.3) | 559 (42.9) | |
Percentages represent percentage of census tracts with a characteristic. Chi-squared test used for to test for lack of independence between unhealthy food quartiles and categorical census tract characteristics while ANOVA was used to test for equivalence of means for population aged 40 years of age and older between quartiles of unhealthy food access. Census tract characteristics were acquired from the 2010 Decennial Census.
In the overall analysis, census tracts with poverty rates >10% to ≤20% (IRR = 1.27, 95% CI: 1.24, 1.29) and >20% (IRR = 1.39, 1.35, 1.42) were significantly more likely to have higher colorectal cancer incidence than census tracts with ≤10% of persons living below the poverty threshold after adjusting for census tract sociodemographic characteristics (Table 2). Also, census tracts with a majority of the population classified as white or male had significantly lower colorectal cancer incidence while census tracts with an older median age than 35 had higher colorectal cancer incidence.
Table 2.
Multivariable Results Examining the Association Between Unhealthy Food Availability and Colorectal Cancer Incidence of Texas Census Tracts (n=5,215)
| Crude Incidence* | Overall | Male | Female | |
|---|---|---|---|---|
| Unhealthy Food Availability Quartiles | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | |
| Quartile 1 (Low) | 71.7 | REF | REF | REF |
| Quartile 2 | 71.3 | 1.03 (1.00, 1.05) | 1.03 (1.00, 1.07) | 1.03 (1.00, 1.06) |
| Quartile 3 | 72.7 | 1.02 (0.99, 1.05) | 1.04 (1.00, 1.07) | 1.01 (0.98, 1.05) |
| Quartile 4 (Highest) | 75.3 | 1.02 (0.99, 1.05) | 1.01 (0.98, 1.05) | 1.03 (1.00, 1.07) |
| p-Trend | 0.7 | 0.6 | 0.4 | |
| Percent White | ||||
| ≤50 | REF | REF | REF | |
| >50 to ≤75 | 0.94 (0.91, 0.97) | 0.98 (0.94, 1.02) | 0.90 (0.87, 0.94) | |
| >75 | 0.95 (0.92, 0.98) | 1.03 (0.99, 1.07) | 0.86 (0.83, 0.90) | |
| Percent Living Below Poverty | ||||
| ≤10 | REF | REF | REF | |
| >10 to ≤20 | 1.27 (1.24, 1.29) | 1.27 (1.24, 1.31) | 1.27 (1.23, 1.30) | |
| >20 | 1.39 (1.35, 1.42) | 1.43 (1.38, 1.48) | 1.35 (1.31, 1.39) | |
| Percent Male | ||||
| ≤50 | REF | REF | REF | |
| >50 | 0.94 (0.92, 0.96) | 0.91 (0.88, 0.93) | 0.96 (0.93, 0.98) | |
| Median Age | ||||
| ≤35 years | REF | REF | REF | |
| >35 years | 1.16 (1.14, 1.19) | 1.13 (1.10, 1.16) | 1.20 (1.17, 1.23) |
Models were also adjusted for calendar year (categorical: 2005–2015). Incidence Rate Ratios (IRR) were calculated by utilizing generalized estimating equations with Poisson distribution, log link function, and an exchangeable correlation structure. Offset statement was added for the log of census tract population 40 years of age and older to allow modeling number of cases as rates.
Bolded values indicate significant difference from reference group, p<0.05.
Note: Overall crude colorectal cancer incidence for 2010 is reported in rates per 100,000 persons.
In the overall unadjusted analysis, there were no significant differences in colorectal cancer incidence between the lowest unhealthy food availability and quartile 2 (IRR = 1.02, 95% CI: 0.99, 1.05), quartile 3 (IRR = 1.01, 95% CI: 0.99, 1.04), and quartile 4 (IRR = 1.01, 95% CI: 0.98, 1.04; Table A.1). Multivariable results examining the association between unhealthy food availability and colorectal cancer incidence are presented in Table 2. In the overall analysis, compared to the lowest availability of unhealthy food, census tracts in quartile 2 were significantly more likely to have a higher incidence of colorectal cancer (IRR = 1.03, 95% CI: 1.00, 1.05), after adjustment for census tract sociodemographic characteristics. However, census tracts in quartile 3 (IRR = 1.02, 95% CI: 0.99, 1.05) and quartile 4 (Quartile 4: IRR = 1.02, 95% CI: 0.99, 1.05) did not have significantly higher or lower colorectal cancer incidence than census tracts with the lowest unhealthy food availability after adjustment for census tract sociodemographic characteristics. Results with above the median unhealthy food availability compared to below revealed no significant differences (IRR = 1.01, 95% CI: 0.99, 1.02, results not shown).
Table 2 also presents multivariable results stratified by sex. There was no statistically significant relationship between unhealthy food availability and colorectal cancer incidence for females. Male specific rates of colorectal cancer were highest in census tracts in the third quartile of unhealthy food availability compared to the lowest availability (IRR = 1.04, 95% CI: 1.00, 1.07) but not in the second (IRR = 1.03, 95% CI: 1.00, 1.07) or fourth (IRR = 1.01, 95% CI: 0.98, 1.05) quartiles compared to census tracts with the lowest availability of unhealthy food. Sensitivity analysis results utilizing a negative binomial distribution were similar (Table A.2).
4. Discussion
Census tracts with higher rates of poverty were found to have higher colorectal cancer incidence. However, there was no consistent association between unhealthy food availability and colorectal cancer incidence. The link between poverty and colorectal cancer incidence has been well established[[27], [28], [29]]. Policies and inequitable access to and distribution of health related resources across communities can result in disparities of colorectal cancer incidence by affecting health insurance coverage rates and colorectal cancer screening rates[[29], [30]].
This study builds on the findings of existing ecological studies of nutrition and colorectal cancer incidence by examining the potential influence of the food environment on colorectal cancer incidence[[16], [17], [18], [19], [20], [21], [22]]. Two possible reasons that an association between the food environment and colorectal cancer incidence were not observed are 1) administrative boundaries may be inadequate proxies for the food environment[[31], [32]], and 2) the availability of unhealthy foods may be modified by factors such as transportation, and dietary preferences[33]. Overall, our results are consistent with one previous prospective cohort study which did not find an association between the food environment within a 1-mile network buffer of an individual’s residence and being diagnosed with colorectal cancer[23].
This study has several strengths. First, it used a population-based registry to capture cases of colorectal cancer with a large minority population. Second, it used a majority (99%) of available census tracts in Texas, which is geographically diverse with large urban and rural areas and examined the relationship between unhealthy food availability in 2005 and colorectal cancer incidence from 2005 to 2015 at the census tract level. One important strength is that an ecological study design allows researchers to examine the relationship between a wide range of exposure and the outcome of interest.
This study has several limitations. First, the findings of an ecological study cannot be used to infer an individual’s risk of developing malignant colorectal cancer or provide information about the biological, sociological, or behavioral mechanisms. Ecological fallacy results when an inference is made about an individual’s risk of disease based on results of aggregated data[34]. Second, utilization of administrative boundaries, like census tracts and zip codes, to quantify environmental exposures can be problematic. These boundaries were developed by government agencies for non-health research purposes and may not be representative of an individual’s residential environment[[35], [36]]. Network buffers, as used by Canchola et al. (2017), may be more accurate proxies for a person’s residential environment. Despite the limitations of using administrative boundaries we did observe significant differences of colorectal cancer incidence across poverty levels.
A third limitation is the potential misclassification of unhealthy food availability. We used the Business Patterns Survey from 2005 to identify limited service restaurants. These stores were operationalized as unhealthy food stores to help quantify the exposure, but this study did not have any information on the food options provided at included stores. One source of potential non-differential misclassification in this study is crosswalking the zip code level exposure to census tracts. Generally, non-differential misclassification of exposure results in estimates being biased toward the null, but in the context of an ecological design with a categorical independent variable, we cannot exclude the possibility of overestimating the effect size[37]. Furthermore, there is not a systematic definition for grouping food stores making it difficult to compare and reproduce research findings[38]. North American Industry Classification System (NAICS) Codes are commonly used to classify stores, but literature has provided more refined systematic methods to group food stores using NAICS codes[[38], [39]]. Lastly, limited service restaurants are only one, non-specific aspect of the food environment. Residential food environments and availability can be better characterized using multiple food sources, such as grocery stores or farmers markets. Fourth, since an ecological study design is an observational study, it is possible that there are important confounding factors that were unmeasured and unaccounted for which can affect the estimates of the food environment with colorectal cancer incidence. Furthermore, categorizing continuous variables, as was done in this study increases the likelihood of residual confounding and false positives, and results in a loss of power[[40], [41]].
5. Conclusion
In conclusion, we did not demonstrate an association between unhealthy food availability and colorectal cancer incidence but did observe disparities across poverty levels. Efforts to limit availability of unhealthy food stores may not be beneficial for reducing colorectal cancer incidence. Future research investigating the food environment and cancer outcomes should develop and validate geographic measures for the food environment and explore associations across different neighborhood boundaries and buffer distances[42]. However, ensuring equitable distribution of health resources and healthy environments for all communities can be a major step towards improving disparities in colorectal cancer incidence.
Supplementary Material
Highlights:
Unhealthy food availability was weakly associated with colorectal cancer incidence
Higher rates of poverty were associated with higher colorectal cancer incidence
Research should examine how the built environment influences colorectal cancer risk
Acknowledgments
We would like to express our gratitude of the assistance we received from the epidemiologists at the Texas Cancer Registry. We would also like to thank the anonymous reviewers who provided thorough comments and feedback to improve the manuscript.
Research funding: Derrick C. Gibson is funded by the T32HS02613301 (Agency for Healthcare Research and Quality, PI: Kuo)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Conflicts of Interest: Derrick C Gibson has no conflicts of interest to disclose. John D. Prochaska has no conflicts of interest to disclose. Xiaoying Yu has no conflicts of interest to disclose. Sapna Kaul has no conflicts of interest to disclose.
References
- [1].Key Statistics for Colorectal Cancer. American Cancer Society https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. Published January 24, 2019. Accessed November 10, 2019.
- [2].Chao A, Thun MJ, Connell CJ, et al. Meat Consumption and Risk of Colorectal Cancer. JAMA 2005;293(2):172–182. 10.1001/jama.293.2.172 [DOI] [PubMed] [Google Scholar]
- [3].Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. International Journal of Cancer 2006;119(11):2657–2664. 10.1002/ijc.22170 [DOI] [PubMed] [Google Scholar]
- [4].van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2009;89(5):1441–1452. 10.3945/ajcn.2008.27120 [DOI] [PubMed] [Google Scholar]
- [5].Wu QJ, Yang Y, Vogtmann E, et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 2013;24(4):1079–1087. 10.1093/annonc/mds601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moore LV, Diez Roux AV, Nettleton JA, Jacobs DR. Associations of the Local Food Environment with Diet Quality—A Comparison of Assessments based on Surveys and Geographic Information SystemsThe Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2008;167(8):917–924. 10.1093/aje/kwm394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Franco M, Diez-Roux AV, Nettleton JA, et al. Availability of healthy foods and dietary patterns: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2009;89(3):897–904. 10.3945/ajcn.2008.26434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morland K, Wing S, Roux AD. The Contextual Effect of the Local Food Environment on Residents’ Diets: The Atherosclerosis Risk in Communities Study. Am J Public Health 2002;92(11):1761–1768. 10.2105/AJPH.92.11.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zenk SN, Lachance LL, Schulz AJ, Mentz G, Kannan S, Ridella W. Neighborhood Retail Food Environment and Fruit and Vegetable Intake in a Multiethnic Urban Population. Am J Health Promot 2009;23(4):255–264. 10.4278/ajhp.071204127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morland KB, Evenson KR. Obesity prevalence and the local food environment. Health & Place 2009;15(2):491–495. 10.1016/j.healthplace.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andrew Rundle, Neckerman Kathryn M., Lance Freeman, et al. Neighborhood Food Environment and Walkability Predict Obesity in New York City. Environmental Health Perspectives 2009;117(3):442–447. 10.1289/ehp.11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Auchincloss AH, Roux AVD, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. Neighborhood Resources for Physical Activity and Healthy Foods and Incidence of Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis. Arch Intern Med 2009;169(18):1698–1704. 10.1001/archinternmed.2009.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med 2015;175(8):1311–1320. 10.1001/jamainternmed.2015.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salois MJ. Obesity and diabetes, the built environment, and the ‘local’ food economy in the United States, 2007. Economics & Human Biology 2012;10(1):35–42. 10.1016/j.ehb.2011.04.001 [DOI] [PubMed] [Google Scholar]
- [15].Kaiser P, Diez Roux AV, Mujahid M, et al. Neighborhood Environments and Incident Hypertension in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2016;183(11):988–997. 10.1093/aje/kwv296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ganjavi M, Faraji B. Late effect of the food consumption on colorectal cancer rate. International Journal of Food Sciences and Nutrition 2019;70(1):98–106. 10.1080/09637486.2018.1472747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jansen MCJF Bueno-de-Mesquita HB, Buzina R, et al. Dietary fiber and plant foods in relation to colorectal cancer mortality: The Seven Countries Study. International Journal of Cancer 1999;81(2):174–179. [DOI] [PubMed] [Google Scholar]
- [18].Kono S Secular trend of colon cancer incidence and mortality in relation to fat and meat intake in Japan. European Journal of Cancer Prevention 2004;13(2):127–132. [DOI] [PubMed] [Google Scholar]
- [19].Koo LC, Mang OWK, Ho JH‐C. An ecological study of trends in cancer incidence and dietary changes in Hong Kong. Nutrition and Cancer 1997;28(3):289–301. 10.1080/01635589709514590 [DOI] [PubMed] [Google Scholar]
- [20].Ognjanovic S, Yamamoto J, Maskarinec G, Marchand LL. NAT2, meat consumption and colorectal cancer incidence: an ecological study among 27 countries. Cancer Causes Control 2006;17(9):1175. 10.1007/s10552-006-0061-3 [DOI] [PubMed] [Google Scholar]
- [21].Stoneham M, Goldacre M, Seagroatt V, Gill L. Olive oil, diet and colorectal cancer: an ecological study and a hypothesis. Journal of Epidemiology & Community Health 2000;54(10):756–760. 10.1136/jech.54.10.756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang CX, Kuroishi T, Huang XE, Inoue M, Tajima K. Correlation between Food Consumption and Colorectal Cancer: An Ecological Analysis in Japan. Asian Pac J Cancer Prev 2002;3(1):77–83. [PubMed] [Google Scholar]
- [23].Canchola AJ, Shariff-Marco S, Yang J, et al. Association between the neighborhood obesogenic environment and colorectal cancer risk in the Multiethnic Cohort. Cancer Epidemiology 2017;50:99–106. 10.1016/j.canep.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Story M, Kaphingst KM, Robinson-O’Brien R, Glanz K. Creating Healthy Food and Eating Environments: Policy and Environmental Approaches. Annual Review of Public Health 2008;29(1):253–272. 10.1146/annurev.publhealth.29.020907.090926 [DOI] [PubMed] [Google Scholar]
- [25].Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109(8). 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].HUD USPS ZIP Code Crosswalk Files | HUD USER U.S. Department of Housing and Urban Development. https://www.huduser.gov/portal/datasets/usps_crosswalk.html. Accessed June 10, 2019. [Google Scholar]
- [27].Siegel RL, Jemal A, Thun MJ, Hao Y, Ward EM. Trends in the Incidence of Colorectal Cancer in Relation to County-Level Poverty among Blacks and Whites. Journal of the National Medical Association 2008;100(12):1441–1444. 10.1016/S0027-9684(15)31544-3 [DOI] [PubMed] [Google Scholar]
- [28].Henry KA, Sherman RL, McDonald K, et al. Associations of Census-Tract Poverty with Subsite-Specific Colorectal Cancer Incidence Rates and Stage of Disease at Diagnosis in the United States. Journal of Cancer Epidemiology 10.1155/2014/823484 [DOI] [PMC free article] [PubMed]
- [29].Hao Y, Jemal A, Zhang X, Ward EM. Trends in colorectal cancer incidence rates by age, race/ethnicity, and indices of access to medical care, 1995–2004 (United States). Cancer Causes Control 2009;20(10):1855. 10.1007/s10552-009-9379-y [DOI] [PubMed] [Google Scholar]
- [30].Lian M, Schootman M, Yun S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health 2008;8(1):358. 10.1186/1471-2458-8-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coulton CJ, Korbin J, Chan T, Su M. Mapping Residents’ Perceptions of Neighborhood Boundaries: A Methodological Note. Am J Community Psychol 2001;29(2):371–383. 10.1023/A:1010303419034 [DOI] [PubMed] [Google Scholar]
- [32].Coulton CJ, Jennings MZ, Chan T. How Big is My Neighborhood? Individual and Contextual Effects on Perceptions of Neighborhood Scale. American Journal of Community Psychology 2013;51(1–2):140–150. 10.1007/s10464-012-9550-6 [DOI] [PubMed] [Google Scholar]
- [33].Inagami S, Cohen DA, Brown AF, Asch SM. Body Mass Index, Neighborhood Fast Food and Restaurant Concentration, and Car Ownership. J Urban Health 2009;86(5):683–695. 10.1007/s11524-009-9379-y34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Piantadosi S, Byar DP, Green SB The ecological fallacy Am. J. Epidemiol, 127 (5) (1988), pp. 893–902 [DOI] [PubMed] [Google Scholar]
- [35].Nicotera N Measuring Neighborhood: A Conundrum for Human Services Researchers and Practitioners. Am J Community Psychol 2007;40(1):26–51. 10.1007/s10464-007-9124-1 [DOI] [PubMed] [Google Scholar]
- [36].Rainham D, McDowell I, Krewski D, Sawada M. Conceptualizing the healthscape: Contributions of time geography, location technologies and spatial ecology to place and health research. Social Science & Medicine 2010;70(5):668–676. 10.1016/j.socscimed.2009.10.035 [DOI] [PubMed] [Google Scholar]
- [37].Brenner H, Savitz DA, Jöckel K-H, Greenland S. Effects of Nondifferential Exposure Misclassification in Ecologic Studies. Am J Epidemiol 1992;135(1):85–95. 10.1093/oxfordjournals.aje.a116205 [DOI] [PubMed] [Google Scholar]
- [38].Gamba RJ, Schuchter J, Rutt C, Seto EYW. Measuring the Food Environment and its Effects on Obesity in the United States: A Systematic Review of Methods and Results. J Community Health 2015;40(3):464–475. 10.1007/s10900-014-9958-z [DOI] [PubMed] [Google Scholar]
- [39].Ohri-Vachaspati P, Martinez D, Yedidia MJ, Petlick N. Improving Data Accuracy of Commercial Food Outlet Databases. Am J Health Promot 2011;26(2):116–122. 10.4278/ajhp.100120-QUAN-21 [DOI] [PubMed] [Google Scholar]
- [40].Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Statistics in Medicine 2006;25(1):127–141. 10.1002/sim.2331 [DOI] [PubMed] [Google Scholar]
- [41].Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol 2012;12(1):21. 10.1186/1471-2288-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lytle LA, Sokol RL. Measures of the food environment: A systematic review of the field, 2007–2015. Health & Place 2017;44:18–34. 10.1016/j.healthplace.2016.12.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


