Abstract
Mandibular reconstructions are complex clinical pictures that require careful planning for functional and aesthetic outcomes. Virtual surgical planning and 3D printing are ideal to achieve a predictable result. Through “hybrid techniques” (prebending plates with 3D‐models) and free software, this goal is within reach for clinics with limited financial resources.
Keywords: computer‐assisted surgery, iliac crest bone graft, mandibular reconstruction, osteomyelitis, surgical guide, virtual planning
Mandibular reconstructions are complex clinical pictures that require careful planning for functional and aesthetic outcomes. Virtual surgical planning and 3D printing are ideal to achieve a predictable result. Through “hybrid techniques” (prebending plates with 3D‐models) and free software, this goal is within reach for clinics with limited financial resources.
1. INTRODUCTION
Mandibular reconstruction after trauma or pathology is one of the cornerstones of oral and maxillofacial surgery. 1 This reconstruction is needed in cases with a large amount of bone loss, comminute fractures, severe traumas, and infections leading to multiple bone sequestrations. 2
In the case of infections of the bone, different risk factors may enhance the speed in which the bone is lost, such as age, sex, poor oral hygiene, comorbidities (diabetes, hyperlipemia, autoimmune diseases), and drug abuse (cocaine, cannabinoid, tobacco smoking, hepatic cirrhosis due alcoholism). 3 , 4 , 5 , 6 , 7 , 8
The four basic principles of successful reconstruction are (a) establish an ideal orthognathic relationship; (b) a flush bone to graft/flap contact; (c) stable bony fixation; and (d) adequate, well‐vascularized soft tissue coverage. 1
To achieve the previously established principles, the maxillofacial literature describes different surgical treatment plans. One temporary option is the use of external fixation of the mandible. It is considered a closed reduction type and provides semirigid fixation for fractured segments. 2 One of its main advantages is to minimize the possible complications when compared to open surgical treatment for reduction and stabilization of fractures. 9
Sometimes, the fractured bone contact cannot be achieved due to the level of damage. In such cases, grafts may work as a bridge to achieve fusion. Oral reconstruction is a difficult task because of the anatomical, functional, and aesthetic aspects that must be considered in the surgery. Autogenous bone is the only graft material that possesses osteoconductive, osteo‐inductive, and osteogenic potential. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10
This kind of surgical treatment is widely performed in cases such as that reviewed in this case study. Technology has allowed maxillofacial dentists to improve surgical processes over the last few years. 1 Virtual surgical planning, computer‐aided manufacturing, and 3D printing give the surgeon multiple advantages such as mirroring the anatomy of the unaffected side, planning osteotomies, manipulating bony segments, fabricating surgical resection guides, and creating reconstruction plates. 1
This case study reports a jaw reconstruction in a 52‐year‐old male patient after a previous surgical reconstruction due to trauma that led to a case of osteomyelitis with severe bone loss on the body of the mandible.
2. CASE HISTORY
In July 2018, a 51‐year‐old male patient was referred to the Department of Oral & Maxillofacial Surgery of the Salvador Hospital, Santiago, Chile. In the clinical history, the patient did not present any comorbidities or prescribed drugs; however, a long history of substance abuse such as alcohol, cocaine, cannabinoids, crack, and tobacco was present. With regard to his surgical history, in 2016 the patient suffered a mandible fracture which was surgically fixated.
When he arrived at the Salvador Hospital, he presented an active submandibular cutaneous fistula and left deviation of the jaw. Intraorally, poor hygiene, severe maxillo‐mandibular discrepancy, and malocclusion were found. Both posterior crossbite and the dental midline deviation were on the left side due to a shortening of the mandibular body in the premolar area. In maximal intercuspation, only left posterior molars were in contact (Figure 1A‐E). A computed tomography (CT) was requested, where an extended bone loss was observed around the left canine and molars. Also, a nonfixated plate could be seen in the same area (Figure 2A‐C).
FIGURE 1.
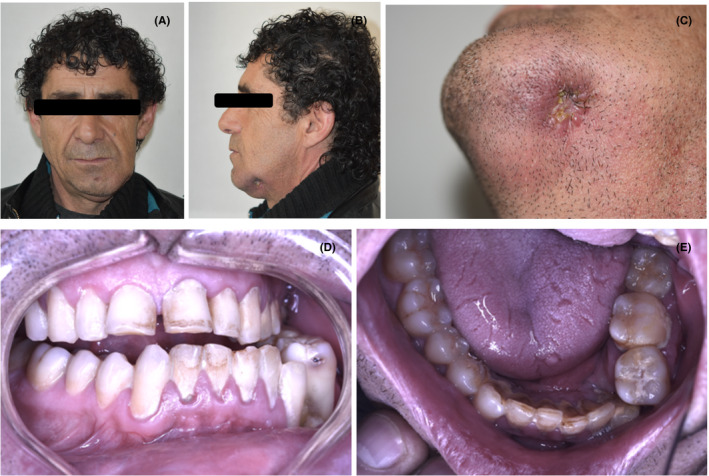
A‐C, Patient on admission, extraoral view. D‐E, Intraoral view of the patient with osteomyelitis associated to mandibular fracture
FIGURE 2.
A‐C, Computed tomography on admission, with bone loss of the left mandibular body
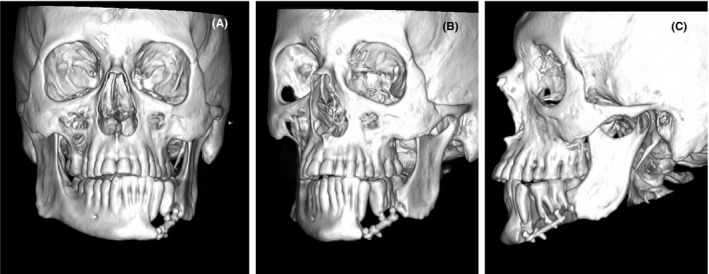
The diagnosis was a suppurative osteomyelitis that went from the left mandibular canine to the second molar of the same side. An extensive surgical cleaning, together with the extraction of the affected bone, teeth, and plate were performed. Two new reconstruction plates, 2.0 and 2.4 profile (Trauma One, ZimmerBiomet. Santiago, Chile), were used to stabilize the remaining mandibular segments (Figure 3A‐C).
FIGURE 3.
A‐C, First surgery postoperative computed tomography, with reconstruction plates
The patient returned in August 2019 complaining of mandibular pain. He reported self‐removal of a loose metallic fragment inside his oral cavity. The patient presented a loosened reconstruction plate that had broken through the oral mucosa and the skin of the chin. In addition, alteration of the mandibular dynamics was observed, caused by a lack of stabilization between the remaining mandibular fragments. (Figure 4A‐B) A new CT showed that only the distal part of the broken 2.0 plate remained and only the posterior part of the 2.4 plate was fixed to the bone. Loss in the contour of the mandible was evidenced both clinically and radiographically. (Figure 4C‐D).
FIGURE 4.
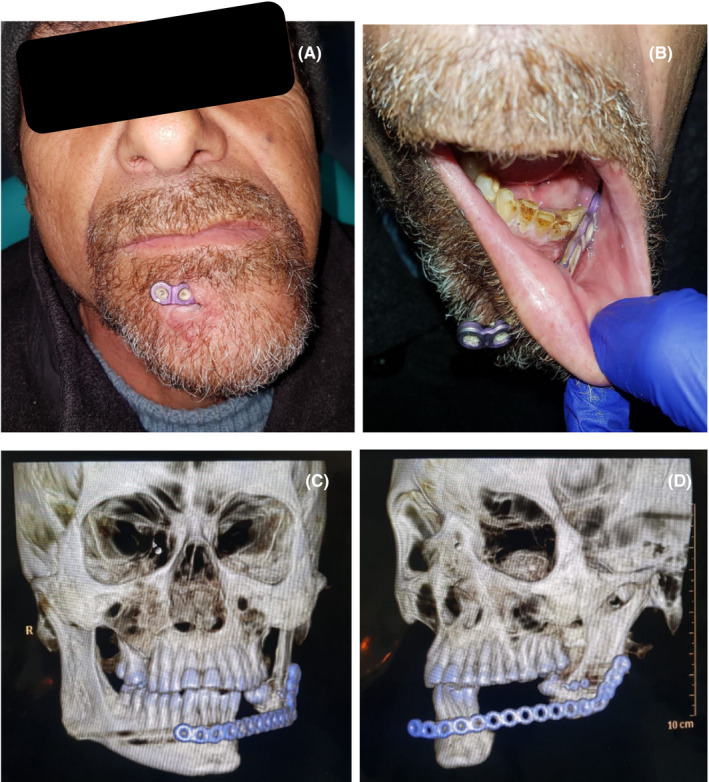
A‐B, Patient after 1 year of first surgery, intra‐ and extraoral view. C‐D, Computed tomography with broken 2.0 plate and lack of anterior fixation of the 2.4 plate
Due to the extensive bone loss, instability of the mandible, and acute symptoms of the patient, it was decided to remove both reconstruction plates, perform surgical cleaning and placement of four external fixations for two months (Kanhui, Empresa Tecnomedical, Santiago, Chile). Two external fixations were placed in the mandibular body of the unaffected side and the remaining two in the left side, more specifically in the condyle and ramus. (Figure 5A‐B).
FIGURE 5.
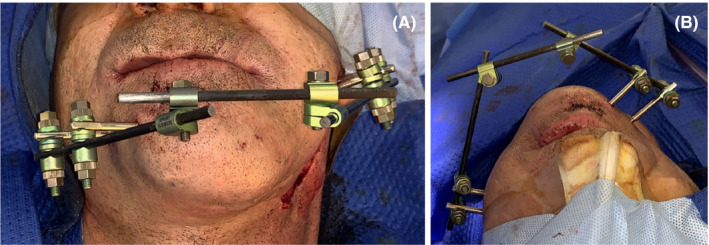
A‐B, Postoperative view with external fixations
During these two months, a three‐dimensional model of the patient's cranium (including maxilla) and mandible was manufactured to preform a 2.4 surgical plate (Kanhui, Empresa Tecnomedical, Santiago, Chile).
The process of preparing the 3D model for surgical preparation involved the following steps: Converting a DICOM file into an STL file, preparing the STL file for printing and then 3D print and postprocess the model.
Starting with the DICOM file, the authors used the free access software 3DSlicer version 4.10.2 to segment the image in its individual parts. Manual segmentation, which is labor intensive, is commonly used for defining the region of interest from the original CT volume by using a slice‐by‐slice approach. Several automatic and semiautomatic segmentation methods have been proposed, among them thresholding to replace manual segmentation and reduce image processing time. This approach is used here with a threshold range of 100‐2700 (Figure 6) to virtually remove metallic artifacts (external fixators), bone marrow, and vertebras present in the original image. Although, this approach is simple, it allows for a rapid elimination of most artifacts. The remaining parts of interest (cranium, mandible, teeth) are then exported as three‐dimensional surfaces in the STL file format.
FIGURE 6.
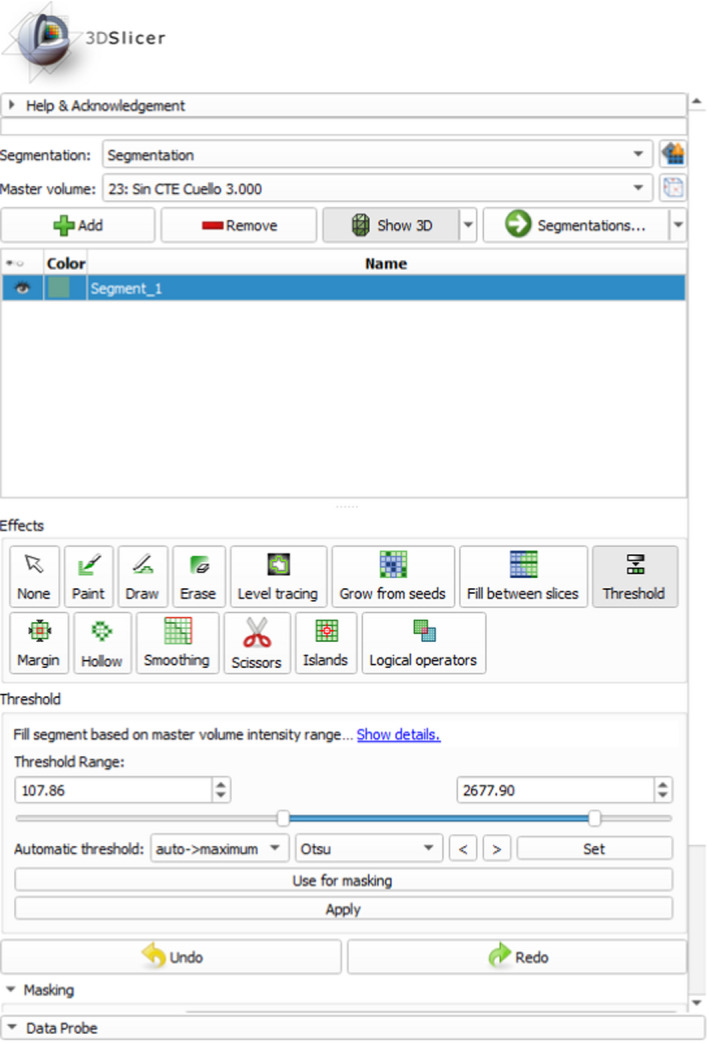
3D Slicer segmentation threshold range
Further manipulation of the .stl file is made using the free to use Autodesk ® MeshMixer™ program. MeshMixer allows for the manual removal of some residual artifacts. Additionally, the bone anatomy of the unaffected side was copied and mirrored to allow for a virtual reconstruction of the bone defect in which the affected ramus was mesially repositioned into the glenoid fossa and segments were modified to re‐establish the dental midline (Figure 7A‐C).
FIGURE 7.
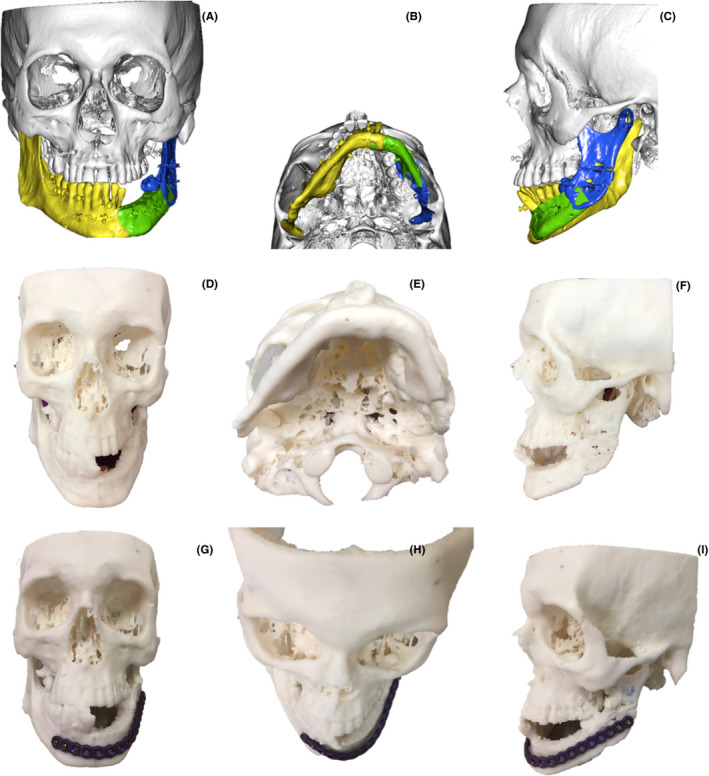
A‐C, Computer‐assisted planning. D‐I, Epoxy resin 3D Printing model, extraoral preformation of the surgical plate
The physical 3D model was printed using a Form 2 printer (Formlabs, Somerville‐Massachusetts, USA), and so required that the prepared STL file was exported to the FormLABS PreForm software to prepare for printing. The 3D model was made using standard Formlabs white resin with a 0.1mm layer height. The print took 35 hours to be completed, and it was then washed and cured before being used to preform the surgical plate. The washing and curing process were done in Formlabs Wash and Cure equipment allowing for a fully automatized postprocessing. The physical 3D model was then handed in to the surgical team for preforming of the surgical plate.
Following the virtual surgical planning, the patient was hospitalized and prepared for surgery. Both procedures, anterior iliac crest graft removal and the mandibular reconstruction, were performed in one operatory step. (Figure 8A) For the mandible reconstruction, skin incisions in the base of the mandible and osteomyocutaneous flaps were made to expose the remaining mandibular bone. Limited resection of the irregular bony margins of the defect was made to provide an optimal graft‐mandible interface. (Figure 8B). The already preformed surgical plate was placed and fixed in sequence for optimal adaptation. (Figure 8C). The preformed iliac crest graft combined with Adbone TCP 3 ‐ 4 mm particulate Xenograft (Kanhui, Empresa Tecnomedical, Santiago, Chile) was placed in the defect and fixed to the plaque with pins located 1.5 cm apart from each other (Figure 8D‐E).
FIGURE 8.
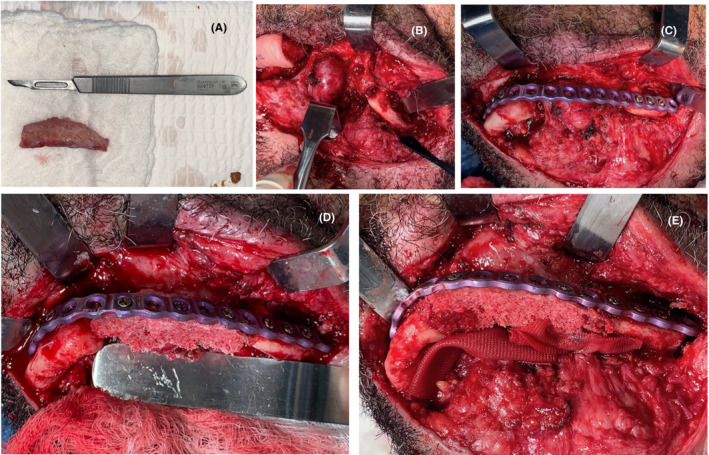
A, Nonvascularized iliac crest graft. B, First approach of the final surgery. C, Preformed reconstruction plate fixed to the mandibular segments. D‐E, Nonvascularized iliac crest graft with xenograft fixed to the plate
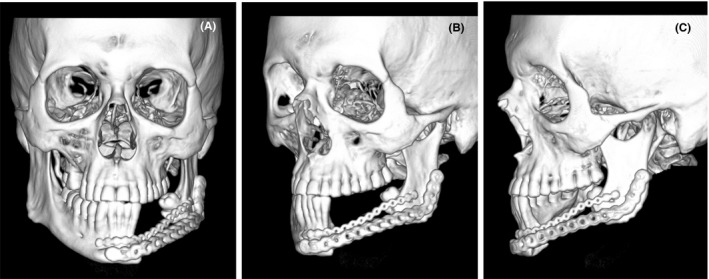
After the surgery, the patient stayed in the hospital 10 days, maintaining a strict hygiene of the skin in the postoperative period and pharmaceutical therapy of 600 mg Clindamycin each 8 h and 1 g Ceftriaxone every 24 h, both intravenous for the first 4 days and only 300 mg oral Clindamycin every 8 h for the next 14 days. He was called for a control two weeks after leaving the hospital, but he did not attend and returned after 3 months instead, where a clinical examination was performed, and a control CT was requested. In the extraoral examination, there was still some residual postsurgical inflammation; nevertheless, there was a decrease in the inflammation that caused facial asymmetry, in addition to the scar, and the soft tissues were healthy and aesthetically adequate (Figure 9A‐B). Intraorally, no signs of periodontal disease, halitosis, erythema, or suppuration were found, the oral mucosa was healthy, symmetrical dental midline, Angle's molar Class I with Class I canine relationship in the right side was achieved (Figure 9C‐D). The mandibular dynamic was asymptomatic with acceptable mandibular range movement and suitable oral functions according to the patient. Regarding the control CT, the graft was successfully preserved, and the plaque maintained the proper mandibular arch relationship. (Figure 10A‐D).
FIGURE 9.
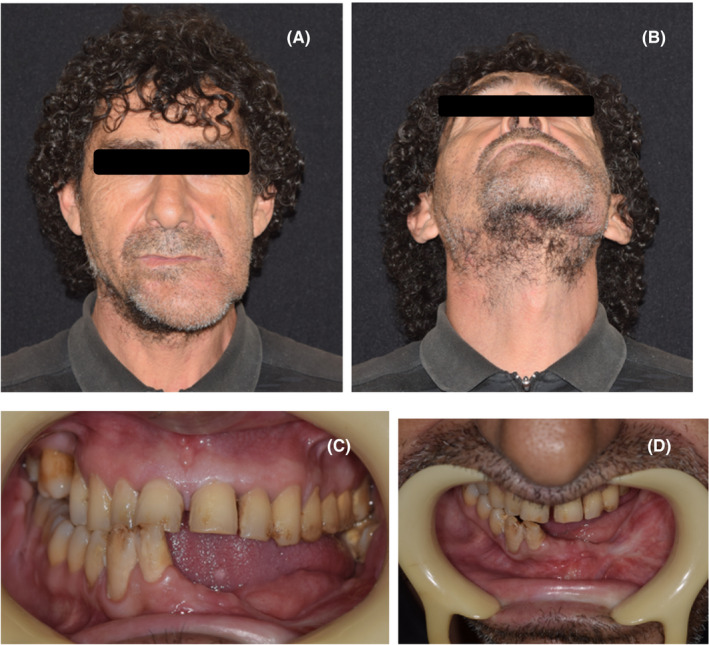
A‐B, 3‐month control after final surgery, extraoral view. C‐D, 3‐month control after final surgery, intraoral view
FIGURE 10.
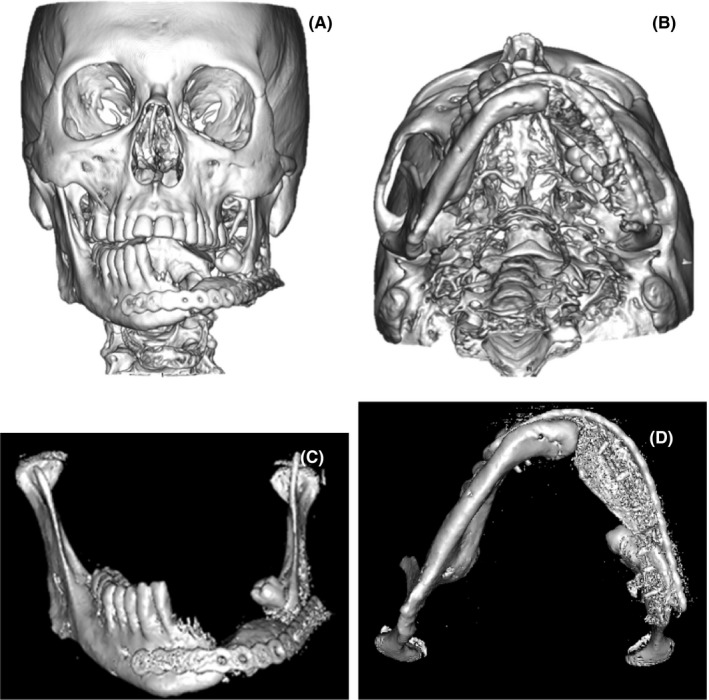
A‐B, 3 months after final surgery, control CT. C‐D, 5 months after final surgery, isolated mandible
3. DISCUSSION
In any type of bone trauma and fracture, there are different factors that must be considered for a successful intervention. In mandibular fractures, the reconstruction must be focused to maximize function and to produce an aesthetically pleasing appearance following primary or secondary surgery. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11
Particularly in this case, the first surgical approach was not successful, deriving in a case of Osteomyelitis with uncomfortable symptoms and complications for the patient and requiring further surgical process. Postsurgical osteomyelitis of the mandible is rare and appears to affect more men than women. The caseload of jaw osteomyelitis seems to have decreased considerably over the last fifty years thanks to the progress made in the field of oral hygiene, the appearance and use of antibiotics, and early screening. 12
In this case, the patient had multiple risk factors for mandibular fracture which should have been identified to prevent the later osteomyelitis. One of them was the smoking habit. Research shows that smoking may have detrimental effects on the skeletal system, leading to lower bone mass and mineral density. Furthermore, smokers submitted to orthopedic surgery have an increased risk of delayed fracture healing, complications (eg, infections and nonunion fractures), and longer hospital stays. 8 In addition, the patient had alcohol induced hepatic cirrhosis that tends to deteriorate both trabecular and cortical bone microarchitecture increasing the susceptibility to low‐trauma fractures. The cannabinoid and cocaine abuse must also be emphasized. Studies have shown that the central nervous system, including neurons and glial cells, plays an important role in regulating bone metabolism and therefore in the pathology of osteoporosis through cannabinoid receptors (CB1 and CB2), which are involved in pain modulation. 6 Cocaine causes both local anesthesia and vasoconstriction. The vasoconstriction may lead to necrosis, chemical irritation, mechanical trauma, bacterial infection, immunosuppression, and osteoblast inhibition. The combination of decreased oxygen tension, inflammation, irritation, and open wounds creates an ideal environment for infection with common. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13
The abuse of multiple drugs and the presence of comorbidities in this case may have increased the susceptibility of the patient to the osteomyelitis after his first intervention and later poor osseointegration of the surgical plates after the second surgery.
In this case, a two‐step surgery with external fixations was chosen, due to the conditions in which the patient arrived at the Hospital. The research on the field of external fixations shows that the main indications for its use are large bone loss caused by tumor resections, infections leading to multiple bone sequestration, comminution, and severe trauma with substance loss. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 In cases such as this, where large bone defects are present, a first approach with internal fixation surgery has an inherent high risk of nonunion and infections. The aim of the external fixators is to maintain the best patient anatomy and reduce pain, before they can benefit from optimal secondary reconstructive surgical procedure. 14 It must also be considered that, because of the general nature and severity of the mandibular fractures treated by external fixation, a high complication rate of up to 35% has been reported. Postoperative infections, cellulitis around the pins, nonunions, malocclusions, and pin loosening are potentially frequent with this fixation technique. External fixation remains a quick, safe, and simple method to treat mandible fractures in selected clinical situations. 15
Recently, computer‐assisted navigation technology has become common in many medical fields. Its advantages include improvements in the accuracy of maxillofacial bone surgery, avoiding injury to vital structures, shortening of the operation time, reducing flap ischemia duration, and is cost‐effective, despite the expensive technology. 1 , 15 , 16 Maxillofacial reconstructive surgery requires a high standard of surgical precision. Dimensional errors are low in 3D printing, with discrepancies between STL models and 3D‐printed models typically smaller than an imaging voxel (< 1 mm), being highly accurate in the surgical planification, compared with conventional surgery. 16 Also, computer‐assisted surgery (CAS) offers the ability to plan osteotomies, mirror the unaffected mandible, evaluate the bone plate relationships for positioning of dental implants, create surgical resection guides, and fabricate patient‐specific reconstruction plates. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 The mandibular reconstruction surgery assisted by virtual planning and 3D printing is highly predicable over time. 17 New technologies are widely spreading in the surgical planning field due to the successful outcomes reported by the literature. Furthermore, free software is now appearing, giving clinics with limited financial resources the chance to use this technology. 18
In cases of large bone loss such as the one seen in this case report, the use of VSP and CAS could be beneficial for predictive outcomes and high standard aesthetics and functionality in the maxillofacial area.
The nonvascularized iliac crest graft was chosen for this case for multiple reasons. The main reason being the severe compromises of blood vessels due to previous surgeries, restricting the chances of a successful anastomosis in a vascularized graft. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 In this case, the facial skin envelope and soft tissue volume was preserved, so no soft tissue graft was needed. 1 Given the size of the bone loss (6cm), the iliac crest graft was used because of the high success rate in less than 7cm defects, presentation of a high‐quality bone bulk, large amounts of bone marrow, and resemblance with the curved lateral surface of the mandible. 10 Iliac crest donor site can be the first choice in isolated, short segment bone defects of mandible without soft tissue defect, which are resulted from trauma or tumor resection. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Also, nonvascularized iliac crest bone grafts used to reconstruct defects of 7 cm or less have an 83% success rate. 1 In future cases like this one, it would be recommended to prepare a virtual bone block that could be later produced by a 3D printer to use as a surgical guide during the bone harvesting. It could be very helpful to achieve an ideal graft form and size to adapt to the defect.
In conclusion, multiple factors can be extrapolated from this case such as habits, comorbidities, and personal adhesion to therapy may be important in the prognosis of the final treatment. External fixations could be a good intermediate alternative for patients that require complex surgery, which may be previously planned due to virtual surgical planning with 3D printing. It is worth remembering, when evaluating the final outcomes of this case, that due to the complications and the difficult management of this patient with severe substance, abuse history did not allow for more that very short‐term follow‐up of the procedure. Nevertheless, the aforementioned, in conjunction with the correct graft choice, could aid a predictable, aesthetically and functionally successful treatment. Through "hybrid techniques" (prebending of standard plates with 3D‐models) and inexpensive software & hardware packages, this goal is now within reach for clinics with limited financial resources.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
MBH, MCH, and DE: surgery planning, execution and follow up, methodology and analysis, writing original draft. PRA: 3D model production, surgery planning, writing original draft. NC and JC: 3D model pre‐processing, 3D model digital manipulation, 3D Printing of mandibular model. GS: 3D model pre‐processing, 3D model digital manipulation, 3D printing of mandibular model, project supervision, writing‐review & editing.
ACKNOWLEDGMENTS
The authors wish to acknowledge the financial support of the “Los Ríos” Regional Government, through its Innovation Competitivity Funds (FIC‐R). Funds allocated to the FIC1720: IngoMED project allowed for the 3D printing of Maxillofacial Biomodels. Published with written consent of the patient.
Barreda Hale M, Romero‐Araya P, Cea Herrera M, et al. Computer‐assisted planning with 3D printing for mandibular reconstruction caused by a mandibular fracture with secondary osteomyelitis: A Case Report. Clin Case Rep. 2021;9:e04410. 10.1002/ccr3.4410
DATA AVAILABILITY STATEMENT
The authors confirm that all relevant data are available within the article and its supplementary materials.
REFERENCES
- 1. Foley BD, Thayer WP, Honeybrook A, et al. Mandibular reconstruction using computer‐aided design and computer‐aided manufacturing: an analysis of surgical results. J Oral Maxillofac Surg. 2013;71:111‐119. [DOI] [PubMed] [Google Scholar]
- 2. Mendes de Alencar MG, De Bortoli MM, Gomes da Silva TC, et al. Suitability of wrist external fixator for treatment of mandibular fracture. J Craniofac Surg. 2018;29(4):e371‐e372. [DOI] [PubMed] [Google Scholar]
- 3. Suei Y, Taguchi A, Tanimoto K, et al. Diagnosis and classification of mandibular osteomyelitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:207‐214. [DOI] [PubMed] [Google Scholar]
- 4. Rubin K. The manifestation of cocaine‐induced midline destructive lesion in bone tissue and its identification in human skeletal remains. Forensic Sci Int. 2013;231(408):e1‐11. [DOI] [PubMed] [Google Scholar]
- 5. Aspera‐Werz RH, Chen T, Ehnert S, et al. Cigarette smoke induces the risk of metabolic bone diseases: transforming growth factor beta signaling impairment via dysfunctional primary cilia affects migration, proliferation, and differentiation of human mesenchymal stem cells. Int J Mol Sci. 2019;20:2896‐2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wanga JJ, Hong‐xia L, Wangc J, et al. Cannabinoid receptors in osteoporosis and osteoporotic pain: a narrative update of review. J Pharm Pharmacol. 2019;71:1469‐1474. [DOI] [PubMed] [Google Scholar]
- 7. Wakolbinger R, Muschitz C, Scheriau G, et al. Bone microarchitecture and bone turnover in hepatic cirrhosis. Osteoporos Int. 2019;30:1195‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Bashaireh AM, Haddad LG, Weaver M, et al. The effect of tobacco smoking on bone mass: an overview of pathophysiologic mechanisms. J. Osteoporos. 2018;2018:12062325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carvalho PH, Da Hora Sales PH, Sombra da Rocha S, et al. Treatment of comminutive fractures by firearm projectiles with adapted wrist external fixator. Oral Maxillofac Surg. 2019;23:501‐505. [DOI] [PubMed] [Google Scholar]
- 10. Akbay E, Aydogan F, et al. Reconstruction of isolated mandibular bone defects with non‐vascularized corticocancellous bone autograft and graft viability. Auris Nasus Larynx. 2014;41:56‐62. [DOI] [PubMed] [Google Scholar]
- 11. Wu J, Sun J, Shen SG, et al. Computer‐assisted navigation: its role in intraoperatively accurate mandibular reconstruction. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:134‐142. [DOI] [PubMed] [Google Scholar]
- 12. Andre CV, Khonsari RH, Ernenwein D, et al. Osteomyelitis of the jaws: a retrospective series of 40 patients. J Stomatol Oral Maxillofac Surg. 2017;118:261‐264. [DOI] [PubMed] [Google Scholar]
- 13. Shuster A, Reiser V, Trejo L, et al. Comparison of the histopathological characteristics of osteomyelitis, medication‐related osteonecrosis of the jaw, and osteoradionecrosis. Int J Oral Maxillofac Surg. 2019;48:17‐22. [DOI] [PubMed] [Google Scholar]
- 14. Marti‐Flich L, Schlund M, Raoul G, et al. Twenty‐four years of experience in management of complex mandibular fractures with low cost, custom‐made mandibular external fixation: A 65‐patient series. J Stomatol Oral Maxillofac Surg. 2019;121:242‐247. [DOI] [PubMed] [Google Scholar]
- 15. Braidy FH, Ziccardi VB, et al. External fixation for mandible fractures. Atlas Oral Maxillofacial Surg Clin N Am. 2009;17:45‐53. [DOI] [PubMed] [Google Scholar]
- 16. Van Baara JC, Forouzanfara T, Libertona NP, et al. Accuracy of computer‐assisted surgery in mandibular reconstruction: a systematic review. Oral Oncol. 2018;84:52‐60. [DOI] [PubMed] [Google Scholar]
- 17. Shilo D, Emodi O, Blanc O, Noy D, Rachmiel A. Printing the future‐updates in 3D printing for surgical applications. Rambam Maimonides Med J. 2018;9(3).e0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Modabber A, Ayoub N, Möhlhenrich S, et al. The accuracy of computer‐assisted primary mandibular reconstruction with vascularized bone flaps: iliac crest bone flap versus osteomyocutaneous fibula flap. Med Devices (Auckl). 2014;7:211‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moura LB, Carvalho PH, Xavier CB, et al. Autogenous non‐vascularized bone graft in segmental mandibular reconstruction: a systematic review. Int J Oral Maxillofac Surg. 2016;45:1388‐1394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all relevant data are available within the article and its supplementary materials.


